Simple Summary
Lung adenocarcinoma, a prevalent histological variant of lung cancer, is experiencing a persistent rise in global incidence. Concurrently, type 2 diabetes presents a significant risk to human health. However, the molecular mechanisms connecting these two diseases remain predominantly unexplored. This study used bioinformatics analysis techniques. Our objective was to identify the potential genes linked to type 2 diabetes and lung adenocarcinoma, as well as to investigate the associated microRNAs (miRNAs) and transcription factor (TF) genes. The research findings indicate that ATR, RFC4, and MCM2 are pivotal genes in the pathogenesis of type 2 diabetes and lung adenocarcinoma. Moreover, hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p exhibit the most significant correlation with these hub genes. These findings enhance the comprehension of the pathogenesis of type 2 diabetes and lung adenocarcinoma while offering critical insights for refining management plans and augmenting treatment strategies for both conditions.
Abstract
Background: Lung adenocarcinoma (LUAD) is a common histopathological variant of non-small cell lung cancer. Individuals with type 2 diabetes (T2DM) face an elevated risk of developing LUAD. We examined the common genomic characteristics between LUAD and T2DM through bioinformatics analysis. Methods: We acquired the GSE40791, GSE25724, GSE10072, and GSE71416 datasets. Differentially expressed genes (DEGs) were identified through R software, particularly its version 4.1.3 and analyzed via gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Subsequently, we analyzed the relationship between immune cell infiltration and DEGs. we constructed a protein–protein interaction network using STRING and visualized it with Cytoscape. Moreover, gene modules were identified utilizing the MCODE plugin, and hub genes were selected through the CytoHubba plugin. Additionally, we evaluated the predictive significance of hub genes using receiver operating characteristic curves and identified the final central hub genes. Finally, we forecasted the regulatory networks of miRNA and transcription factors for the central hub genes. Results: A total of 748 DEGs were identified. Analysis of immune infiltration showed a notable accumulation of effector-memory CD8 T cells, T follicular helper cells, type 1 T helper cells, activated B cells, natural killer cells, macrophages, and neutrophils in both LUAD and T2DM. Moreover, these DEGs were predominantly enriched in immune-related pathways, including the positive regulation of I-κB kinase/NF-κB signaling, positive regulation of immunoglobulin production, cellular response to interleukin-7, and cellular response to interleukin-4. The TGF-β signaling pathway was significantly important among them. Additionally, seven hub genes were identified, including ATR, RFC4, MCM2, NUP155, NUP107, NUP85, and NUP37. Among them, ATR, RFC4, and MCM2 were identified as pivotal hub genes. Additionally, hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p were associated with LUAD and T2DM. SP1 (specific protein 1) and KDM5A (lysine-specific demethylase 5A) regulated MCM2, ATR, and RFC4. Conclusions: Our study elucidates the common mechanisms of immune response, TGF-β signaling pathway, and natural killer cells in LUAD and T2DM, and identifies ATR, RFC4, and MCM2 as key potential biomarkers and therapeutic targets for the comorbidity of these two conditions.
1. Introduction
Lung cancer is among the most common malignancies and continues to be a primary cause of cancer-related mortality globally [1]. The incidence and mortality rates have been consistently rising [2], with an estimated 20,000 new cases and 20,000 deaths each year [3]. The five-year survival rate for lung cancer varies internationally, ranging from 10% to 20% in most nations, with Japan exhibiting the highest rate at 33% [4]. Lung cancer is categorized into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with NSCLC representing roughly 80% of cases [5]. Lung adenocarcinoma (LUAD) is the predominant histological variant among NSCLC subtypes. Although early-stage LUAD patients may benefit from surgical resection, patients in advanced stages necessitate alternative therapies. The advent of molecular-targeted therapies and immune checkpoint inhibitors has considerably broadened treatment alternatives for LUAD [6,7]. Comprehending the molecular mechanisms that govern alterations in gene expression in LUAD is essential for enhancing patient survival rates.
Diabetes mellitus (DM) is a chronic metabolic condition marked by consistently elevated blood glucose levels. The two main subtypes are type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), each characterized by unique pathophysiological mechanisms [8]. T1DM arises from the autoimmune destruction of pancreatic β-cells, resulting in complete insulin deficiency and a lifelong requirement for insulin therapy. Conversely, T2DM is marked by insulin resistance in peripheral tissues, including the liver, muscles, and adipose tissue, alongside progressive dysfunction of pancreatic β-cells, ultimately leading to insufficient insulin secretion. Diabetes is linked to microvascular complications, including nephropathy [9] and retinopathy [10], but also with an increased risk of cancers, including lung, breast, and pancreatic cancer [11], breast cancer [12], and pancreatic cancer [13]. Yang et al. examined clinical data from hospitalized T2DM patients diagnosed with diverse cancers over a five-year period and determined that lung cancer, specifically LUAD, was the predominant malignancy among this cohort [14].
Lai et al. additionally reported that T2DM is associated with reduced serum 25-hydroxyvitamin D3 levels, with diminished levels correlating with an elevated risk of LUAD [15]. Guo et al. discovered that in LUAD, the long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 modulates downstream functional proteins, affecting the progression of diabetes and its complications [16]. This lncRNA competitively binds to microRNAs (miRNAs), influencing downstream transcription factors (TFs) associated with inflammation and apoptosis, which may contribute to the progression of LUAD and diabetes-related complications. These findings indicate a potential bidirectional relationship between LUAD and T2DM, wherein diabetes may facilitate cancer initiation and progression. Nonetheless, the exact mechanisms underlying this association remain ambiguous.
This study aimed to investigate the shared molecular mechanisms between LUAD and T2DM. We acquired datasets (GSE40791, GSE25724, GSE10072, and GSE71416) from the Gene Expression Omnibus (GEO) database, encompassing samples from LUAD patients, T2DM patients, and healthy controls. Differentially expressed genes (DEGs) were identified, and the common DEGs between LUAD and T2DM were subjected to further analysis. Considering the possible association between these diseases and immune dysregulation, we conducted single-sample gene set enrichment analysis (ssGSEA) to evaluate immune infiltration patterns in both LUAD and T2DM. Furthermore, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed to clarify the biological functions and pathways related to the common DEGs. A protein–protein interaction (PPI) network was established to identify hub genes, along with their corresponding miRNAs and transcription factors (TFs). Our findings elucidate the common molecular mechanisms linking LUAD and T2DM, presenting potential targets for forthcoming diagnostic and therapeutic approaches.
2. Materials and Methods
2.1. Data Collection
We established the following as our screening criteria: ensuring data loss in either the samples or the probes, the data align with the research objectives, and in the experimental design, there are scientifically sound and reasonable blank and positive controls. Based on these criteria, we precisely searched for datasets related to “lung adenocarcinoma” and “diabetes” in the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/ (accessed on 5 January 2024)) of the National Center for Biotechnology Information (NCBI). After a series of meticulous screenings, we finally identified four datasets, namely GSE40791 (LUAD), GSE25724 (T2DM), GSE10072 (LUAD), and GSE71416 (T2DM), for further in-depth analysis. The sequencing platform used for GSE40791 (LUAD) and GSE71416 (T2DM) was GPL570, whereas GPL96 was used for GSE25724 (T2DM) and GSE10072 (LUAD).
GSE40791 (LUAD) dataset: This dataset consists of 194 samples, with 100 samples from non-lung adenocarcinoma cases and 94 samples from lung adenocarcinoma cases.
GSE25724 (T2DM) dataset: Contains 13 samples, among which 7 are non-type 2 diabetes samples and 6 are type 2 diabetes samples.
GSE10072 (LUAD) dataset: There are a total of 107 samples, with 49 samples from non-lung adenocarcinoma cases and 58 samples from lung adenocarcinoma cases.
GSE71416 (T2DM) dataset: Includes 20 samples, 6 from non-diabetes samples and 14 from diabetes samples. The GSE71416 (T2DM) and GSE10072 (LUAD) datasets respectively serve as external validation datasets for the corresponding diseases. By performing validation analyses on this dataset, we assessed the stability and consistency of key genes across different datasets and sample conditions, thereby enhancing the reliability of the study results.
2.2. Identification of DEGs
The R packages “limma” and “GEOquery” (version 4.1.3) were used for data normalization and probe annotation of the GSE40791 and GSE25724 datasets. DEGs were identified using an adjusted p-value threshold of <0.05 and an absolute log2 fold change > 1.5. The R package “ggplot” was used to generate volcano plots for visualizing the DEGs in the datasets. The heat map was generated using the “heatmap” function in R. Venn diagram analysis was conducted using the online tool (http://www.liuxiaoyuyuan.cn/ (accessed on 7 January 2024)) to extract the DEGs commonly observed between GSE40791 and GSE25724.
2.3. Immune Infiltration Analysis
The ssGSEA (http://www.biocloudservice.com/home.html (accessed on 10 February 2024)) score was used to quantify immune cell infiltration in LUAD and T2DM tissues, providing an infiltration level for each dataset sample. The ssGSEA score was used to quantify the infiltration of immune cells in LUAD or T2DM tissues and determine the level of immune infiltration in each dataset sample. Spearman correlation analysis was conducted to determine the correlation between DEGs and 28 immune cells.
2.4. Enrichment and Pathway Analysis
Using the DAVID online tool (https://davidbioinformatics.nih.gov/ (accessed on 13 February 2024)), we performed gene ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on the common DEGs to elucidate their functions. The enriched GO terms and KEGG pathways with a p-value < 0.05 were analyzed and visually presented using both bubble and bar graphs.
2.5. Construction of Protein–Protein Interaction (PPI) Network
We employed the protein interaction gene search tool STRING (http://string-db.org/ (accessed on 15 February 2024)) for the analysis of PPI of the common DEGs. We extracted PPI pairs with interaction scores above 0.9 and visualized them using Cytoscape 3.9.1 (https://cytoscape.org/release_notes_3_9_1.html (accessed on 17 February 2024)). Furthermore, we used the Cytoscape plugin MCODE to identify significant functional modules, with the following selection criteria: K-core of 2, degree cutoff of 2, maximum depth of 100, and node score cutoff of 0.2.
2.6. Identification of Hub Genes
The CytoHubba (https://cytoscape.org/release_notes_3_9_1.html (accessed on 20 February 2024)) plugin of Cytoscape was used to identify hub genes from the PPI network, applying four algorithms—MNC, MCC, EPC, and Degree—to confirm the key hub genes. A Venn diagram was used for visualization.
2.7. Receiver Operating Characteristic (ROC) Curve Analysis of Hub Genes
The pROC software package (version 4.1.3) in R language was used to generate ROC curves to evaluate the predictive values of hub genes in two test datasets, including GSE10072 (LUAD) and GSE71416 (T2DM).
2.8. Prediction of miRNAs for the Central Hub Genes
To gain a deeper understanding of the relationship between miRNAs and target genes of T2DM and LUAD, we used the NetworkAnalyst dataset (https://www.networkanalyst.ca/ (accessed on 5 March 2024)) to predict the miRNAs of the central hub genes. The KEGG enrichment analysis of three common miRNAs (hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p) was conducted using miRPath4.0 (http://www.microrna.gr/miRPathv4 (accessed on 8 March 2024)) to identify signaling pathways.
2.9. Construction of Hub Gene-TF Regulatory Network
To investigate the interactions between TFs and central hub genes and evaluate the influence of TFs on the expression and functional pathways of central hub genes, we employed NetworkAnalyst. We predicted the TFs for central hub genes from this database and visualized the transcriptional regulatory network using the Cytoscape software.
3. Results
3.1. Identification of Common DEGs Between T2DM and LUAD
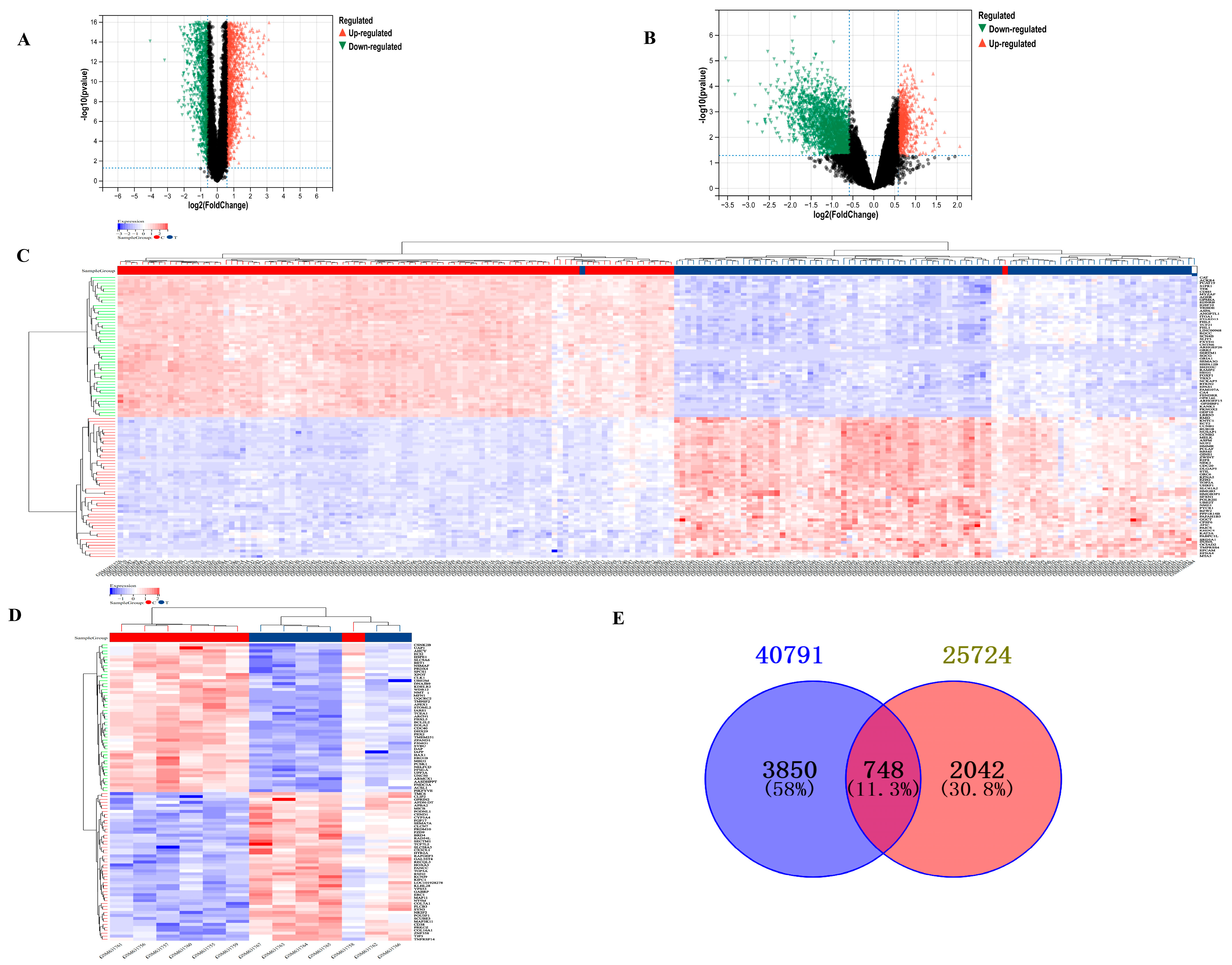
After screening based on the criteria of p-value < 0.05 and |log2 fold change| > 1.5, a total of 4598 DEGs from GSE40791 and 2790 DEGs from GSE25724 were identified, which are presented in volcano plots (Figure 1A,B) and heat maps (Figure 1C,D), respectively. Furthermore, using Venn diagram analysis, 748 DEGs were identified at the intersection of LUAD DEGs and TDM DEGs, after excluding genes with opposite expression trends (Figure 1E). There were 99 upregulated DEGs and 526 downregulated DEGs.
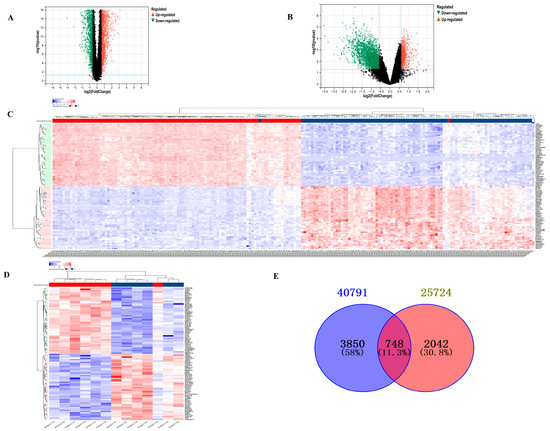
Figure 1.
Analysis of DEGs between T2DM and LUAD. (A) Volcano plot showing DEGs of LUAD from GSE40791. (B) Volcano plot showing DEGs of T2DM from GSE25724. (C) Heat map showing DEGs of T2DM from GSE40791. (D) Heat map showing DEGs of LUAD from GSE25724. (E) Venn diagram showing common DEGs between T2DM and LUAD.
3.2. Immune Infiltration Analysis in LUAD and T2DM
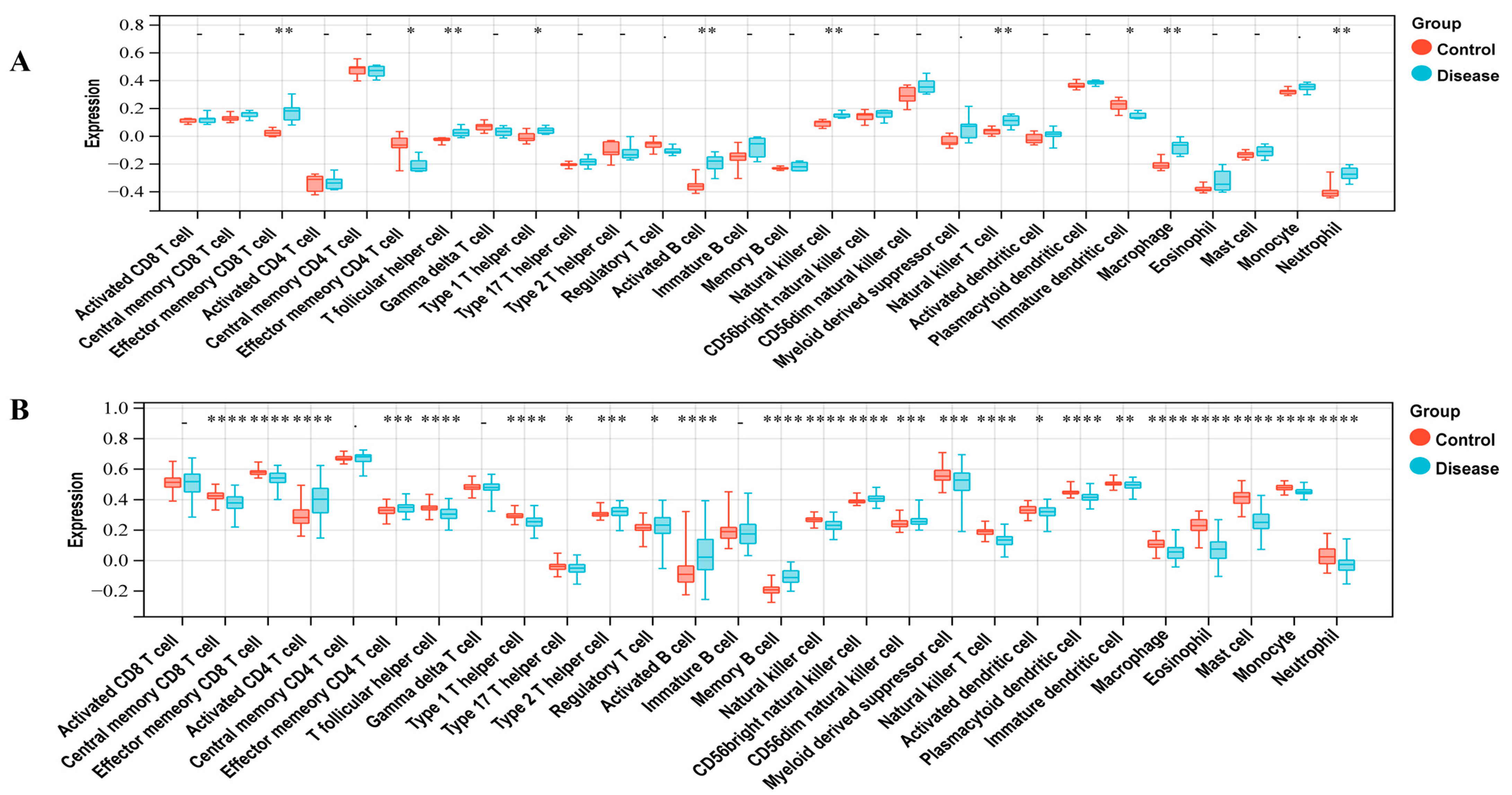
To investigate the immunocyte infiltration in the control and case groups of LUAD and T2DM, we performed the ssGSEA analysis. Figure 2A demonstrates the correlation between LUAD and immune cells, including Central memory CD8 T cell, Effector memory CD8 T cell, Activated CD4 T cell, Effector memory CD4 T cell, T follicular helper cell, Type 1 T helper cell, Type 17 T helper cell, Type 2 T helper cell, Regulatory T cell, Activated B cell, Memory B cell, Natural killer cell, CD56bright natural killer cell, CD56dim natural killer cell, Myeloid-derived suppressor cell, Natural killer T cell, Activated dendritic cell, Plasmacytoid dendritic cell, Immature dendritic cell, Macrophage, Eosinophil, Mast cell, Monocyte, and Neutrophil. As shown in Figure 2B, T2DM was correlated with immune cells, including the Effector memory CD8 T cell, Effector memory CD4 T cell, T follicular helper cell, Type 1 T helper cell, Activated B cell, Natural killer cell, Natural killer T cell, Immature dendritic cell, Macrophage, and Neutrophil.
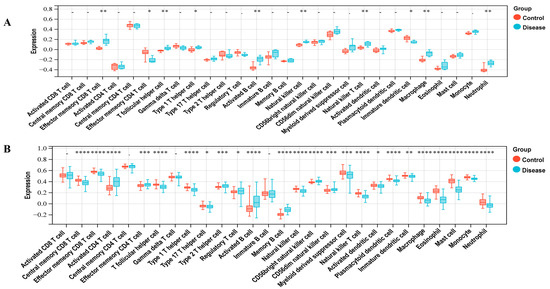
Figure 2.
Immune infiltration analysis. (A) Correlation between LUAD with immune cells. (B) Correlation between T2DM with immune cells. * represents a p-value < 0.05; ** represents a p-value < 0.01; *** represents a p-value < 0.001; **** represents a p-value < 0.0001.
3.3. Enrichment Analysis of Common DEGs
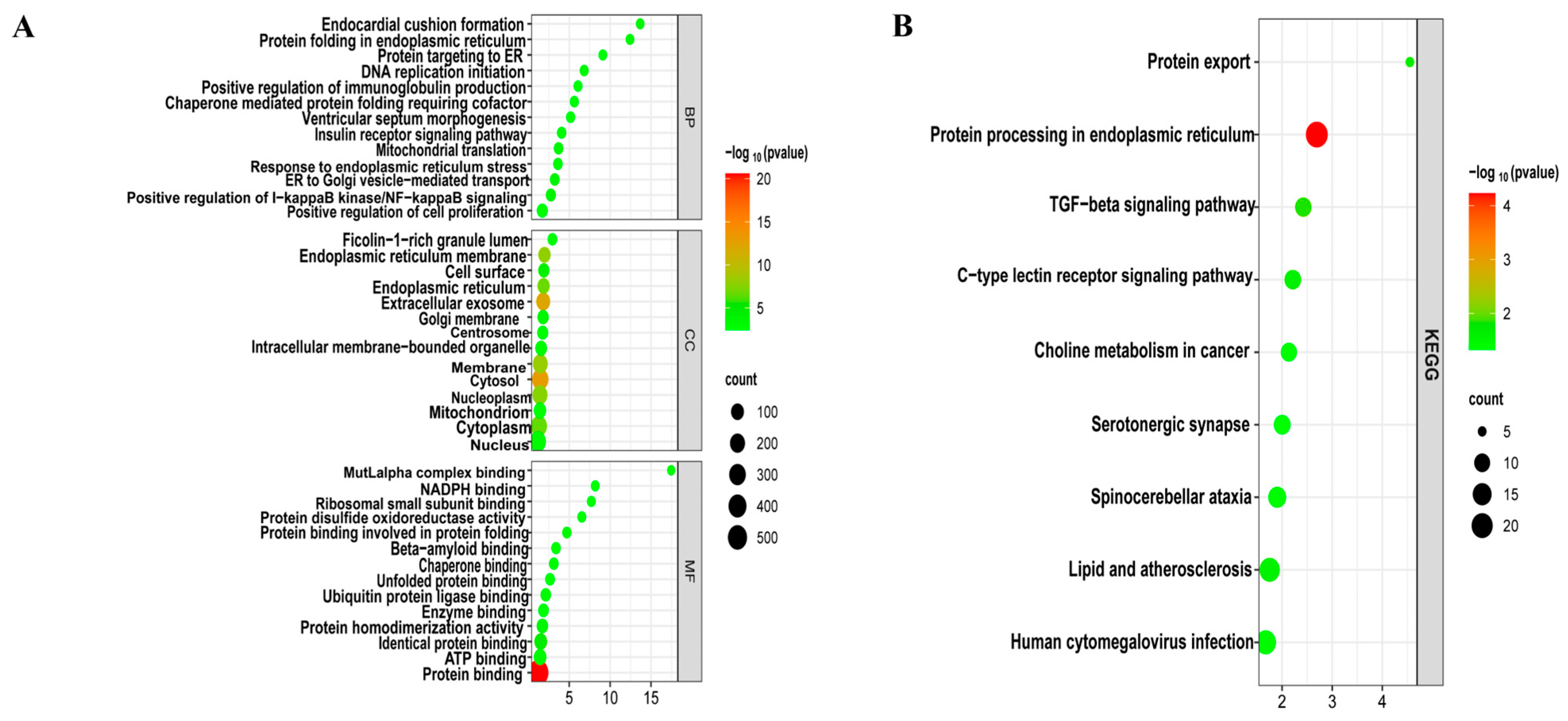
To decipher the biological functions, GO (Figure 3A) and KEGG (Figure 3B) functional enrichment analyses were performed using the DAVID online tool on 748 common DEGs of LUAD and T2DM. These genes were primarily involved in biological processes such as the positive regulation of I-κB kinase/NF-κB signaling, positive regulation of immunoglobulin production, positive regulation of cell proliferation, cellular response to interleukin-7, cellular response to interleukin-4, and positive regulation of TOR signaling. Molecular functions of these genes included protein binding, lipid binding, protein homodimerization activity, and ubiquitin protein ligase binding, among others. In terms of cellular components, these genes were predominantly associated with the cytosol, extracellular vesicles, endoplasmic reticulum membrane, and cytoplasm. Additionally, KEGG pathway analysis revealed involvement in the transforming growth factor-beta (TGF-β) signaling pathway, C-type lectin receptor signaling pathway, lipid metabolism, and atherosclerosis.
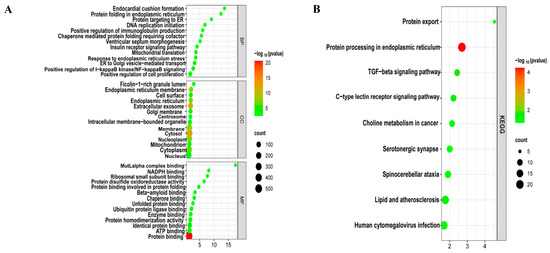
Figure 3.
Enrichment analysis of common DEGs. (A) GO enrichment analysis. Top GO terms in biological process (BF), cellular component (CC), and molecular function (MF) are displayed. (B) KEGG pathway enrichment analysis. Top 9 pathways are presented.
3.4. PPI Network
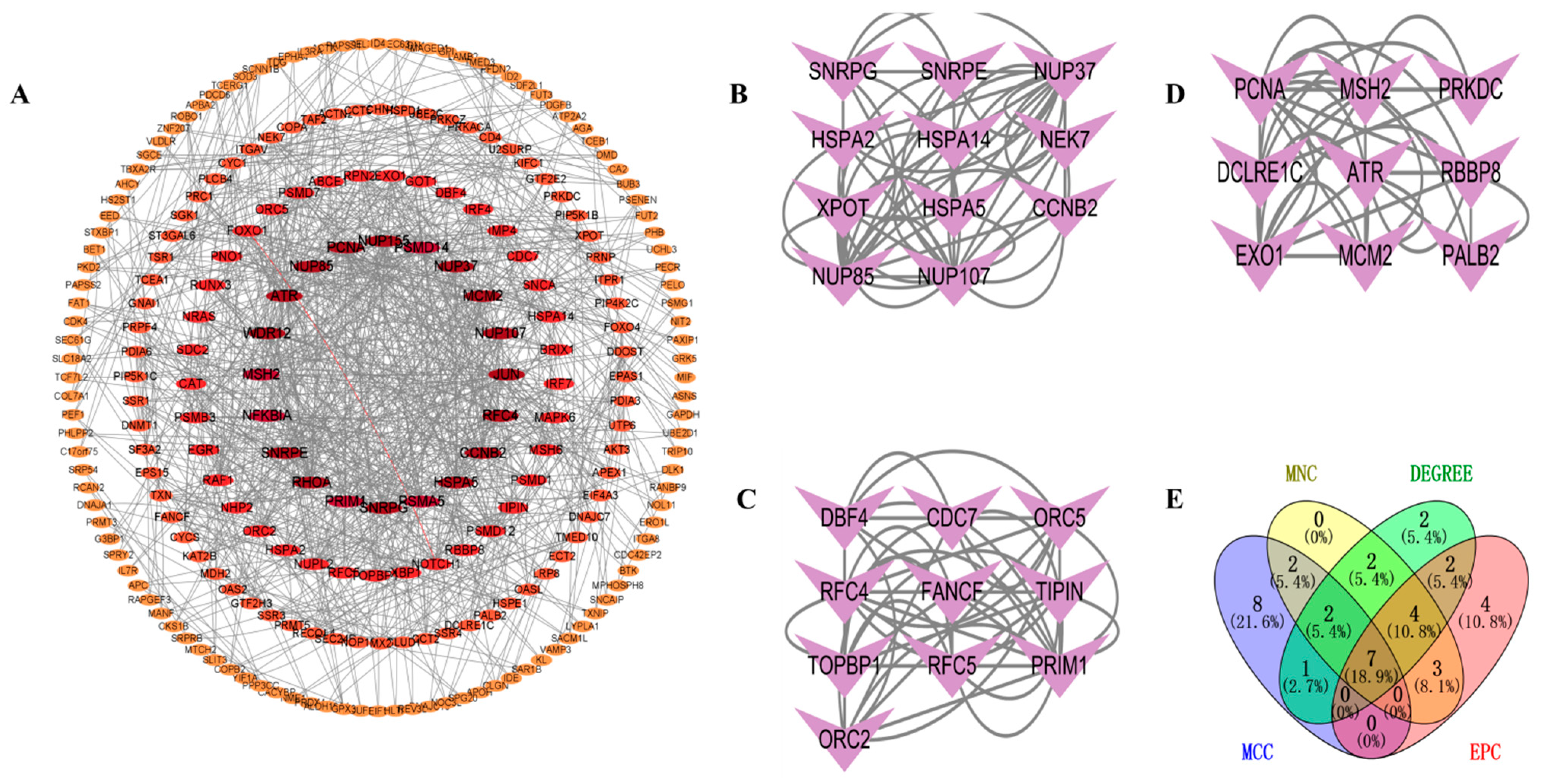
To investigate the interactions between DEGs, we constructed a PPI network using the STRING database. A visual interaction score of 0.9 was set as the minimum threshold for constructing the PPI network. This PPI network consisted of 739 nodes and 577 edges, with a PPI enrichment p-value < 2.75 × 10−5 (Figure 4A). Using the MCODE plugin in Cytoscape, we identified three significant modules (Figure 4B–D) comprising 11 nodes and 56 edges, 10 nodes, and 46 edges, and 9 nodes and 34 edges, respectively. By employing four algorithms (MCC, MNC, Degree, and EPC) in the CytoHubba plugin, we obtained the top 20 hub genes and visualized the overlapping central hub genes among these algorithms using a Venn diagram. Finally, seven central hub genes were identified, including MCM2, RFC4, ATR, NUP155, NUP107, NUP85, and NUP37 (Figure 4E).
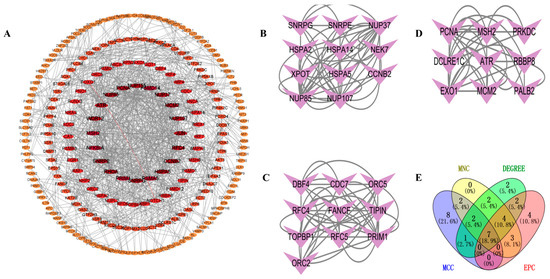
Figure 4.
PPI network analysis. (A) PPI network. (B) Significant module 1. (C) Significant module 2. (D) Significant module 3. (E) Venn diagram of MNC, DEGREE, EPC, and MCC.
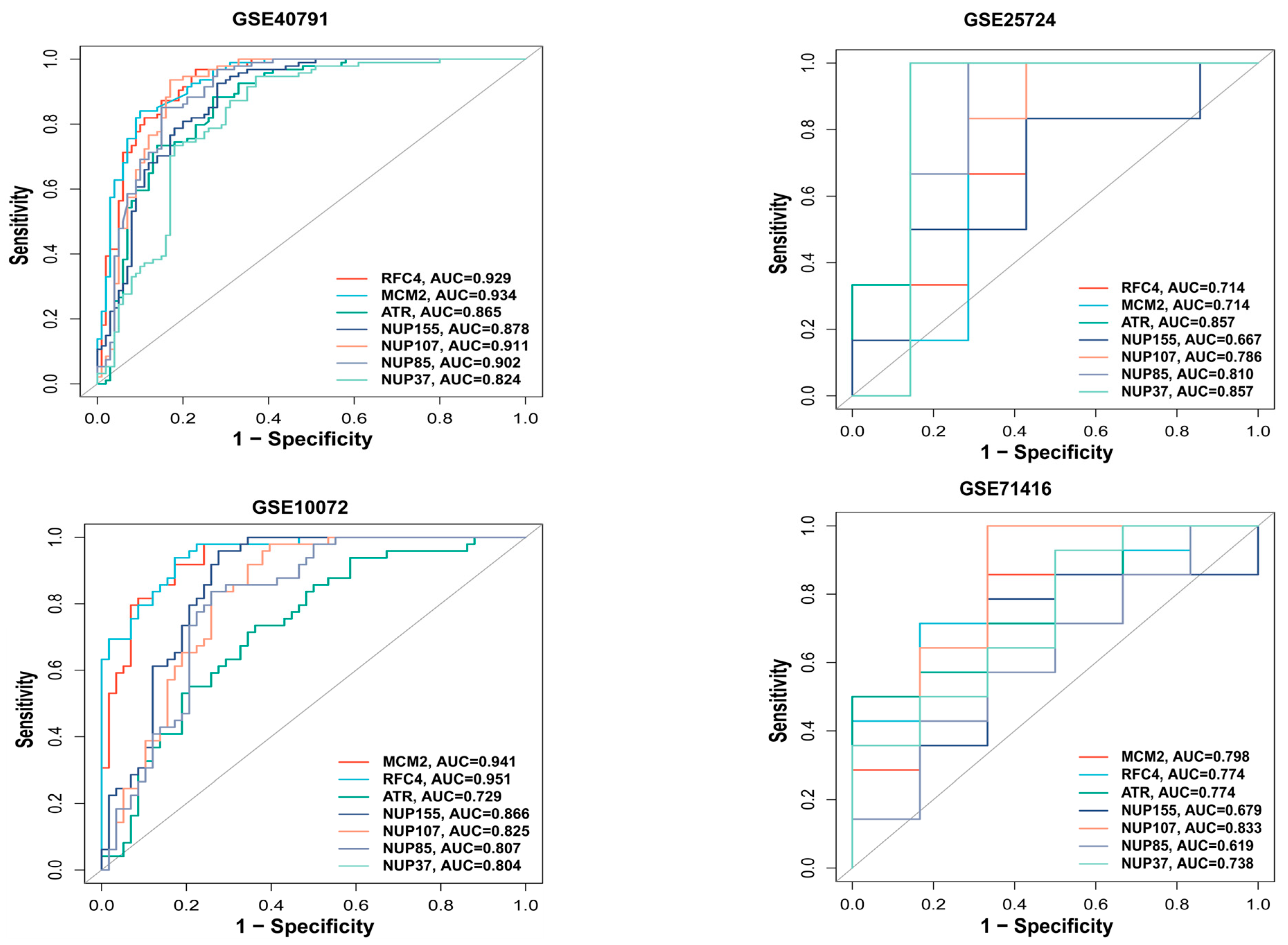
3.5. ROC Analysis of Hub Genes
We used the pROC package in R to plot the ROC curve for the hub genes and evaluate their predictive accuracy. Our training set included GSE40791 and GSE25724, while the validation set consisted of GSE10072 (LUAD) and GSE71416 (T2DM). In all the datasets, including GSE10072 and GSE71416, the area under the curve values of the three hub genes, RFC4, MCM2, and ATR, were all greater than 0.7, indicating their high predictive reliability (Figure 5). Therefore, we selected these three hub genes as the central hub genes.
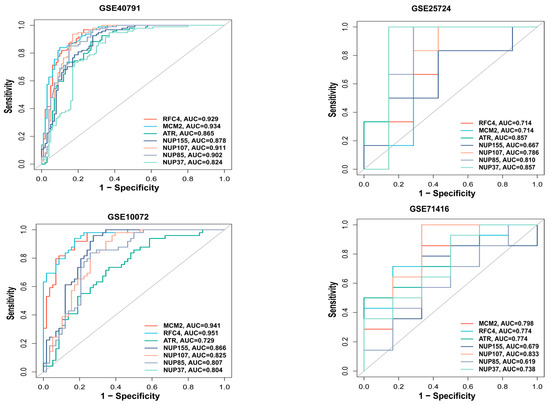
Figure 5.
ROC analysis of hub genes. ROC curve for hub genes from the training set (GSE40791 and GSE25724) and the validation set (GSE10072 and GSE71416) were plotted with the pROC package in R.
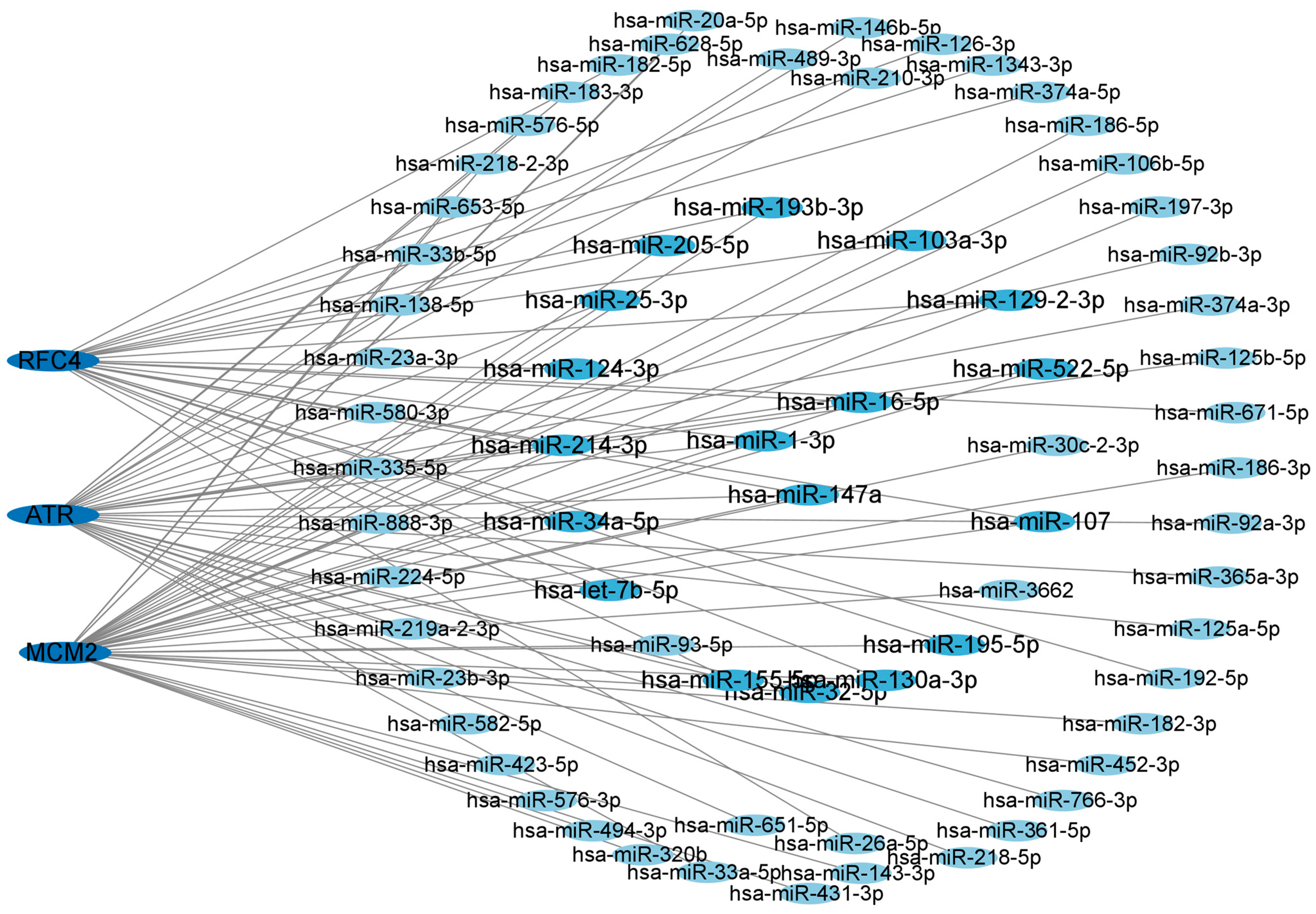
3.6. Network of Central Hub Genes and miRNAs
To gain a deeper understanding of the relationship between miRNAs and target genes of T2DM and LUAD, we constructed and utilized the miRNA–target gene network using the NetworkAnalyst dataset. We displayed the central hub genes and the associated regulatory miRNAs in Figure 6. The results revealed that the degree of hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p were greater than 3, indicating a stronger association with T2DM and LUAD. Therefore, these candidate miRNAs may provide a solid foundation for understanding the molecular mechanisms of T2DM and LUAD, as well as revealing a series of promising shared targets in T2DM and LUAD.
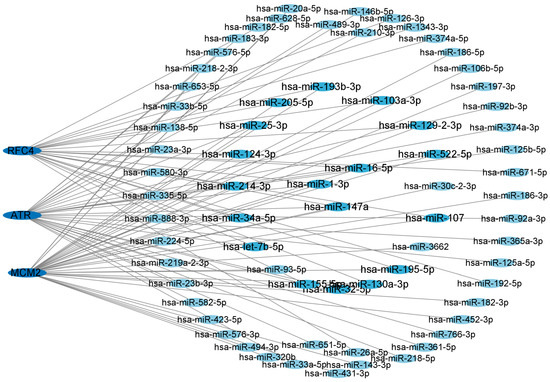
Figure 6.
Network of central hub genes and miRNAs. miRNA–target gene network was constructed using the NetworkAnalyst dataset. Central hub genes were RFC4, MCM2, and ATR.
3.7. KEGG Pathway Enrichment Analysis of hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p
We performed KEGG enrichment analysis on hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p using miRPath4.0. The results demonstrated that multiple signaling pathways were identified, including the TGF-β signaling pathway, Rap1 signaling pathway, MAPK signaling pathway, IL-17 signaling pathway, PI3K-Akt signaling pathway, and other signaling pathways (Table 1). Therefore, these three miRNAs may play a role in the dysregulation of signal transduction and immune system pathways in T2DM and LUAD.

Table 1.
Enriched KEGG pathway.
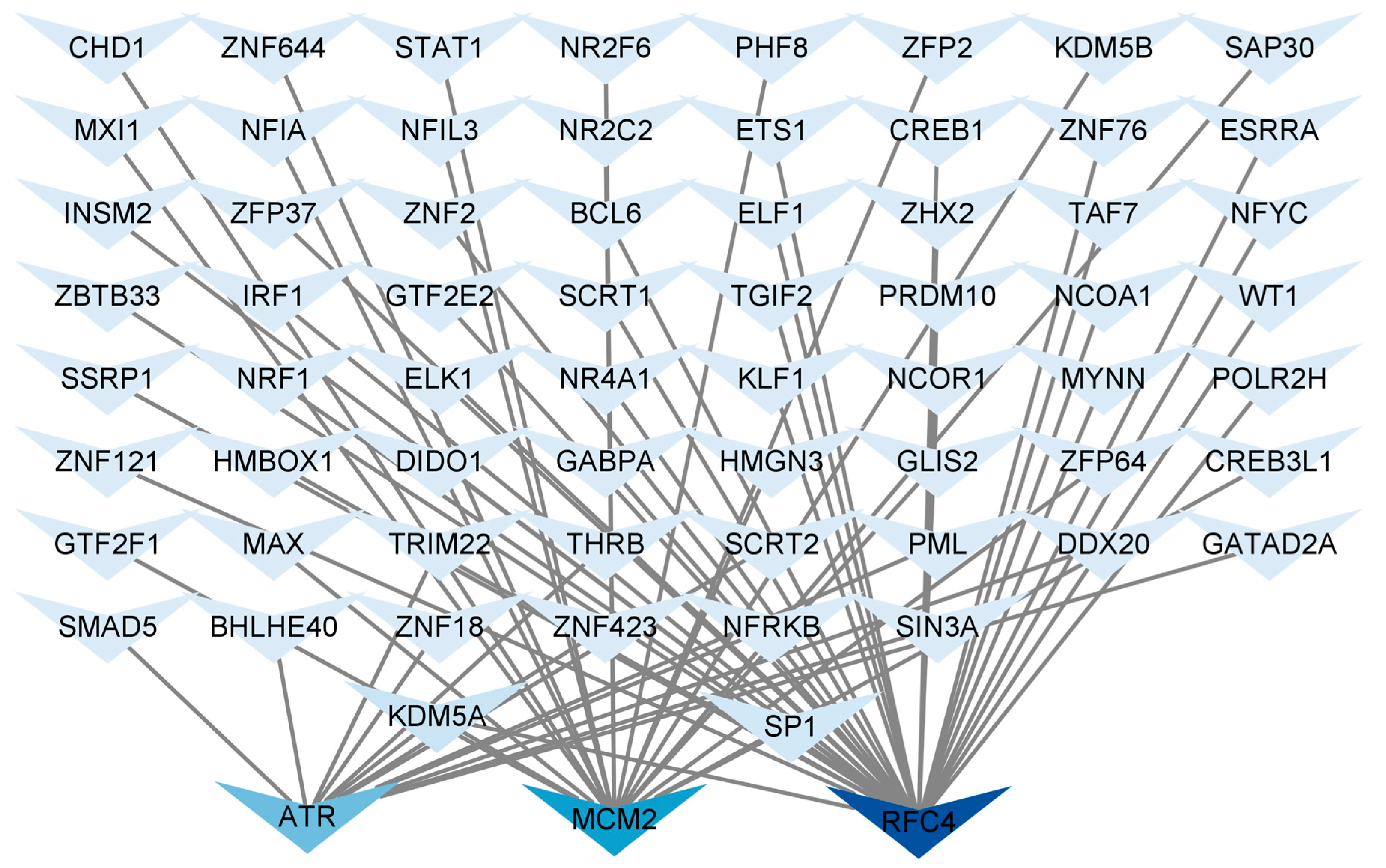
3.8. Construction of TF Regulatory Network
Identifying common TFs will help us understand the possible mechanisms related to T2DM and LUAD. We used NetworkAnalyst 3.0 to analyze the interactions between the central hub genes and TFs and evaluate the impact of TFs on the expression and functional pathways of the central hub genes. A hub gene–TF regulatory network consisting of 67 TFs was constructed (Figure 7). ATR was regulated by 13 TFs, MCM2 was regulated by 20 TFs, and RFC4 was regulated by 34 TFs. Additionally, in the hub gene-TF regulatory network, both specificity protein 1 (SP1) and lysine-specific demethylase 5A (KDM5A) regulated the central hub genes. Therefore, these two regulatory factors were identified as key TFs that were crucial for guiding gene expression and the physiological and pathological processes of T2DM and LUAD.
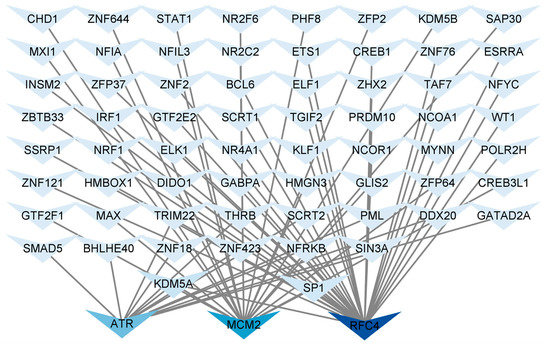
Figure 7.
Regulatory network of central hub genes and TFs. Hub gene–TF regulatory network was constructed using the NetworkAnalyst. Central hub genes were RFC4, MCM2, and ATR.
4. Discussion
Diabetes mellitus (DM) is a chronic metabolic condition with substantial genetic foundations, although its exact etiology and pathogenesis are still not fully understood. As of now, roughly 463 million adults between the ages of 20 and 79 are afflicted by diabetes, constituting 9.3% of the global population within this demographic [17]. Although diabetes is typically linked to microvascular complications, there is growing evidence connecting it to multiple cancers. Lung adenocarcinoma (LUAD) has been recognized as a significant complication linked to extended diabetes duration [15,18]. Research has established correlations between type 2 diabetes mellitus (T2DM) and various malignancies, including cancer [19], breast cancer [20], endometrial cancer [21], hepatocellular carcinoma [22], pancreatic cancer [23], lung cancer [24], renal cell carcinoma [25], and cervical cancer [26,27]. Considering that genetic factors significantly affect T2DM susceptibility, it is posited that diabetes may not only coexist with but also facilitate cancer development [28]. However, the specific molecular mechanisms connecting diabetes and cancer remain predominantly unexamined. Consequently, our research examines the interaction between T2DM and LUAD.
We conducted a bioinformatics analysis using datasets GSE24724 and GSE40791 from the GEO database to explore the common molecular mechanisms linking T2DM and LUAD. A total of 738 DEGs exhibiting analogous expression patterns in T2DM and LUAD were identified and analyzed for functional enrichment. GO and KEGG pathway analyses indicated that these DEGs were primarily enriched in immune-related pathways, encompassing the positive regulation of I-κB kinase/NF-κB signaling, enhancement of immunoglobulin production, cellular responses to interleukin-7 and interleukin-4, positive regulation of the TOR signaling pathway, the TGF-β signaling pathway, and the C-type lectin receptor signaling pathway. These findings underscore the pivotal role of inflammation in the pathogenesis of both type 2 diabetes mellitus and lung adenocarcinoma. The TGF-β signaling pathway is recognized for its regulation of cell proliferation, differentiation, apoptosis, migration, and tumor progression [29,30]. In early-stage cancer, TGF-β demonstrates tumor-suppressive effects by inducing G1 cell cycle arrest and apoptosis [31]. Subsequently, TGF-β facilitates EMT, stemness, metastasis, drug resistance, and malignant progression [32]. Elevated glucose levels in T2DM have been demonstrated to increase pro-inflammatory cytokines, such as TNF-α, IFN-γ, and TGF-β, which may augment TGF-β pathway activation and heighten the risk of LUAD [33].
Analysis of immune infiltration demonstrated substantial correlations among LUAD, T2DM, and various immune cell populations, including effector memory CD8+ T cells, T follicular helper cells, type 1 T helper cells, activated B cells, NK cells, macrophages, and neutrophils. NK cells, comprising 5% to 19% of peripheral blood lymphocytes, are crucial in anti-tumor immunity via the secretion of cytokines and chemokines [34,35]. Decreased NK cell activity has been associated with heightened cancer susceptibility [36], and modified NK cell function has been noted in obesity and T2DM [37,38]. In type 2 diabetes mellitus, natural killer cells demonstrate diminished cytotoxicity and decreased population levels. Additionally, they promote TGF-β pathway activation through the secretion of TNF-α and IFN-γ, thereby advancing LUAD progression [39,40,41]. Impaired NK cell function may therefore contribute to both elevated cancer risk and increased cancer incidence in patients with T2DM [35].
ATR is expressed in multiple tumor types. Inhibition of ATR and TOP1 is reported to augment the immunogenicity of small-cell lung cancer [42]. Furthermore, research has demonstrated the elevated expression of MCM2 in various cancers, including breast cancer [43] and hepatocellular carcinoma [44], suggesting that MCM2 may represent a viable therapeutic target for numerous cancer types. RFC4 has been documented in nasopharyngeal carcinoma [45], oral squamous cell carcinoma [46], and various other malignancies. RFC4 has been identified as a potential pathogenic mechanism in T2DM [47]. We used the CytoHubba plugin of Cytoscape to identify potential hub genes. Seven genes were identified: ATR, MCM2, RFC4, NUP155, NUP107, NUP85, and NUP37. Subsequently, we delineated the ROC curves for these seven prospective hub genes. The findings indicated that the AUC values for ATR, RFC4, and MCM2 exceeded 0.7 in both validation datasets. Consequently, we identified these three genes as the primary hub genes for subsequent investigation.
MicroRNAs are being extensively researched for their functions in development, diagnosis, and prognosis across various domains. A recent study indicated that miR-1 serves as a dependable predictor of myocardial lipotoxicity in T2DM [48]. Hsa-mir-1-3p is ranked third in non-small cell lung cancer relative to normal lung tissue [49]. Hsa-mir16-5p is a crucial biomarker for the diagnosis of Alzheimer’s disease [50]. Currently, there is no report associating hsa-mir16-5p with LUAD and T2DM. In our study, we developed a central hub gene–miRNA network to investigate the potential regulatory mechanisms of central hub genes in T2DM and LUAD. The findings indicated that hsa-mir147a, hsa-mir16-5p, and hsa-mir-1-3p exhibited the greatest connectivity with the three central hub genes. This signifies their significance in the possible mechanisms of LUAD and T2DM. No studies exist regarding the role of mir-147a in LUAD and T2DM. It has been reported that miR-147a modulates the progression of NSCLC by targeting CC chemokine ligand 5 [51]. Furthermore, miR-147a directly interacts with myocardial infarction-associated transcript (MIAT), and E2F transcription factor 3 (E2F3) has been established as the target gene of miR-147a [52]. Altered expression of MIAT is implicated in cellular apoptosis and the pathogenesis of several diseases, including myocardial infarction, microvascular dysfunction, diabetes, and cancer [53]. miR-147a significantly inhibits E2F3 expression, while the competitive interaction between MIAT and miR-147a liberates E2F3. Thus, the MIAT/miR-147a/E2F3 axis may promote cellular proliferation and fibrosis in the progression of diabetic nephropathy [52].
Transcription factors are crucial in modulating gene expression. In the hub gene-TF regulatory network, we noted that SP1 and KDM5A exhibited a greater interaction frequency with the three central hub genes. SP1 is a transcription factor of the Sp/KLF family that operates by binding to GC-rich sequences in gene regulatory regions. SP1 regulates cell proliferation, apoptosis, differentiation, and angiogenesis [54]. SP1 is significantly overexpressed in gastric cancer [55], meningioma [56], lung adenocarcinoma [57], liver cancer [58], and pancreatic cancer [59]. SP1 engages with the gene regulatory regions that encode components of the MAPK, p38, JAK/STAT, and PI3K/Akt signaling pathways, thereby influencing cell proliferation, differentiation, and apoptosis [60]. KDM5A/RBP2 is a demethylase that suppresses NOTCH signaling, sustains neuroendocrine differentiation, and facilitates the development of small-cell lung cancer [61].
T2DM and LUAD share a complex bidirectional relationship that significantly influences patient diagnosis, prognosis, and treatment. The fundamental molecular mechanisms likely entail complex and interconnected interactions among various signaling pathways that are essential in the pathogenesis of both diseases. This study identifies ATR, RFC4, and MCM2 as pivotal crosstalk genes that significantly influence T2DM and LUAD. ATR is essential for preserving the genomic stability of pancreatic islet β-cells. After ATR malfunction, insulin secretion will be compromised. MCM2 participates in the regulation of the cell cycle and DNA replication. Aberrant MCM2 function can interfere with normal cellular metabolism, significantly impacting the pathogenesis of T2DM. If ATR undergoes mutations or exhibits abnormal functionality, it will result in the disruption of the regulatory mechanisms governing cell proliferation, thereby posing a latent risk for the development of lung adenocarcinoma. RFC4 is crucial in the DNA repair mechanism. When RFC4 malfunctions, the cell’s capacity to repair DNA damage is markedly diminished, thereby hastening the onset and progression of LUAD. Aberrant MCM2 function disrupts the normal cell cycle and exacerbates the progression of LUAD. These pivotal crosstalk genes create intricate molecular linkages between the two diseases, offering a novel perspective and theoretical foundation for a more profound comprehension of the pathogenesis of T2DM and LUAD, as well as the formulation of more efficacious diagnostic and therapeutic strategies.
5. Conclusions
This bioinformatics study identified common genetic traits between T2DM and LUAD, elucidating potential molecular mechanisms involved in their pathogenesis. Our analysis indicates that dysregulated immune responses and abnormal TGF-β signaling may serve as shared pathogenic mechanisms connecting LUAD and T2DM. Moreover, ATR, MCM2, and RFC4 have emerged as prospective therapeutic targets for both conditions, necessitating further exploration. Our findings establish a basis for future research focused on creating innovative therapeutic and preventive approaches for T2DM and LUAD.
Author Contributions
N.N., D.D. and W.Z. designed the study. S.H. and Y.P. performed bioinformatic analysis. N.M. and A.B. contributed to the conception of the study and drafted the manuscript. A.A. contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Tianshan Talents-Youth Science and Technology Innovation Talents Training Program of Xinjiang Autonomous Region, grant number 2022TSYCCX0035; National Natural Science Foundation of China, grant number 81660696; “Fourteenth Five-Year Plan” Key Discipline Construction Project of Xinjiang Autonomous Region, grant number 2021; Xinjiang Key Laboratory of Natural Medicines Active Components and Drug Release Technology, grant number XJDX1713; Xinjiang Key Laboratory of Biopharmaceuticals and Medical Devices, grant number 2023; and Engineering Research Center of Xinjiang and Central Asian Medicine Resources, Ministry of Education, grant number 2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets (GSE40791, GSE25724, GSE, GSE10072, GSE71416) for this study can be found in the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/ (accessed on 5 January 2024)) and the STRING database (https://cn.string-db.org/ (accessed on 15 February 2024)). All the data in this paper support the results of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuhn, E.; Morbini, P.; Cancellieri, A.; Damiani, S.; Cavazza, A.; Comin, C.E. Adenocarcinoma classification: Patterns and prognosis. Pathologica 2018, 110, 5–11. [Google Scholar] [PubMed]
- Chen, P.; Quan, Z.; Song, X.; Gao, Z.; Yuan, K. MDFI is a novel biomarker for poor prognosis in LUAD. Front. Oncol. 2022, 12, 1005962. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, D.; Yan, C.; Su, H.; Zhang, X.; Shi, Y.; Ying, K. METTL3 promotes lung adenocarcinoma tumor growth and inhibits ferroptosis by stabilizing SLC7A11 m6A modification. Cancer Cell Int. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, D.; Shen, J.; Niu, Z.; Hu, M.; Zeng, H.; Yang, Z.; Zhang, Z.; Zhao, C.; Cao, G. Analysis of the incidence and mortality of lung cancer in countries with different Human Development Index. Shanghai J. Prev. Med. 2023, 35, 305–313. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar]
- Liang, Y.; Wang, H.; Chen, B.; Mao, Q.; Xia, W.; Zhang, T.; Song, X.; Zhang, Z.; Xu, L.; Dong, G.; et al. circDCUN1D4 suppresses tumor metastasis and glycolysis in lung adenocarcinoma by stabilizing TXNIP expression. Mol. Ther. Nucleic Acids 2020, 23, 355–368. [Google Scholar]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, s1470–s2045. [Google Scholar]
- Sapra, A.; Bhandari, P. Diabetes Mellitus; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Palumbo, C.; Nicolaci, N.; La Manna, A.A.; Branek, N.; Pissano, M.N. Asociación de diabetes insípida central y diabetes mellitus tipo 2 [Association between central diabetes insipidus and type 2 diabetes mellitus]. Medicina 2018, 78, 127–130. (In Spanish) [Google Scholar]
- Oh, A.J.; Javaheri, M.; Hosseini, H.; Prasad, P.S. Purtscher-like retinopathy in a 19-year-old with maturity-onset diabetes of the young: A case report. J. Med. Case Rep. 2023, 17, 255. [Google Scholar] [CrossRef]
- Stan, M.C.; Mireştean, C.C.; Stoica, D.; Popescu, F.C.; Bădulescu, F. Lung cancer and type 2 diabetes experience in Dolj County (southwest region of Romania)—A clinical, bioclinical and pathological study. Rom. J. Morphol. Embryol. 2023, 64, 411–417. [Google Scholar] [CrossRef]
- Chou, P.C.; Choi, H.H.; Huang, Y.; Fuentes-Mattei, E.; Velazquez-Torres, G.; Zhang, F.; Phan, L.; Lee, J.; Shi, Y.; Bankson, J.A.; et al. Impact of diabetes on promoting the growth of breast cancer. Cancer Commun. 2021, 41, 414–431. [Google Scholar] [CrossRef]
- Pizzato, M.; Turati, F.; Rosato, V.; La Vecchia, C. Exploring the link between diabetes and pancreatic cancer. Expert Rev. Anticancer Ther. 2019, 19, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, C.; Shen, H.; Wu, W.; Tian, Z.; Xu, Q.; Cao, C.; Ye, S.; Ban, L.; Tong, X.; et al. Discovery and validation of PZP as a novel serum biomarker for screening lung adenocarcinoma in type 2 diabetes mellitus patients. Cancer Cell Int. 2021, 21, 162. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, E.; Huang, Y. Analysis of the correlation between serum 25-hydroxyvitamin D3 levels and the occurrence of lung adenocarcinoma in type 2 diabetes. New World Diabetes 2022, 25, 35–38. [Google Scholar] [CrossRef]
- Guo, F.; Ma, H.; Lan, W.; Yao, L. Research progress on long non-coding RNA-transcript 1 associated with lung adenocarcinoma metastasis in diabetes and its complications. Int. J. Pharm. Res. 2020, 47, 798–803. [Google Scholar] [CrossRef]
- Russo, M.P.; Grande-Ratti, M.F.; Burgos, M.A.; Molaro, A.A.; Bonella, M.B. Prevalence of diabetes, epidemiological characteristics and vascular complications. Arch. Cardiol. Mex. 2023, 93, 30–36. (In English) [Google Scholar] [CrossRef]
- Sciacca, L.; Vigneri, R.; Tumminia, A.; Frasca, F.; Squatrito, S.; Frittitta, L.; Vigneri, P. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 808–815. [Google Scholar] [CrossRef]
- Wei, J.; Wu, Y.; Zhang, X.; Sun, J.; Li, J.; Li, J.; Yang, X.; Qiao, H. Type 2 diabetes is more closely associated with risk of colorectal cancer based on elevated DNA methylation levels of ADCY5. Oncol. Lett. 2022, 24, 206. [Google Scholar] [CrossRef]
- Ashley, L.; Robb, K.A.; O’Connor, D.B.; Platt, R.; Price, M.; Robinson, O.; Travis, E.; Lipscombe, L.; Ajjan, R.; Birch, R. Increased Breast and Colorectal Cancer Risk in Type 2 Diabetes: Awareness Among Adults With and Without Diabetes and Information Provision on Diabetes Websites. Ann. Behav. Med. 2023, 57, 386–398. [Google Scholar] [CrossRef]
- Zabuliene, L.; Kaceniene, A.; Steponaviciene, L.; Linkeviciute-Ulinskiene, D.; Stukas, R.; Arlauskas, R.; Vanseviciute-Petkeviciene, R.; Smailyte, G. Risk of Endometrial Cancer in Women with Diabetes: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2021, 10, 3453. [Google Scholar] [CrossRef]
- Liu, G.M.; Zeng, H.D.; Zhang, C.Y.; Xu, J.W. Key genes associated with diabetes mellitus and hepatocellular carcinoma. Pathol. Res. Pract. 2019, 215, 152510. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, L.; Zheng, F.; Chen, X.; Li, L. A review of the last decade: Pancreatic cancer and type 2 diabetes. Arch. Physiol. Biochem. 2023, 130, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Li, J.; Hang, T.; Duan, P. Association of the key genes in the pathophysiology between the Type 2 diabetes and Lung cancer. Neuro Endocrinol. Lett. 2021, 42, 63–69. [Google Scholar] [PubMed]
- Sayyid, R.K.; Fleshner, N.E. Diabetes Mellitus Type 2: A Driving Force for Urological Complications. Trends Endocrinol. Metab. 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Papadimitriou, N.; Markozannes, G.; Cividini, S.; Kakourou, A.; Gill, D.; Rizos, E.C.; Monori, G.; Ward, H.A.; Kyrgiou, M.; et al. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1218–1228. [Google Scholar] [CrossRef]
- Yuan, S.; Kar, S.; Carter, P.; Vithayathil, M.; Mason, A.M.; Burgess, S.; Larsson, S.C. Is Type 2 Diabetes Causally Associated with Cancer Risk? Evidence from a Two-Sample Mendelian Randomization Study. Diabetes 2020, 69, 1588–1596. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Lin, W.; Deng, M.; Shi, L.; An, Y.; Yang, J.; Zhou, C.; Tong, Q.; Yang, W. Comprehensive Analysis of Diabetes Mellitus-Related Gene Expression and Associated Prognoses in Human Lung Cancer. Curr. Cancer Drug Targets 2023, 23, 889–899. [Google Scholar] [CrossRef]
- Zhao, M.; Mishra, L.; Deng, C.X. The role of TGF-β/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018, 14, 111–123. [Google Scholar] [CrossRef]
- Chan, M.K.; Chung, J.Y.; Tang, P.C.; Chan, A.S.; Ho, J.Y.; Lin, T.P.; Chen, J.; Leung, K.T.; To, K.F.; Lan, H.Y.; et al. TGF-β signaling networks in the tumor microenvironment. Cancer Lett. 2022, 550, 215925. [Google Scholar] [CrossRef]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and extracellular TGF-β signaling in cancer: Some recent topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Bing, L.; Xie, Y.; Yu, W. Dioscin Promotes Proliferation of Pancreatic Beta Cells via Wnt/β-Catenin Signaling Pathways. Clin. Lab. 2018, 64, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Piątkiewicz, P.; Bernat-Karpińska, M.; Miłek, T.; Rabijewski, M.; Rosiak, E. NK cell count and glucotransporter 4 (GLUT4) expression in subjects with type 2 diabetes and colon cancer. Diabetol. Metab. Syndr. 2016, 8, 38. [Google Scholar] [CrossRef]
- Jung, Y.S.; Kwon, M.J.; Park, D.I.; Sohn, C.I.; Park, J.H. Association between natural killer cell activity and the risk of colorectal neoplasia. J. Gastroenterol. Hepatol. 2018, 33, 831–836. [Google Scholar] [CrossRef]
- Naujoks, W.; Quandt, D.; Hauffe, A.; Kielstein, H.; Bähr, I.; Spielmann, J. Characterization of Surface Receptor Expression and Cytotoxicity of Human NK Cells and NK Cell Subsets in Overweight and Obese Humans. Front. Immunol. 2020, 11, 573200. [Google Scholar] [CrossRef]
- Wang, H.; Cao, K.; Liu, S.; Xu, Y.; Tang, L. Tim-3 Expression Causes NK Cell Dysfunction in Type 2 Diabetes Patients. Front. Immunol. 2022, 13, 852436. [Google Scholar] [CrossRef]
- Conroy, M.J.; Dunne, M.R.; Donohoe, C.L.; Reynolds, J.V. Obesity-associated cancer: An immunological perspective. Proc. Nutr. Soc. 2016, 75, 125–138. [Google Scholar] [CrossRef]
- Xu, W.; Chen, L.; Liu, J.; Zhang, Z.; Wang, R.; Zhang, Q.; Li, H.; Xiang, J.; Fang, L.; Xu, P.; et al. LINC00152 induced by TGF-β promotes metastasis via HuR in lung adenocarcinoma. Cell Death Dis. 2022, 13, 772. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, L.; Yan, X.X.; Li, Y.; Chen, X.Y.; Hu, X.H.; Bu, Q.; Lv, X.P. Identification of a TGF-β signaling-related gene signature for prediction of immunotherapy and targeted therapy for lung adenocarcinoma. World J. Surg. Oncol. 2022, 20, 183. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhao, Z.; Miao, N.; Liu, G.; Deng, L.; Wei, S.; Hou, J. Immunogenicity of small-cell lung cancer associates with STING pathway activation and is enhanced by ATR and TOP1 inhibition. Cancer Med. 2023, 12, 4864–4881. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tan, Z.; Huang, Z.; Pan, Y.; Zhang, W.; Wang, J. Expression Profile and Prognostic Values of Mini-Chromosome Maintenance Families (MCMs) in Breast Cancer. Med. Sci. Monit. 2020, 26, e923673. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.X.; Pan, Y.Y.; You, C.G. CDK1, CCNB1, CDC20, BUB1, MAD2L1, MCM3, BUB1B, MCM2, and RFC4 May Be Potential Therapeutic Targets for Hepatocellular Carcinoma Using Integrated Bioinformatic Analysis. BioMed Res. Int. 2019, 2019, 1245072. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Feng, L.; Wei, J.; Wang, G.; Wu, L. Knockdown of RFC4 inhibits the cell proliferation of nasopharyngeal carcinoma in vitro and in vivo. Front. Med. 2023, 17, 132–142. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Xie, X. RFC4 promotes the progression and growth of Oral Tongue squamous cell carcinoma in vivo and vitro. J. Clin. Lab. Anal. 2021, 35, e23761. [Google Scholar] [CrossRef]
- Che, X.; Zhao, R.; Xu, H.; Liu, X.; Zhao, S.; Ma, H. Differently Expressed Genes (DEGs) Relevant to Type 2 Diabetes Mellitus Identification and Pathway Analysis via Integrated Bioinformatics Analysis. Med. Sci. Monit. 2019, 25, 9237–9244. [Google Scholar] [CrossRef]
- De Gonzalo-Calvo, D.; van der Meer, R.W.; Rijzewijk, L.J.; Smit, J.W.; Revuelta-Lopez, E.; Nasarre, L.; Escola-Gil, J.C.; Lamb, H.J.; Llorente-Cortes, V. Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Sci. Rep. 2017, 7, 47. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Luo, H.; Zhang, J.; Wang, J.; Guo, H. Identification of the differential expression of genes and upstream microRNAs in small cell lung cancer compared with normal lung based on bioinformatics analysis. Medicine 2020, 99, e19086. [Google Scholar] [CrossRef]
- Hashemi, K.S.; Aliabadi, M.K.; Mehrara, A.; Talebi, E.; Hemmati, A.A.; Rezaeiye, R.D.; Ghanbary, M.J.; Motealleh, M.; Dayeri, B.; Alashti, S.K. A meta-analysis of microarray datasets to identify biological regulatory networks in Alzheimer’s disease. Front. Genet. 2023, 14, 1225196. [Google Scholar] [CrossRef]
- Lu, Y.; Luan, X.R. miR-147a suppresses the metastasis of non-small-cell lung cancer by targeting CCL5. J. Int. Med. Res. 2020, 48, 300060519883098. [Google Scholar] [CrossRef]
- Ji, T.T.; Qi, Y.H.; Li, X.Y.; Tang, B.; Wang, Y.K.; Zheng, P.X.; Li, W.; Qu, X.; Feng, L.; Bai, S.J. Loss of lncRNA MIAT ameliorates proliferation and fibrosis of diabetic nephropathy through reducing E2F3 expression. J. Cell Mol. Med. 2020, 24, 13314–13323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Xiong, Y. Long noncoding RNA MIAT promotes non-small cell lung cancer progression by sponging miR-149-5p and regulating FOXM1 expression. Cancer Cell Int. 2020, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS 2015, 282, 224–258. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, Z.; Zhou, F.; Cui, J.; Wang, L.; Wang, L. High co-expression of Sp1 and HER-2 is correlated with poor prognosis of gastric cancer patients. Surg. Oncol. 2015, 24, 220–225. [Google Scholar] [CrossRef]
- Liu, P.C.; Lieu, A.S.; Lin, C.J.; Tsai, H.P.; Chai, C.Y.; Kwan, A.L. High Expression of Sp1 is Associated with Recurrence of Meningioma. World Neurosurg. 2021, 149, e1056–e1060. [Google Scholar] [CrossRef]
- Li, J.; Qi, C.; Shao, S.; Chen, Y.; Peng, Z.; Shen, Q.; Zhang, Z. SP1 transcriptionally regulates UBE2N expression to promote lung adenocarcinoma progression. Mol. Biomed. 2023, 4, 7. [Google Scholar] [CrossRef]
- Liu, L.; Ji, P.; Qu, N.; Pu, W.L.; Jiang, D.W.; Liu, W.Y.; Li, Y.Q.; Shi, R.L. The impact of high co-expression of Sp1 and HIF1α on prognosis of patients with hepatocellular cancer. Oncol. Lett. 2016, 12, 504–512. [Google Scholar] [CrossRef]
- Hu, H.; Wu, L.L.; Han, T.; Zhuo, M.; Lei, W.; Cui, J.J.; Jiao, F.; Wang, L.W. Correlated high expression of FXR and Sp1 in cancer cells confers a poor prognosis for pancreatic cancer: A study based on TCGA and tissue microarray. Oncotarget 2017, 8, 33265–33275. [Google Scholar] [CrossRef]
- Ivanenko, K.A.; Prassolov, V.S.; Khabusheva, E.R. Transcription Factor Sp1 in the Expression of Genes Encoding Components of MAPK, JAK/STAT, and PI3K/Akt Signaling Pathways. Mol. Biol. 2022, 56, 832–847. (In Russian) [Google Scholar] [CrossRef]
- Oser, M.G.; Sabet, A.H.; Gao, W.; Chakraborty, A.A.; Schinzel, A.C.; Jennings, R.B.; Fonseca, R.; Bonal, D.M.; Booker, M.A.; Flaifel, A.; et al. The KDM5A/RBP2 histone demethylase represses NOTCH signaling to sustain neuroendocrine differentiation and promote small cell lung cancer tumorigenesis. Genes Dev. 2019, 33, 1718–1738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).