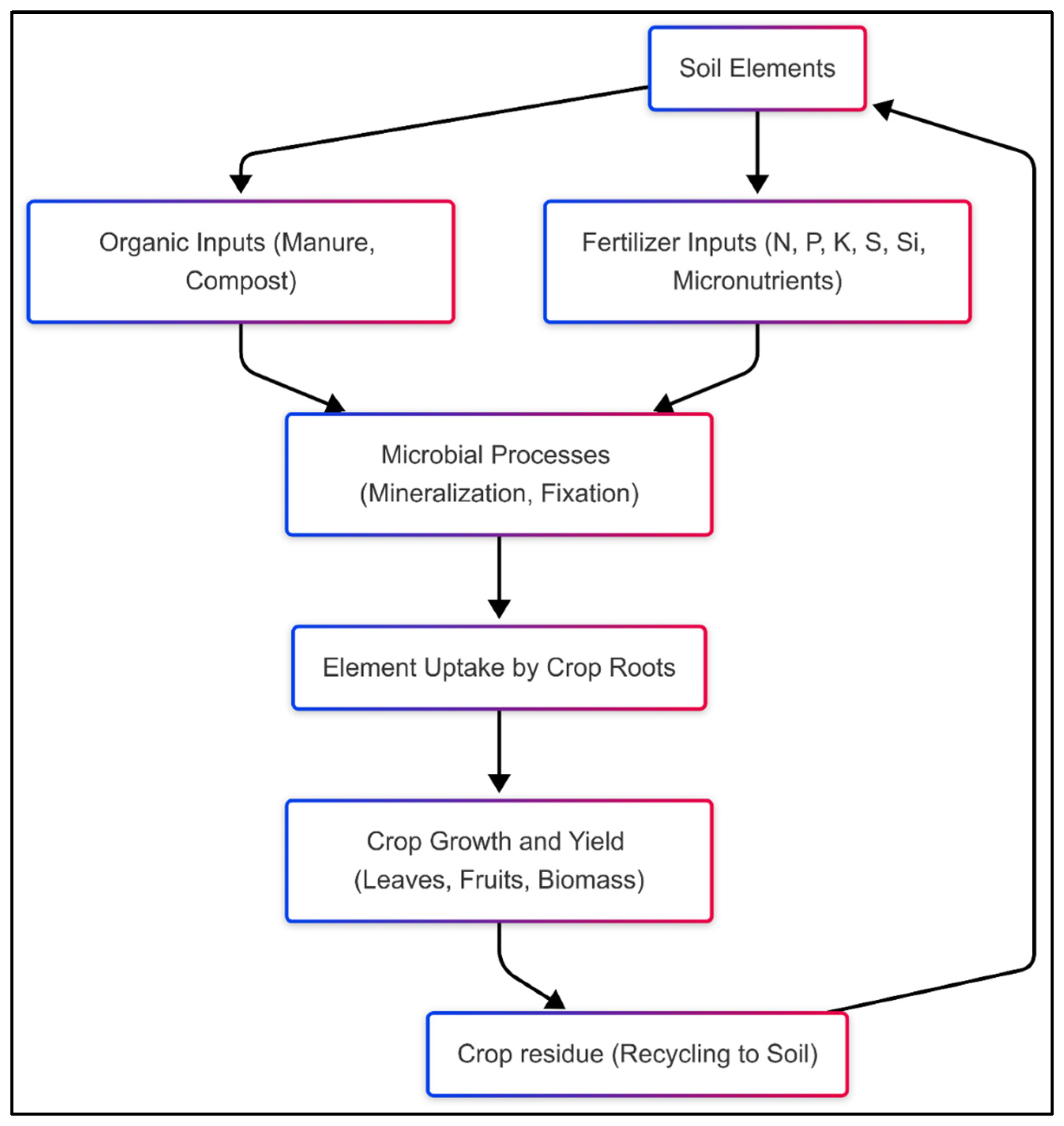
Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions
Simple Summary
Abstract
1. Introduction
2. A Short Overview of Carbon, Nitrogen, Phosphorus, Sulfur, and Silicon Cycles in Plant–Soil Systems
2.1. Carbon Cycle
2.1.1. Carbon Profile
2.1.2. Carbon Respiration and Sequestration in Soils
2.1.3. Impact of Land-Use on Carbon Cycling
2.2. Nitrogen Cycle
2.2.1. Nitrogen Profile
2.2.2. Nitrogen Fixation, Nitrification, and Denitrification
2.2.3. Nitrogen Supply in Agricultural Plant–Soil Systems
2.3. Phosphorus Cycle
2.3.1. Phosphorus Profile
2.3.2. Availability of Phosphorus in Soils
2.3.3. Global P Resources and Strategies to Reduce the Need for Synthetic P Fertilizers in Agricultural Plant–Soil Systems
2.4. Sulfur Cycle
2.4.1. Sulfur Profile
2.4.2. Sulfur Transformation Processes
2.4.3. Sulfur Management in Agricultural Plant–Soil Systems
2.5. Silicon Cycle
2.5.1. Silicon Profile
2.5.2. Biosilicification and Its Role in Silicon Cycling
2.5.3. The Threat of Anthropogenic Desilication and How to Prevent It
3. Interactions Between Biogeochemical Cycles
3.1. Underlying Mechanisms
3.2. Role of Microbial Communities
4. Implications for Ecosystem Services
4.1. Soil Fertility and Crop Production
4.2. Carbon Sequestration and Climate Mitigation
4.3. Biodiversity and Ecosystem Resilience
5. Challenges and Future Perspectives
5.1. Anthropogenic Impacts on Biogeochemical Cycles
5.2. Technological Advancements
5.3. Research Gaps and Future Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Meng, H.; Gu, J. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Janes-Bassett, V.; Davies, J.; Rowe, E.; Tipping, E. Simulating long-term carbon nitrogen and phosphorus biogeochemical cycling in agricultural environments. Sci. Total Environ. 2020, 714, 136599. [Google Scholar] [CrossRef] [PubMed]
- Auguères, A.-S.; Loreau, M. Can Organisms Regulate Global Biogeochemical Cycles? Ecosystems 2015, 18, 813–825. [Google Scholar] [CrossRef]
- Beare, M.; Coleman, D.; Crossley, D.; Hendrix, P.; Odum, E. A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 1995, 170, 5–22. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.; Mao, Q.; Gilliam, F.; Luo, Y.; Turner, B.; Zhou, G.; Mo, J. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef]
- Xu, Z. Unravelling the Biogeochemical Cycles of Carbon and Nutrients in Forest Ecosystems: Innovative Approaches with Advanced Stable Isotope and NMR Techniques; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Di, H.; Condron, L.; Frossard, E. Isotope techniques to study phosphorus cycling in agricultural and forest soils: A review. Biol. Fertil. Soils 1997, 24, 1–12. [Google Scholar] [CrossRef]
- Struyf, E.; Smis, A.; Van Damme, S.; Garnier, J.; Govers, G.; Van Wesemael, B.; Conley, D.J.; Batelaan, O.; Frot, E.; Clymans, W.; et al. Historical land use change has lowered terrestrial silica mobilization. Nat. Commun. 2010, 1, 129. [Google Scholar] [CrossRef]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil-Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon Cycling in Soils Revisited. Plants 2021, 10, 295. [Google Scholar] [CrossRef]
- Paredes, S.; Lebeis, S. Giving back to the community: Microbial mechanisms of plant–soil interactions. Funct. Ecol. 2016, 30, 1043–1052. [Google Scholar] [CrossRef]
- Arcand, M.; Helgason, B.; Lemke, R. Microbial crop residue decomposition dynamics in organic and conventionally managed soils. Appl. Soil Ecol. 2016, 107, 347–359. [Google Scholar] [CrossRef]
- Shibata, H.; Branquinho, C.; McDowell, W.; Mitchell, M.; Monteith, D.; Tang, J.; Arvola, L.; Cruz, C.; Cusack, D.; Halada, L.; et al. Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: The need for long-term and site-based research. AMBIO 2015, 44, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Isson, T.; Planavsky, N.; Coogan, L.; Stewart, E.; Ague, J.; Bolton, E.; Zhang, S.; McKenzie, N.; Kump, L. Evolution of the Global Carbon Cycle and Climate Regulation on Earth. Glob. Biogeochem. Cycles 2020, 34, e2018GB006061. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Schaller, J. Biological impacts on silicon availability and cycling in agricultural plant-soil systems. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–324. [Google Scholar]
- Street-Perrott, F.A.; Barker, P.A. Biogenic silica: A neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf. Process. Landf. 2008, 33, 1436–1457. [Google Scholar] [CrossRef]
- Haynes, R.J. Significance and Role of Si in Crop Production. Adv. Agron. 2017, 146, 83–166. [Google Scholar] [CrossRef]
- Guignard, M.; Leitch, A.; Acquisti, C.; Eizaguirre, C.; Elser, J.; Hessen, D.; Jeyasingh, P.; Neiman, M.; Richardson, A.; Soltis, P.; et al. Impacts of Nitrogen and Phosphorus: From Genomes to Natural Ecosystems and Agriculture. Front. Ecol. Evol. 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Smith, P.; Cotrufo, M.; Rumpel, C.; Paustian, K.; Kuikman, P.; Elliott, J.; McDowell, R.; Griffiths, R.; Asakawa, S.; Bustamante, M.; et al. Biogeochemical cycles and biodiversity as key drivers of ecosystem services provided by soils. Soil 2015, 1, 665–685. [Google Scholar] [CrossRef]
- Olalekan, R.M.; Ilesanmi, A.; Alima, O.; Omini, D.; Raimi, A.-A.G. Exploring How Human Activities Disturb the Balance of Biogeochemical Cycles: Evidence from the Carbon, Nitrogen and Hydrologic Cycles. SSRN Electron. J. 2021, 2, 24–44. [Google Scholar] [CrossRef]
- Tong, S.; Bambrick, H.; Beggs, P.J.; Chen, L.; Hu, Y.; Ma, W.; Steffen, W.; Tan, J. Current and future threats to human health in the Anthropocene. Environ. Int. 2022, 158, 106892. [Google Scholar] [CrossRef]
- Fontaine, S.; Abbadie, L.; Aubert, M.; Barot, S.; Bloor, J.; Derrien, D.; Duchene, O.; Gross, N.; Henneron, L.; Roux, X.L.; et al. Plant–soil synchrony in nutrient cycles: Learning from ecosystems to design sustainable agrosystems. Glob. Chang. Biol. 2023, 30, e17034. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Cole, J.J.; Finzi, A.C.; Holland, E.A. Introduction to coupled biogeochemical cycles. Front. Ecol. Environ. 2011, 9, 5–8. [Google Scholar] [CrossRef]
- Conley, D.J. Terrestrial ecosystems and the global biogeochemical silica cycle. Glob. Biogeochem. Cycles 2002, 16, 68-1–68-8. [Google Scholar] [CrossRef]
- Dodds, W.; Whiles, M. Nitrogen, Sulfur, Phosphorus, and Other Nutrients; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Van Cappellen, P. Biomineralization and global biogeochemical cycles. Rev. Mineral. Geochem. 2003, 54, 357–381. [Google Scholar] [CrossRef]
- Stemmet, M.; De Bruyn, J.; Zeeman, P. The uptake of carbon dioxide by plant roots. Plant Soil 1962, 17, 357–364. [Google Scholar] [CrossRef]
- Shimono, H.; Kondo, M.; Evans, J.R. Internal transport of CO2 from the root-zone to plant shoot is pH dependent. Physiol. Plant. 2019, 165, 451–463. [Google Scholar] [CrossRef]
- Bardgett, R.; De Deyn, G.; Ostle, N. Plant–soil interactions and the carbon cycle. J. Ecol. 2009, 97, 838–839. [Google Scholar] [CrossRef]
- Kudeyarov, V. Soil respiration and carbon sequestration: A review. Eurasian Soil Sci. 2023, 56, 1191–1200. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A. Global carbon budget 2024. Earth Syst. Sci. Data 2025, 17, 965–1039. [Google Scholar] [CrossRef]
- Nissan, A.; Alcolombri, U.; Peleg, N.; Galili, N.; Jimenez-Martinez, J.; Molnar, P.; Holzner, M. Global warming accelerates soil heterotrophic respiration. Nat. Commun. 2023, 14, 3452. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Kästner, M.; Miltner, A.; Thiele-Bruhn, S.; Liang, C. Microbial Necromass in Soils—Linking Microbes to Soil Processes and Carbon Turnover. Front. Environ. Sci. 2021, 9, 756378. [Google Scholar] [CrossRef]
- Berner, R.A. Weathering, plants, and the long-term carbon cycle. Geochim. Cosmochim. Acta 1992, 56, 3225–3231. [Google Scholar] [CrossRef]
- Knapp, W.J.; Tipper, E.T. The efficacy of enhancing carbonate weathering for carbon dioxide sequestration. Front. Clim. 2022, 4, 928215. [Google Scholar] [CrossRef]
- Padbhushan, R.; Kumar, U.; Sharma, S.; Rana, D.; Kumar, R.; Kohli, A.; Kumari, P.; Parmar, B.; Kaviraj, M.; Sinha, A.; et al. Impact of Land-Use Changes on Soil Properties and Carbon Pools in India: A Meta-analysis. Front. Environ. Sci. 2022, 9, 794866. [Google Scholar] [CrossRef]
- Edmondson, J.; Davies, Z.; McHugh, N.; Gaston, K.; Leake, J. Organic carbon hidden in urban ecosystems. Sci. Rep. 2012, 2, srep00963. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Webster, G.; Krouse, H. Nitrogen movement and transformation in soils. Plant Soil 1969, 31, 224–237. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J. Symbiotic nitrogen fixation by rhizobia-the roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef]
- Yansheng, C.; Fengliang, Z.; Zhongyi, Z.; Tongbin, Z.; Hua-Yun, X. Biotic and abiotic nitrogen immobilization in soil incorporated with crop residue. Soil Tillage Res. 2020, 202, 104664. [Google Scholar] [CrossRef]
- Hill, R.; Rinker, R.; Wilson, H.D. Atmospheric nitrogen fixation by lightning. J. Atmos. Sci. 1980, 37, 179–192. [Google Scholar] [CrossRef]
- Singh, H.B. Reactive nitrogen in the troposphere. Environ. Sci. Technol. 1987, 21, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotechnol. 2018, 50, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, N.; Dong, H.; Harland, J.; Hunt, A.; White, C. Reversing nitrogen fixation. Nat. Rev. Chem. 2018, 2, 278–289. [Google Scholar] [CrossRef]
- Verstraete, W.; Focht, D. Biochemical Ecology of Nitrification and Denitrification; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar] [CrossRef]
- Withers, P.J.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Schulte-Uebbing, L.F.; Beusen, A.H.; Bouwman, A.F.; De Vries, W. From planetary to regional boundaries for agricultural nitrogen pollution. Nature 2022, 610, 507–512. [Google Scholar] [CrossRef]
- Meisinger, J.; Schepers, J.; Raun, W. Crop nitrogen requirement and fertilization. Nitrogen Agric. Syst. 2008, 49, 563–612. [Google Scholar]
- Smil, V. Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Kolbe, H. Comparative analysis of soil fertility, productivity and sustainability of organic farming in Central Europe—Part 1: Effect of medium manifestations on conversion, fertilizer types and cropping systems. Agronomy 2022, 12, 2001. [Google Scholar] [CrossRef]
- Shafi, M.; Shah, A.; Bakht, J.; Shah, M.; Mohammad, W. Integrated effect of inorganic and organic nitrogen sources on soil fertility and productivity of maize. J. Plant Nutr. 2012, 35, 524–537. [Google Scholar] [CrossRef]
- Herencia, J.F.; Ruiz-Porras, J.C.; Melero, S.; Garcia-Galavis, P.A.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels, crop macronutrient concentrations, and yield. Agron. J. 2007, 99, 973–983. [Google Scholar] [CrossRef]
- Mugendi, D.N.; Waswa, B.S.; Mucheru-Muna, M.W.; Kimetu, J.M.; Palm, C. Comparative analysis of the current and potential role of legumes in integrated soil fertility management in East Africa. In Fighting Poverty in Sub-Saharan Africa: The Multiple Roles of Legumes in Integrated Soil Fertility Management; Springer: Dordrecht, The Netherlands, 2011; pp. 151–173. [Google Scholar]
- Mapfumo, P. Comparative analysis of the current and potential role of legumes in integrated soil fertility management in southern Africa. In Fighting Poverty in Sub-Saharan Africa: The Multiple Roles of Legumes in Integrated Soil Fertility Management; Springer: Dordrecht, The Netherlands, 2011; pp. 175–200. [Google Scholar]
- Hammad, H.M.; Khaliq, A.; Abbas, F.; Farhad, W.; Fahad, S.; Aslam, M.; Shah, G.M.; Nasim, W.; Mubeen, M.; Bakhat, H.F. Comparative effects of organic and inorganic fertilizers on soil organic carbon and wheat productivity under arid region. Commun. Soil Sci. Plant Anal. 2020, 51, 1406–1422. [Google Scholar] [CrossRef]
- Azam, F. Comparative effects of organic and inorganic nitrogen sources applied to a flooded soil on rice yield and availability of N. Plant Soil 1990, 125, 255–262. [Google Scholar] [CrossRef]
- Mtambanengwe, F.; Mapfumo, P.; Vanlauwe, B. Comparative short-term effects of different quality organic resources on maize productivity under two different environments in Zimbabwe. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Springer: Dordrecht, The Netherlands, 2007; pp. 575–588. [Google Scholar] [CrossRef]
- Föllmi, K. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Sci. Rev. 1996, 40, 55–124. [Google Scholar] [CrossRef]
- Amadou, I.; Faucon, M.; Houben, D. Role of Soil Minerals on Organic Phosphorus Availability and Phosphorus Uptake by Plants. Geoderma 2022, 428, 116125. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef]
- U.S.G.S. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2023; 210p.
- Scholz, R.W.; Wellmer, F.-W.; Mew, M.; Steiner, G. The dynamics of increasing mineral resources and improving resource efficiency: Prospects for mid-and long-term security of phosphorus supply. Resour. Conserv. Recycl. 2025, 213, 107993. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 2017, 140, 945–963. [Google Scholar] [CrossRef]
- Hedley, C. The role of precision agriculture for improved nutrient management on farms. J. Sci. Food Agric. 2015, 95, 12–19. [Google Scholar] [CrossRef]
- Pätzold, S.; Leenen, M.; Frizen, P.; Heggemann, T.; Wagner, P.; Rodionov, A. Predicting plant available phosphorus using infrared spectroscopy with consideration for future mobile sensing applications in precision farming. Precis. Agric. 2020, 21, 737–761. [Google Scholar] [CrossRef]
- Owen, D.; Williams, A.P.; Griffith, G.W.; Withers, P.J. Use of commercial bio-inoculants to increase agricultural production through improved phosphrous acquisition. Appl. Soil Ecol. 2015, 86, 41–54. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.; Hawkesford, M.J.; De Kok, L.J. Atmospheric H2S and SO2 as sulfur sources for Brassica juncea and Brassica rapa: Regulation of sulfur uptake and assimilation. Environ. Exp. Bot. 2016, 124, 1–10. [Google Scholar] [CrossRef]
- Zhong, Q.; Shen, H.; Yun, X.; Chen, Y.; Ren, Y.a.; Xu, H.; Shen, G.; Du, W.; Meng, J.; Li, W. Global sulfur dioxide emissions and the driving forces. Environ. Sci. Technol. 2020, 54, 6508–6517. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef]
- Xia, Y.; Lü, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dong, X.; Lu, R.; Zhou, Y.; Zheng, P.-F.; Feng, D.; Wang, Y. Microbial ecology of sulfur cycling near the sulfate-methane transition of deep-sea cold seep sediments. Environ. Microbiol. 2021, 23, 6844–6858. [Google Scholar] [CrossRef]
- Galloway, J. Acid deposition: Perspectives in time and space. Water Air Soil Pollut. 1995, 85, 15–24. [Google Scholar] [CrossRef]
- Hinckley, E.-L.S.; Crawford, J.T.; Fakhraei, H.; Driscoll, C.T. A shift in sulfur-cycle manipulation from atmospheric emissions to agricultural additions. Nat. Geosci. 2020, 13, 597–604. [Google Scholar] [CrossRef]
- Sharma, R.K.; Cox, M.S.; Oglesby, C.; Dhillon, J.S. Revisiting the role of sulfur in crop production: A narrative review. J. Agric. Food Res. 2024, 15, 101013. [Google Scholar] [CrossRef]
- Kovar, J.L.; Grant, C.A. Nutrient cycling in soils: Sulfur. In Soil Management: Building a Stable Base for Agriculture; Wiley: Hoboken, NJ, USA, 2011; pp. 103–115. [Google Scholar]
- Gilbert, F. The place of sulfur in plant nutrition. Bot. Rev. 1951, 17, 671–691. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids management and utilizations: A review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Gahane, D.; Mandavgane, S.A. Biogenic potassium: Sources, method of recovery, and sustainability assessment. Rev. Chem. Eng. 2024, 40, 707–722. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef]
- Dürr, H.H.; Meybeck, M.; Hartmann, J.; Laruelle, G.G.; Roubeix, V. Global spatial distribution of natural riverine silica inputs to the coastal zone. Biogeosciences 2011, 8, 597–620. [Google Scholar] [CrossRef]
- Struyf, E.; Conley, D.J. Emerging understanding of the ecosystem silica filter. Biogeochemistry 2012, 107, 9–18. [Google Scholar] [CrossRef]
- Bartoli, F. The biogeochemical cycle of silicon in two temperate forest ecosystems. In Ecological Bulletins; Oikos Editorial Office: Lund, Sweden, 1983; pp. 469–476. [Google Scholar]
- Gérard, F.; Mayer, K.U.; Hodson, M.J.; Ranger, J. Modelling the biogeochemical cycle of silicon in soils: Application to a temperate forest ecosystem. Geochim. Cosmochim. Acta 2008, 72, 741–758. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Yu, C.; Ding, F. Quantification of different silicon fractions in broadleaf and conifer forests of northern China and consequent implications for biogeochemical Si cycling. Geoderma 2020, 361, 114036. [Google Scholar] [CrossRef]
- Puppe, D. Review on protozoic silica and its role in silicon cycling. Geoderma 2020, 365, 114224. [Google Scholar] [CrossRef]
- Puppe, D.; Ehrmann, O.; Kaczorek, D.; Wanner, M.; Sommer, M. The protozoic Si pool in temperate forest ecosystems—Quantification, abiotic controls and interactions with earthworms. Geoderma 2015, 243–244, 196–204. [Google Scholar] [CrossRef]
- Puppe, D.; Wanner, M.; Sommer, M. Data on euglyphid testate amoeba densities, corresponding protozoic silicon pools, and selected soil parameters of initial and forested biogeosystems. Data Brief 2018, 21, 1697–1703. [Google Scholar] [CrossRef]
- Wanner, M.; Elmer, M.; Sommer, M.; Funk, R.; Puppe, D. Testate amoebae colonizing a newly exposed land surface are of airborne origin. Ecol. Indic. 2015, 48, 55–62. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Wanner, M.; Sommer, M. Dynamics and drivers of the protozoic Si pool along a 10-year chronosequence of initial ecosystem states. Ecol. Eng. 2014, 70, 477–482. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Sommer, M. As time goes by—Spatiotemporal changes of biogenic Si pools in initial soils of an artificial catchment in NE Germany. Appl. Soil Ecol. 2016, 105, 9–16. [Google Scholar] [CrossRef]
- Puppe, D.; Höhn, A.; Kaczorek, D.; Wanner, M.; Wehrhan, M.; Sommer, M. How big is the influence of biogenic silicon pools on short-term changes in water-soluble silicon in soils? Implications from a study of a 10-year-old soil–plant system. Biogeosciences 2017, 14, 5239–5252. [Google Scholar] [CrossRef]
- Aoki, Y.; Hoshino, M.; Matsubara, T. Silica and testate amoebae in a soil under pine–oak forest. Geoderma 2007, 142, 29–35. [Google Scholar] [CrossRef]
- Sommer, M.; Jochheim, H.; Höhn, A.; Breuer, J.; Zagorski, Z.; Busse, J.; Barkusky, D.; Meier, K.; Puppe, D.; Wanner, M.; et al. Si cycling in a forest biogeosystem—The importance of transient state biogenic Si pools. Biogeosciences 2013, 10, 4991–5007. [Google Scholar] [CrossRef]
- Creevy, A.L.; Fisher, J.; Puppe, D.; Wilkinson, D.M. Protist diversity on a nature reserve in NW England—With particular reference to their role in soil biogenic silicon pools. Pedobiologia 2016, 59, 51–59. [Google Scholar] [CrossRef]
- Wanner, M.; Birkhofer, K.; Fischer, T.; Shimizu, M.; Shimano, S.; Puppe, D. Soil Testate Amoebae and Diatoms as Bioindicators of an Old Heavy Metal Contaminated Floodplain in Japan. Microb. Ecol. 2020, 79, 123–133. [Google Scholar] [CrossRef]
- Qin, Y.; Puppe, D.; Payne, R.; Li, L.; Li, J.; Zhang, Z.; Xie, S. Land-use change effects on protozoic silicon pools in the Dajiuhu National Wetland Park, China. Geoderma 2020, 368, 114305. [Google Scholar] [CrossRef]
- Qin, Y.; Puppe, D.; Zhang, L.; Sun, R.; Li, P.; Xie, S. How Does Sphagnum Growing Affect Testate Amoeba Communities and Corresponding Protozoic Si Pools? Results from Field Analyses in SW China. Microb. Ecol. 2021, 82, 459–469. [Google Scholar] [CrossRef]
- Qin, Y.; Puppe, D.; Li, H.; Li, H.; Mazei, Y.; Tsyganov, A.N.; Man, B.; Huang, X.; Gu, Y.; Xie, S. Peatland degradation in Asia threatens the biodiversity of testate amoebae (Protozoa) with consequences for protozoic silicon cycling. Geoderma 2022, 420, 115870. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Poulton, P.R.; McGrath, S.P.; Meunier, J.-D. Long-term removal of wheat straw decreases soil amorphous silica at Broadbalk, Rothamsted. Plant Soil 2012, 352, 173–184. [Google Scholar] [CrossRef]
- Meunier, J.; Guntzer, F.; Kirman, S.; Keller, C. Terrestrial plant-Si and environmental changes. Mineral. Mag. 2008, 72, 263–267. [Google Scholar] [CrossRef]
- Vandevenne, F.; Struyf, E.; Clymans, W.; Meire, P. Agricultural silica harvest: Have humans created a new loop in the global silica cycle? Front. Ecol. Environ. 2012, 10, 243–248. [Google Scholar] [CrossRef]
- Schaller, J.; Puppe, D. Heat improves silicon availability in mineral soils. Geoderma 2021, 386, 114909. [Google Scholar] [CrossRef]
- Schaller, J.; Webber, H.; Ewert, F.; Stein, M.; Puppe, D. The transformation of agriculture towards a silicon improved sustainable and resilient crop production. npj Sustain. Agric. 2024, 2, 27. [Google Scholar] [CrossRef]
- Li, Z.; Delvaux, B. Phytolith-rich biochar: A potential Si fertilizer in desilicated soils. GCB Bioenergy 2019, 11, 1264–1282. [Google Scholar] [CrossRef]
- Puppe, D.; Kaczorek, D.; Schaller, J.; Barkusky, D.; Sommer, M. Crop straw recycling prevents anthropogenic desilication of agricultural soil–plant systems in the temperate zone–Results from a long-term field experiment in NE Germany. Geoderma 2021, 403, 115187. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Qin, Z.; Wu, L.; Yin, L.; Van Zwieten, L.; Song, A.; Ran, X.; Yu, C.; Wang, H. Phytolith-rich straw application and groundwater table management over 36 years affect the soil-plant silicon cycle of a paddy field. Plant Soil 2020, 454, 343–358. [Google Scholar] [CrossRef]
- Haynes, R.J. What effect does liming have on silicon availability in agricultural soils? Geoderma 2019, 337, 375–383. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Müller, K.; Wang, H. Biogeochemical silicon cycle and carbon sequestration in agricultural ecosystems. Earth-Sci. Rev. 2014, 139, 268–278. [Google Scholar] [CrossRef]
- Ostle, N.; Smith, P.; Fisher, R.; Woodward, I.; Fisher, J.; Smith, J.; Galbraith, D.; Levy, P.; Meir, P.; McNamara, N.; et al. Integrating plant–soil interactions into global carbon cycle models. J. Ecol. 2009, 97, 851–863. [Google Scholar] [CrossRef]
- Burgin, A.; Yang, W.; Hamilton, S.; Silver, W. Beyond carbon and nitrogen: How the microbial energy economy couples elemental cycles in diverse ecosystems. Front. Ecol. Environ. 2011, 9, 44–52. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.; Guhr, A.; Borer, E.; Seabloom, E.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Finzi, A.; Austin, A.; Cleland, E.; Frey, S.; Houlton, B.; Wallenstein, M. Responses and feedbacks of coupled biogeochemical cycles to climate change: Examples from terrestrial ecosystems. Front. Ecol. Environ. 2011, 9, 61–67. [Google Scholar] [CrossRef]
- Waring, B.; Weintraub, S.; Sinsabaugh, R. Ecoenzymatic stoichiometry of microbial nutrient acquisition in tropical soils. Biogeochemistry 2013, 117, 101–113. [Google Scholar] [CrossRef]
- Walker, A.; Walker, A.; Beckerman, A.; Gu, L.; Kattge, J.; Cernusak, L.; Domingues, T.; Scales, J.; Wohlfahrt, G.; Wullschleger, S.; et al. The relationship of leaf photosynthetic traits—Vcmax and Jmax—To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef]
- Tang, B.; Rocci, K.; Lehmann, A.; Rillig, M. Nitrogen increases soil organic carbon accrual and alters its functionality. Glob. Chang. Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef]
- Mehnaz, K.; Corneo, P.; Keitel, C.; Dijkstra, F. Carbon and phosphorus addition effects on microbial carbon use efficiency, soil organic matter priming, gross nitrogen mineralization and nitrous oxide emission from soil. Soil Biol. Biochem. 2019, 134, 175–186. [Google Scholar] [CrossRef]
- Treguer, P.J.; De La Rocha, C.L. The world ocean silica cycle. Ann. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef] [PubMed]
- Derry, L.A.; Kurtz, A.C.; Ziegler, K.; Chadwick, O.A. Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 2005, 433, 728–731. [Google Scholar] [CrossRef]
- Zondervan, J.R.; Hilton, R.G.; Dellinger, M.; Clubb, F.J.; Roylands, T.; Ogrič, M. Rock organic carbon oxidation CO2 release offsets silicate weathering sink. Nature 2023, 623, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Textor, C.; Graf, H.-F.; Timmreck, C.; Robock, A. Emissions from volcanoes. In Emissions of Atmospheric Trace Compounds; Springer: Berlin/Heidelberg, Germany, 2004; pp. 269–303. [Google Scholar]
- Mather, T.; Allen, A.; Davison, B.; Pyle, D.; Oppenheimer, C.; McGonigle, A. Nitric acid from volcanoes. Earth Planet. Sci. Lett. 2004, 218, 17–30. [Google Scholar] [CrossRef]
- Newman, E. Phosphorus inputs to terrestrial ecosystems. J. Ecol. 1995, 83, 713–726. [Google Scholar] [CrossRef]
- Nanzyo, M. Unique properties of volcanic ash soils. Glob. Environ. Res.-Engl. Ed. 2002, 6, 99–112. [Google Scholar]
- Zhu, Y.; Toon, O.B.; Jensen, E.J.; Bardeen, C.G.; Mills, M.J.; Tolbert, M.A.; Yu, P.; Woods, S. Persisting volcanic ash particles impact stratospheric SO2 lifetime and aerosol optical properties. Nat. Commun. 2020, 11, 4526. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Riley, W.; Tang, J.; Bouskill, N. Plant responses to elevated CO2 under competing hypotheses of nitrogen and phosphorus limitations. Ecol. Appl. 2024, 34, e2967. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Tariq, A.; Chen, H.; He, Q.; Guan, Y.; Pan, K.; Chen, S.; Li, J.; Zhao, C.; Wang, H.; et al. Effect of nitrogen and phosphorus application on agricultural soil food webs. Arch. Agron. Soil Sci. 2017, 63, 1176–1186. [Google Scholar] [CrossRef]
- Netherway, T. From Forests to Microbiomes: The Mediation of Plant-Soil Systems by Root-Symbiotic Fungi. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2023. [Google Scholar]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Ohkama-Ohtsu, N.; Wasaki, J. Recent progress in plant nutrition research: Cross-talk between nutrients, plant physiology and soil microorganisms. Plant Cell Physiol. 2010, 51, 1255–1264. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, A.; Chen, Y.; Xu, Z.; Liu, Y.; Yao, Y.; Wang, Y.; Jia, B. Beneficial microorganisms: Regulating growth and defense for plant welfare. Plant Biotechnol. J. 2025, 23, 986–998. [Google Scholar] [CrossRef]
- Joshi, D.; Kaushik, A.; Kumar, R.; Arya, A.; Santoyo, G.; Singh, V.K.; Kashyap, N.; Solanki, M.K.; Kumari, M.; Bhardwaj, N. Improving Plant Performance Through Microbiome Manipulation: The Potential Role of Current Bioengineering Approaches. Bacteria 2025, 4, 12. [Google Scholar] [CrossRef]
- Santana, M.M.; Dias, T.; Gonzalez, J.M.; Cruz, C. Transformation of organic and inorganic sulfur–adding perspectives to new players in soil and rhizosphere. Soil Biol. Biochem. 2021, 160, 108306. [Google Scholar] [CrossRef]
- Sinsabaugh, R. Enzymic analysis of microbial pattern and process. Biol. Fertil. Soils 2004, 17, 69–74. [Google Scholar] [CrossRef]
- Ren, Q.; Song, H.-S.; Yuan, Z.; Ni, X.; Li, C. Changes in Soil Enzyme Activities and Microbial Biomass after Revegetation in the Three Gorges Reservoir, China. Forests 2018, 9, 249. [Google Scholar] [CrossRef]
- Brehm, U.; Gorbushina, A.; Mottershead, D. The role of microorganisms and biofilms in the breakdown and dissolution of quartz and glass. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 219, 117–129. [Google Scholar] [CrossRef]
- White, J.; Reddy, K. Nitrification and denitrification rates of Everglades wetland soils along a phosphorus-impacted gradient. J. Environ. Qual. 2003, 32, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.; Singh, P.; Chakraborty, D.; Mishra, S.; Pattnaik, R. Insight Into the Role of PGPR in Sustainable Agriculture and Environment. Front. Sustain. Food Syst. 2021, 5, 667150. [Google Scholar] [CrossRef]
- Rousk, J.; Bengtson, P. Microbial regulation of global biogeochemical cycles. Front. Microbiol. 2014, 5, 103. [Google Scholar] [CrossRef]
- Hobbie, S.E. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 1992, 7, 336–339. [Google Scholar] [CrossRef]
- Favero, V.O.; De Carvalho, R.H.; Motta, V.M.; Leite, A.B.C.; Coelho, M.; Xavier, G.; Rumjanek, N.; Urquiaga, S. Bradyrhizobium as the Only Rhizobial Inhabitant of Mung Bean (Vigna radiata) Nodules in Tropical Soils: A Strategy Based on Microbiome for Improving Biological Nitrogen Fixation Using Bio-Products. Front. Plant Sci. 2021, 11, 602645. [Google Scholar] [CrossRef]
- Ghosh, P.; Rathinasabapathi, B. Phosphorus solubilization and plant growth enhancement by arsenic-resistant bacteria. Chemosphere 2015, 134, 1–6. [Google Scholar] [CrossRef]
- Jensen, C.N.G.; Pang, J.K.Y.; Gottardi, M.; Kračun, S.; Svendsen, B.; Nielsen, K.F.; Kovács, Á.; Moelbak, L.; Fimognari, L.; Husted, S.; et al. Bacillus subtilis promotes plant phosphorus (P) acquisition through P solubilization and stimulation of root and root hair growth. Physiol. Plant. 2024, 176, e14338. [Google Scholar] [CrossRef]
- Shrestha, N.; Hadano, S.; Kamachi, T.; Okura, I. Dinitrogen production from ammonia by Nitrosomonas europaea. Appl. Catal. A Gen. 2002, 237, 33–39. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Dungait, J.A.J. Soil Microbiology and Nutrient Cycling. In Soil Microbiology and Sustainable Crop Production; Dixon, G.R., Tilston, E.L., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 59–80. [Google Scholar]
- Zhang, M.; O’Connor, P.J.; Zhang, J.; Ye, X. Linking soil nutrient cycling and microbial community with vegetation cover in riparian zone. Geoderma 2021, 384, 114801. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Dikilitas, M.; Abdel-Azeem, A.M.; Ahluwalia, A.S.; Saxena, A.K. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal. Agric. Biotechnol. 2021, 33, 102009. [Google Scholar] [CrossRef]
- Haghverdi, K.; Kooch, Y. Effects of diversity of tree species on nutrient cycling and soil-related processes. CATENA 2019, 178, 335–344. [Google Scholar] [CrossRef]
- Singh, S.B.; Carroll-Portillo, A.; Lin, H.C. Desulfovibrio in the Gut: The Enemy within? Microorganisms 2023, 11, 1772. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D.; Davis, B.; Beveridge, T.J. Role of Thiobacillus and sulfate-reducing bacteria in iron biocycling in oxic and acidic mine tailings. FEMS Microbiol. Ecol. 1996, 21, 11–24. [Google Scholar] [CrossRef]
- Schulze, E.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Global Biogeochemical Cycles; Springer: Berlin/Heidelberg, Germany, 2019; pp. 827–841. [Google Scholar] [CrossRef]
- Cleveland, C.; Townsend, A.; Taylor, P.; Álvarez-Clare, S.; Bustamante, M.; Chuyong, G.; Dobrowski, S.; Grierson, P.; Harms, K.; Houlton, B.; et al. Relationships among net primary productivity, nutrients and climate in tropical rain forest: A pan-tropical analysis. Ecol. Lett. 2011, 14, 939–947. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Role of Soil Microbes in Biogeochemical Cycle for Enhancing Soil Fertility; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–157. [Google Scholar]
- Blanco, J.; Zavala, M.; Imbert, J.; Castillo, F. Sustainability of forest management practices: Evaluation through a simulation model of nutrient cycling. For. Ecol. Manag. 2005, 213, 209–228. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, Q.H.; Xie, Z.B.; Darboux, F.; Holden, N.M. The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope Ultisol. Catena 2016, 138, 30–37. [Google Scholar] [CrossRef]
- Song, Z.; McGrouther, K.; Wang, H. Occurrence, turnover and carbon sequestration potential of phytoliths in terrestrial ecosystems. Earth-Sci. Rev. 2016, 158, 19–30. [Google Scholar] [CrossRef]
- Schaller, J.; Kleber, M.; Puppe, D.; Stein, M.; Sommer, M.; Rillig, M.C. The importance of reactive silica for maintaining soil health. Plant Soil 2025, 1–12. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.; Doherty, A.; Von Caemmerer, S.; Farquhar, G. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Lal, R. Soil degradation as a reason for inadequate human nutrition. Food Secur. 2009, 1, 45–57. [Google Scholar] [CrossRef]
- Bossio, A.; Cook-Patton, S.; Ellis, P.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Wood, S.; Zomer, R.; Unger, M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Crowther, T.; Hoogen, J.; Wan, J.; Mayes, M.; Mayes, M.; Keiser, A.; Keiser, A.; Mo, L.; Averill, C.; Averill, C.; et al. The global soil community and its influence on biogeochemistry. Science 2019, 365, 772. [Google Scholar] [CrossRef]
- Jarecki, M.; Lal, R. Crop Management for Soil Carbon Sequestration. Crit. Rev. Plant Sci. 2003, 22, 471–502. [Google Scholar] [CrossRef]
- Adekiya, A.; Alori, E.; Ogunbode, T.; Sangoyomi, T.; Oriade, O. Enhancing Organic Carbon Content in Tropical Soils: Strategies for Sustainable Agriculture and Climate Change Mitigation. Open Agric. J. 2023, 17, e18743315282476. [Google Scholar] [CrossRef]
- Hodson, M.J. The Relative Importance of Cell Wall and Lumen Phytoliths in Carbon Sequestration in Soil: A Hypothesis. Front. Earth Sci. 2019, 7, 167. [Google Scholar] [CrossRef]
- de Tombeur, F.; Hodson, M.J.; Saunders, M.; Clode, P.L. How important is carbon sequestration in phytoliths within the soil? Plant Soil 2024, 505, 185–198. [Google Scholar] [CrossRef]
- Porwollik, V.; Rolinski, S.; Heinke, J.; Von Bloh, W.; Schaphoff, S.; Müller, C. The role of cover crops for cropland soil carbon, nitrogen leaching, and agricultural yields—A global simulation study with LPJmL (V. 5.0-tillage-cc). Biogeosciences 2021, 19, 957–977. [Google Scholar] [CrossRef]
- Kutos, S.; Stricker, E.; Cooper, A.; Ryals, R.; Creque, J.; Machmuller, M.; Kroegar, M.; Silver, W. Compost amendment to enhance carbon sequestration in rangelands. J. Soil Water Conserv. 2023, 78, 163–177. [Google Scholar] [CrossRef]
- Montagnini, F.; Nair, P. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004, 61–62, 281–295. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, L.; Chen, B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133 Pt B, 105211. [Google Scholar] [CrossRef]
- Swift, M.; Andrén, O.; Brussaard, L.; Briones, M.; Coûteaux, M.; Ekschmitt, K.; Kjøller, A.; Loiseau, P.; Smith, P. Global change, soil biodiversity, and nitrogen cycling in terrestrial ecosystems: Three case studies. Glob. Chang. Biol. 1998, 4, 729–743. [Google Scholar] [CrossRef]
- Wan, N.F.; Zheng, X.-R.; Fu, L.; Kiær, L.; Zhang, Z.; Chaplin-Kramer, R.; Dainese, M.; Tan, J.; Qiu, S.; Hu, Y.-Q.; et al. Global synthesis of effects of plant species diversity on trophic groups and interactions. Nat. Plants 2020, 6, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Siddique, I.; Vieira, I.; Schmidt, S.; Lamb, D.; De Carvalho, C.J.R.; Figueiredo, R.; Blomberg, S.; Davidson, E. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories. Ecology 2010, 91, 2121–2131. [Google Scholar] [CrossRef]
- Moura, R.F.; Sternberg, M.; Vorst, C.; Katz, O. Plant silicon content as a proxy for understanding plant community properties and ecosystem structure. Ecosphere 2024, 15, e4907. [Google Scholar] [CrossRef]
- Kleinert, A.; Benedito, V.; Morcillo, R.; Dames, J.; Cornejo-Rivas, P.; Zuniga-Feest, A.; Delgado, M.; Muñoz, G. Morphological and symbiotic root modifications for mineral acquisition from nutrient-poor soils. In Root Biology; Springer: Cham, Switzerland, 2018; pp. 85–142. [Google Scholar]
- Griffiths, B.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef]
- Rao, I.M.; Miles, J.W.; Beebe, S.E.; Horst, W.J. Root adaptations to soils with low fertility and aluminium toxicity. Ann. Bot. 2016, 118, 593–605. [Google Scholar] [CrossRef]
- Etesami, H. Enhancing soil microbiome resilience: The mitigating role of silicon against environmental stresses. Front. Agron. 2024, 6, 1465165. [Google Scholar] [CrossRef]
- Putra, R.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Is it time to include legumes in plant silicon research? Funct. Ecol. 2020, 34, 1142–1157. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen journey in plants: From uptake to metabolism, stress response, and microbe interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Wilcox, K.; Koerner, S.; Hoover, D.; Borkenhagen, A.; Burkepile, D.; Collins, S.; Hoffman, A.; Kirkman, K.; Knapp, A.; Strydom, T.; et al. Rapid recovery of ecosystem function following extreme drought in a South African savanna-grassland. Ecology 2020, 101, e02983. [Google Scholar] [CrossRef]
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Moiseenko, T. Evolution of biogeochemical cycles under anthropogenic loads: Limits impacts. Geochem. Int. 2017, 55, 841–860. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biogeochemical C and N cycles in urban soils. Environ. Int. 2009, 35, 1–8. [Google Scholar] [CrossRef]
- Iqbal, S.; Riaz, U.; Murtaza, G.; Jamil, M.; Ahmed, M.; Hussain, A.; Abbas, Z. Chemical fertilizers, formulation, and their influence on soil health. In Microbiota and Biofertilizers: A Sustainable Continuum for Plant Soil Health; Springer: Cham, Switzerland, 2021; pp. 1–15. [Google Scholar]
- Howe, J.A.; McDonald, M.D.; Burke, J.; Robertson, I.; Coker, H.; Gentry, T.J.; Lewis, K.L. Influence of fertilizer and manure inputs on soil health: A review. Soil Secur. 2024, 16, 100155. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Jiang, Y. Response of Nitrogen Losses to Excessive Nitrogen Fertilizer Application in Intensive Greenhouse Vegetable Production. Sustainability 2019, 11, 1513. [Google Scholar] [CrossRef]
- Clymans, W.; Struyf, E.; Govers, G.; Vandevenne, F.; Conley, D.J. Anthropogenic impact on amorphous silica pools in temperate soils. Biogeosciences 2011, 8, 2281–2293. [Google Scholar] [CrossRef]
- Conley, D.J.; Likens, G.E.; Buso, D.C.; Saccone, L.; Bailey, S.W.; Johnson, C.E. Deforestation causes increased dissolved silicate losses in the Hubbard Brook Experimental Forest. Glob. Chang. Biol. 2008, 14, 2548–2554. [Google Scholar] [CrossRef]
- Maavara, T.; Dürr, H.H.; Van Cappellen, P. Worldwide retention of nutrient silicon by river damming: From sparse data set to global estimate. Glob. Biogeochem. Cycles 2014, 28, 842–855. [Google Scholar] [CrossRef]
- Laruelle, G.G.; Roubeix, V.; Sferratore, A.; Brodherr, B.; Ciuffa, D.; Conley, D.; Dürr, H.; Garnier, J.; Lancelot, C.; Le Thi Phuong, Q. Anthropogenic perturbations of the silicon cycle at the global scale: Key role of the land-ocean transition. Glob. Biogeochem. Cycles 2009, 23, GB4031. [Google Scholar] [CrossRef]
- Sharpley, A.; McDowell, R.; Kleinman, P. Phosphorus loss from land to water: Integrating agricultural and environmental management. Plant Soil 2001, 237, 287–307. [Google Scholar] [CrossRef]
- Niklaus, P.A. Climate change effects on biogeochemical cycles, nutrients, and water supply. In Agroecosystems in a Changing Climate; CRC Press: Boca Raton, FL, USA, 2007; pp. 11–52. [Google Scholar]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-analysis of heavy metal effects on soil enzyme activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Díaz, R.; Nestlerode, J.; Díaz, M. A Global Perspective on the Effects of Eutrophication and Hypoxia on Aquatic Biota and Water Quality; U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- Hong, S.; Cong, N.; Ding, J.; Piao, S.; Liu, L.; Peñuelas, J.; Chen, A.; Quine, T.; Zeng, H.; Houlton, B. Effects of Afforestation on Soil Carbon and Nitrogen Accumulation Depend on Initial Soil Nitrogen Status. Glob. Biogeochem. Cycles 2022, 37, e2022GB007490. [Google Scholar] [CrossRef]
- Zagural’skaya, L.; Zaybchenko, S. Impact of industrial pollution on soil microbial activity in boreal forests of the Kostomusksha region. Eurasian Soil Sci. 1994, 26, 67–74. [Google Scholar]
- Puppe, D.; Kaczorek, D.; Stein, M.; Schaller, J. Silicon in Plants: Alleviation of Metal (loid) Toxicity and Consequential Perspectives for Phytoremediation. Plants 2023, 12, 2407. [Google Scholar] [CrossRef]
- Chai, L.; Huang, M.; Fan, H.; Wang, J.; Jiang, D.; Zhang, M.; Huang, Y. Urbanization altered regional soil organic matter quantity and quality: Insight from excitation emission matrix (EEM) and parallel factor analysis (PARAFAC). Chemosphere 2019, 220, 249–258. [Google Scholar] [CrossRef]
- Adimassu, Z.; Tamene, L.; Degefie, D. The influence of grazing and cultivation on runoff, soil erosion, and soil nutrient export in the central highlands of Ethiopia. Ecol. Process. 2020, 9, 23. [Google Scholar] [CrossRef]
- Toming, K.; Liu, H.; Soomets, T.; Uuemaa, E.; Nõges, T.; Kutser, T. Estimation of the Biogeochemical and Physical Properties of Lakes Based on Remote Sensing and Artificial Intelligence Applications. Remote Sens. 2024, 16, 464. [Google Scholar] [CrossRef]
- Reddy, T.; Shiva, S.S.; Reddy, R.; Meghana, S.; Prasanna, K.; Sai, T.; Reddy, S. Design and Developing AI-Driven Agro-sage for Optimal Precision Agriculture. In Proceedings of the 2024 5th International Conference on Smart Electronics and Communication (ICOSEC), Trichy, India, 18–20 September 2024; pp. 1538–1542. [Google Scholar]
- Wehrhan, M.; Puppe, D.; Kaczorek, D.; Sommer, M. Spatial patterns of aboveground phytogenic Si stocks in a grass-dominated catchment—Results from UAS-based high-resolution remote sensing. Biogeosciences 2021, 18, 5163–5183. [Google Scholar] [CrossRef]
- Wehrhan, M.; Rauneker, P.; Sommer, M. UAV-Based Estimation of Carbon Exports from Heterogeneous Soil Landscapes--A Case Study from the CarboZALF Experimental Area. Sensors 2016, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Soil Moisture and Nutrient Monitoring. IEEE Access 2021, 9, 14095–14121. [Google Scholar] [CrossRef]
- Sivakumar, V.; Vimal, S.; Baskar, V.; Murugan, S.; Vadivel, M. IoT and GIS Integration for Real-Time Monitoring of Soil Health and Nutrient Status. In Proceedings of the 2023 International Conference on Self Sustainable Artificial Intelligence Systems (ICSSAS), Erode, India, 18–20 October 2023; pp. 1265–1270. [Google Scholar] [CrossRef]




| Nitrogen Source | Soil Fertility Contribution | Crop Productivity Contribution | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Synthetic Fertilizers | High immediate nutrient availability | High yield response | Quick release, tailored compositions | Risk of leaching, environmental harm | [53] |
| Manure | Slow nutrient release | Moderate to high, depending on quality | Organic matter improvement | Variable nutrient content | [54] |
| Compost | Slow and steady nutrient release | Moderate | Enhances soil structure | Requires time for production | [55] |
| Cover Crops | Long-term improvement | Indirect, through soil health | Erosion control, organic matter boost | Requires land-use during growth period | [56] |
| Leguminous Plants | Biological nitrogen fixation | High in compatible systems | Self-sustaining nitrogen source | Limited to suitable crops | [57] |
| Biofertilizers | Variable, depends on microbial activity | Variable | Environmentally friendly | Requires optimal conditions | [58] |
| Organic-Inorganic Mix | Balanced nutrient availability | High yield response | Improves nutrient-use efficiency | Complex management | [59] |
| High-Quality Organic Resources | Moderate to high | High, particularly in low-fertility soils | Reduces dependency on synthetic inputs | Requires high-quality material | [60] |
| Soil Amendment | Source | Impact on Plant–Soil System | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Elemental Sulfur | Naturally mined sulfur deposits | Lowers soil pH, improves nutrient availability | Long-term sulfur supply, pH adjustment | Requires microbial oxidation for effect | [55] |
| Gypsum (CaSO4·2H2O) | By-product of industrial processes or mined | Supplies calcium and sulfur; improves soil structure | Reduces aluminum toxicity in acidic soils | Limited to soils needing calcium | [54] |
| Ammonium Sulfate | By-product of fertilizer manufacturing | Rapid sulfur and nitrogen source | Quick nutrient release | Potential to acidify soils | [53] |
| Sulfur-Coated Urea | Industrially coated nitrogen fertilizer | Slow-release sulfur and nitrogen source | Provides consistent nutrient availability | Expensive to produce | [59] |
| Organic Matter | Plant residues, manure, compost | Gradual sulfur release through decomposition | Improves soil organic matter and soil fertility | Variable sulfur content | [58] |
| Biosolids | Treated sewage sludge | Supplies sulfur and organic matter | Recycling waste material | May contain “heavy metals” or contaminants | [81] |
| Potassium Sulfate | By-product of potash mining | Provides potassium and sulfur | Improves potassium levels | Limited to crops needing potassium | [82] |
| Sulfuric Acid | Industrial sulfur by-product | Lowers soil pH quickly in alkaline soils | Rapid correction of high soil pH | Risk of over-acidification | [57] |
| Year | Ecosystem | Organism | Si Pool Size | Biosilicification Rate | Reference |
|---|---|---|---|---|---|
| 2007 | Various forests | Testate amoebae | Up to 0.8 kg Si ha−1 | Up to 106 kg Si ha−1 yr−1 | [100] |
| 2013 | Beech forest ‘Beerenbusch’ | ” | 1.9 kg Si ha−1 | 17 kg Si ha−1 yr−1 | [101] |
| 2014 | Initial ecosystem states (different artificial catchments) | ” | Up to 0.7 kg Si ha−1 | Up to 16 kg Si ha−1 yr−1 | [97] |
| 2015 | Various forests | ” | Up to 4.7 kg Si ha−1 | Up to 80 kg Si ha−1 yr−1 | [94] |
| 2016 | Initial ecosystem states (artificial catchment ‘Chicken Creek’) | ” | Up to 0.06 kg Si ha−1 | -- | [98] |
| ” | ” | Diatoms | Up to 0.3 kg Si ha−1 | -- | ” |
| ” | ” | Sponges | Up to 0.2 kg Si ka−1 | -- | ” |
| ” | Various habitats in a nature reserve (artificial catchment ‘Mere Sands Wood’) | Testate amoebae | Up to 82 ng Si g−1 dm | -- | [102] |
| ” | ” | Diatoms | Up to 58 ng Si g−1 dm | -- | ” |
| 2017 | Initial ecosystem states (artificial catchment ‘Chicken Creek’) | Testate amoebae | Up to 0.4 kg Si ha−1 | -- | [99] |
| ” | ” | Diatoms | Up to 1.6 kg Si ha−1 | -- | ” |
| ” | ” | Sponges | Up to 0.5 kg Si ha−1 | -- | ” |
| 2020 | Floodplain (Watarase retarding basin) | Testate amoebae | Up to 2.9 μg Si g−1 dm | -- | [103] |
| ” | ” | Diatoms | Up to 12.8 μg Si g−1 dm | -- | ” |
| ” | Peatland and cropland sites (Dajiuhu National Wetland Park) | Testate amoebae | Up to 5.3 μg Si g−1 dm | -- | [104] |
| 2021 | Natural and cultivated Sphagnum sites | ” | Up to 0.1 μg Si per 150 testate amoeba shells | -- | [105] |
| 2022 | Various peatlands | ” | Up to 97 ng Si per 150 testate amoeba shells | -- | [106] |
| Microbial Species (Scientific Name) | Functional Role | Nutrient Cycling Process | Key Outputs/Impacts | Reference |
|---|---|---|---|---|
| Azotobacter vinelandii | Nitrogen fixation | Atmospheric N2 → Ammonia | Enhances soil nitrogen availability for plants. | [149] |
| Bradyrhizobium japonicum | Symbiotic nitrogen fixation | Forms nodules on legumes | Supplies nitrogen directly to host plants. | [150] |
| Pseudomonas fluorescens | Phosphate solubilization | Converts insoluble phosphorus | Increases bioavailability of phosphorus for plant uptake. | [151] |
| Bacillus subtilis | Phosphate solubilization | Organic phosphorus mineralization | Supports plant growth by enhancing soil phosphorus levels. | [152] |
| Nitrosomonas europaea | Nitrification | Ammonia → Nitrites | Facilitates conversion of nitrogen into usable forms, influencing nutrient cycling. | [153] |
| Nitrobacter winogradskyi | Nitrification | Nitrites → Nitrates | Ensures availability of nitrate for plant uptake but increases leaching risks. | [154] |
| Paraburkholderia phytofirmans | Plant growth promotion | Enhances phosphorus and nitrogen | Improves nutrient acquisition, fostering plant growth. | [155] |
| Rhizobium leguminosarum | Symbiotic nitrogen fixation | Forms nodules on legumes | Converts atmospheric nitrogen for host plants, improving soil fertility. | [156] |
| Frankia spp. | Nitrogen fixation in actinorhizal plants | Atmospheric N2 → Ammonia | Supports nitrogen levels in non-leguminous plants. | [157] |
| Desulfovibrio desulfuricans | Sulfate reduction | Sulfate (SO42−) → Hydrogen sulfide (H2S) | Contributes to sulfur cycling in anaerobic environments, impacting soil and water chemistry. | [158] |
| Thiobacillus thioparus | Sulfur oxidation | Elemental sulfur → Sulfate (SO42−) | Increases soil sulfate levels, promoting plant sulfur uptake. | [159] |
| Soil Management Practice | Description | Carbon Sequestration Potential | Impact on Soil Health | Reference |
|---|---|---|---|---|
| Conservation Tillage | Reduced tillage to minimize soil disturbance. | Moderate to high | Improves soil structure, reduces erosion, and enhances organic matter retention. | [149] |
| Cover Cropping | Planting cover crops during off-season periods. | High | Increases organic carbon inputs and reduces nutrient leaching. | [176] |
| Compost Addition | Application of compost to soils. | High | Enhances microbial activity, nutrient availability, and organic carbon. | [177] |
| Agroforestry | Integration of trees with agricultural crops. | Very high | Promotes biodiversity, reduces soil erosion, and increases carbon storage. | [178] |
| Biochar Amendment | Adding pyrolyzed biomass to soil. | High | Increases soil carbon stability, improves water retention, and supports microbial growth. | [179] |
| Crop straw recycling | Application of chopped straw to soil. | Moderate to high (long-term effects) | Replenishes plant available Si, reduces the need for N fertilizers, and increases organic carbon inputs in the long term. | [113] |
| Crop Rotation | Alternating crops to improve soil nutrient balance. | Moderate | Reduces pest buildup, enhances nitrogen use efficiency, and improves soil structure. | [154] |
| Integrated Livestock Management | Combining livestock and crop systems. | Moderate to high | Enhances nutrient recycling and boosts organic matter input through manure. | [155] |
| No-Tillage | Avoiding plowing entirely to maintain soil integrity. | High | Reduces erosion, improves water infiltration, and increases organic matter retention. | [156] |
| Perennial Grass Systems | Using perennial grasses for soil coverage. | Very high | Reduces erosion, improves soil structure, and enhances long-term carbon storage. | [157] |
| Threat | Impact on Biogeochemical Cycles | Potential Mitigation Strategies | Reference |
|---|---|---|---|
| Excessive Fertilizer Use | Disrupts nitrogen and phosphorus cycles; causes eutrophication. | Precision agriculture, optimized fertilizer application, and crop-specific nutrient management. | [149] |
| Deforestation | Reduces carbon sequestration and alters nitrogen and silicon cycling. | Reforestation, afforestation, and agroforestry practices. | [198,205] |
| Desilication | Loss of Si from agricultural plant–soil systems. | Crop straw recycling, application of amorphous silica. | [111,113] |
| Industrial Pollution | Releases “heavy metals” and toxic compounds, affecting microbial activity and soil health. | Pollution control measures, phytoremediation, and stricter industrial regulations. | [206,207] |
| Urbanization | Alters land-use, leading to loss of soil organic matter and nutrient imbalances. | Urban green spaces, soil restoration projects, and sustainable urban planning. | [208] |
| Overgrazing by Livestock | Depletes soil nutrients and increases erosion, disrupting nutrient cycling. | Rotational grazing, controlled stocking rates, and land rehabilitation. | [209] |
| Waste Mismanagement | Accumulation of organic waste disrupts carbon and nitrogen cycles. | Composting, recycling, and waste-to-energy technologies. | [154] |
| Mining Activity | Causes soil degradation and disrupts phosphorus and sulfur cycles. | Land reclamation, sustainable mining practices, and ecosystem restoration. | [155] |
| Climate Change | Accelerates nutrient leaching and alters carbon, nitrogen, and water cycles. | Carbon capture technologies, renewable energy, and climate-smart agriculture. | [156] |
| Aquatic Pollution | Disturbs nutrient cycling in water bodies, leading to hypoxia. | Wetland restoration, buffer strips, and controlled effluent discharge. | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, W.; Ayaz, A.; Puppe, D. Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology 2025, 14, 433. https://doi.org/10.3390/biology14040433
Zaman W, Ayaz A, Puppe D. Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology. 2025; 14(4):433. https://doi.org/10.3390/biology14040433
Chicago/Turabian StyleZaman, Wajid, Asma Ayaz, and Daniel Puppe. 2025. "Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions" Biology 14, no. 4: 433. https://doi.org/10.3390/biology14040433
APA StyleZaman, W., Ayaz, A., & Puppe, D. (2025). Biogeochemical Cycles in Plant–Soil Systems: Significance for Agriculture, Interconnections, and Anthropogenic Disruptions. Biology, 14(4), 433. https://doi.org/10.3390/biology14040433