Kin-Mediated Male Choice and Alternative Reproductive Tactics in Spider Mites
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Male-Male Competition, No-Choice
2.3. Male Choice, No Competition
2.4. Body Size Measurements
2.5. Statistical Analyses
3. Results
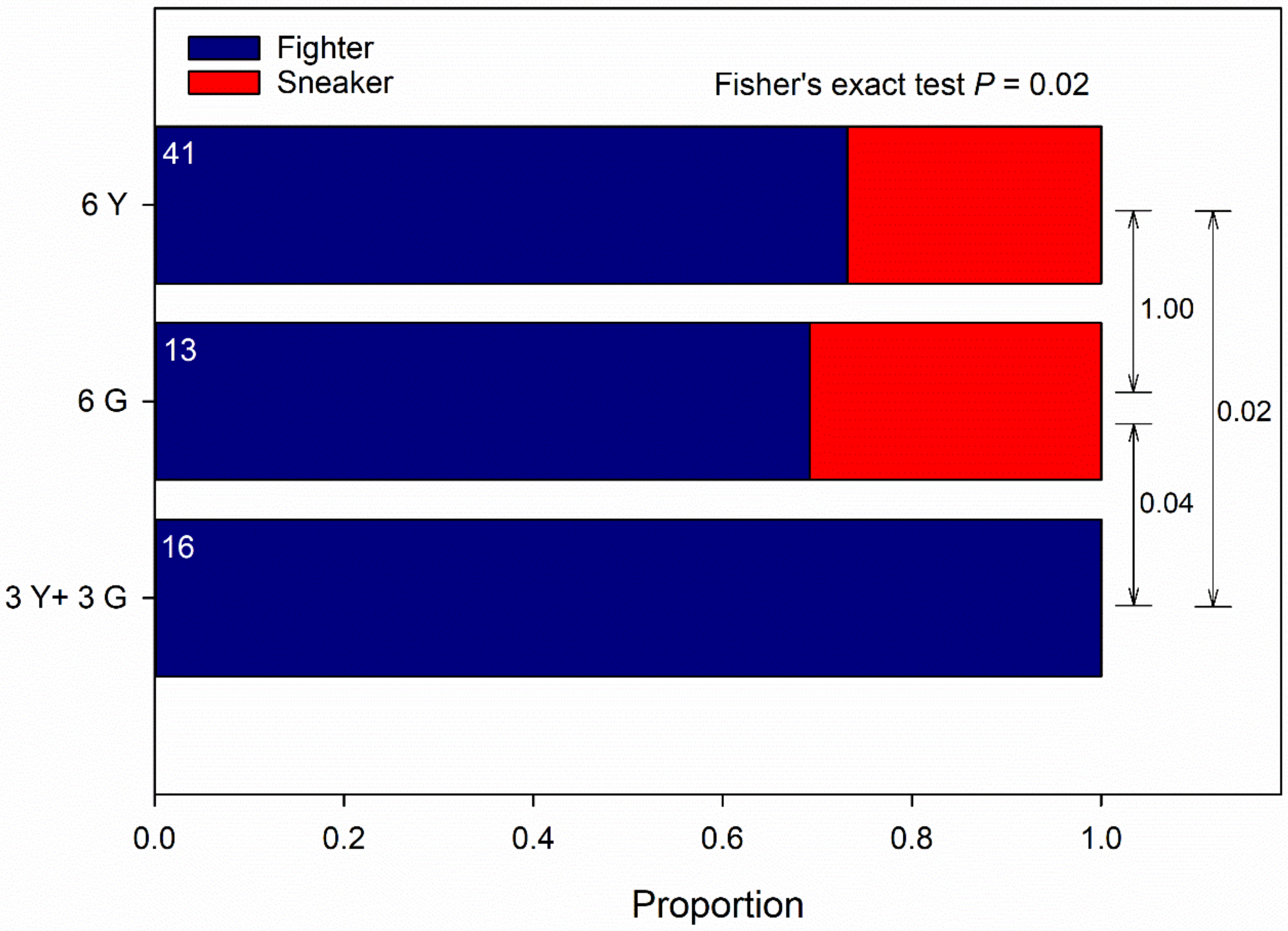
3.1. Male-Male Competition, No-Choice
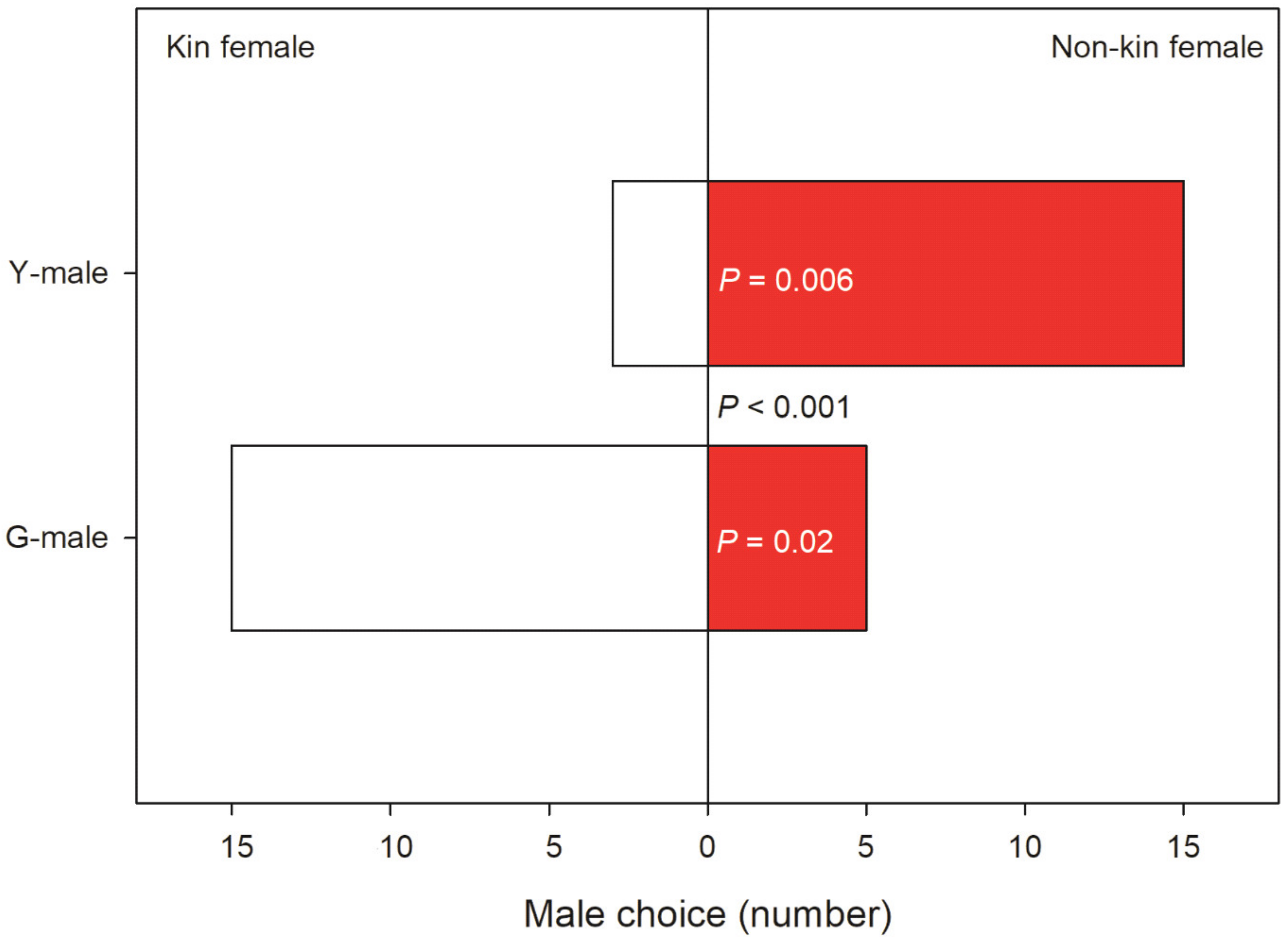
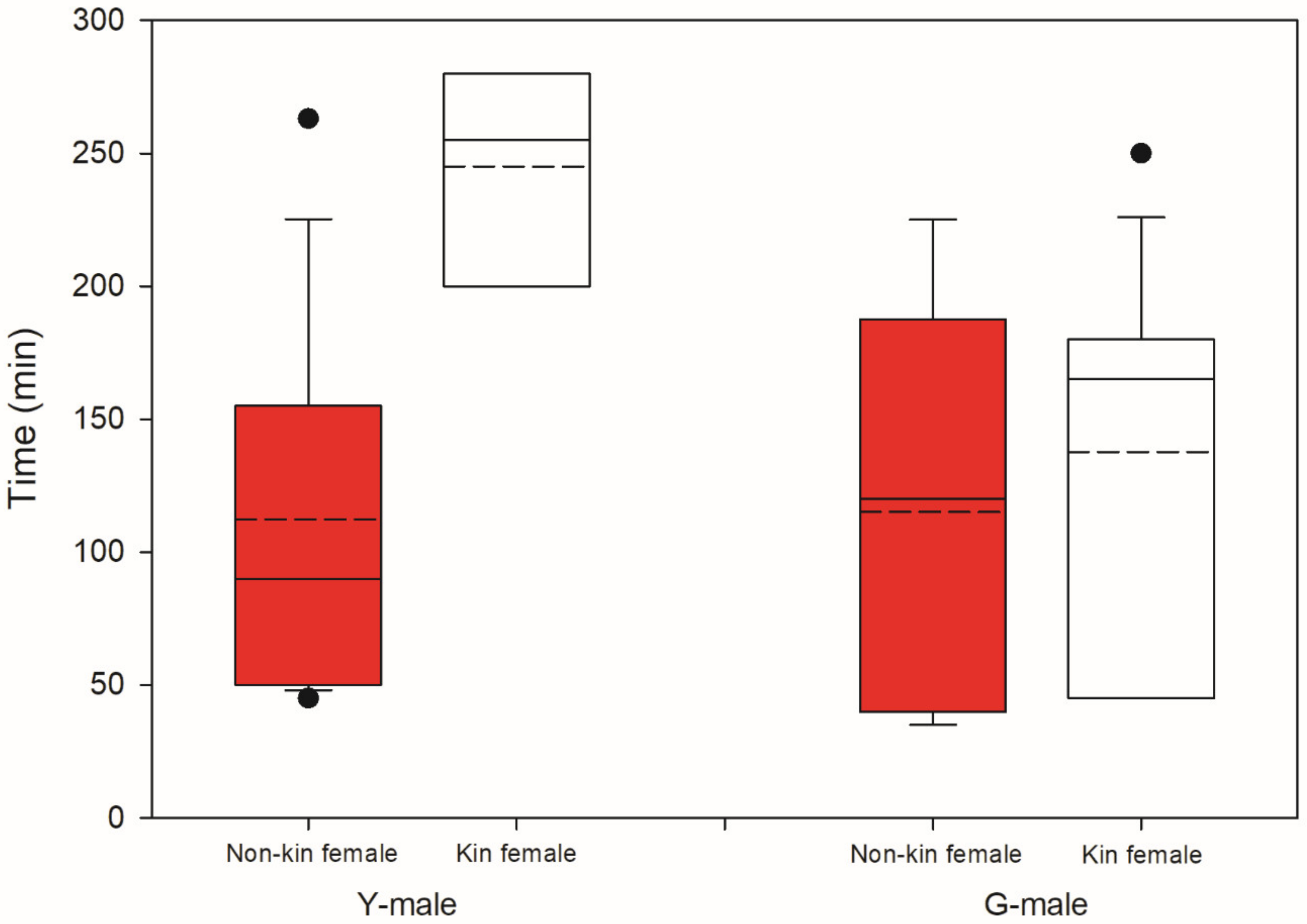
3.2. Male Choice, No Competition
3.3. Body Size
4. Discussion
4.1. Proximate Mechanisms
4.2. Male-Male Competition, No-Choice
4.3. Male Choice, No Competition
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trivers, R.L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B.G., Ed.; Aldine Publishing Company: Chicago, IL, USA, 1972; pp. 136–179. [Google Scholar]
- Edward, D.A.; Chapman, T. The evolution and significance of male mate choice. Trends Ecol. Evol. 2011, 26, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kokko, H.; Brooks, R.; Jennions, M.D.; Morley, J. The evolution of mate choice and mating biases. Proc. R. Soc. B Biol. Sci. 2003, 270, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.G.; Rice, W.R. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. B Boil. Sci. 2006, 273, 917–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oku, K. Sexual selection and mating behavior in spider mites of the genus Tetranychus (Acari: Tetranychidae). Appl. Èntomol. Zooöl. 2013, 49, 1–9. [Google Scholar] [CrossRef]
- Yoshioka, T.; Yano, S. Do Tetranychus urticae males avoid mating with familiar females? J. Exp. Biol. 2014, 217, 2297–2300. [Google Scholar] [CrossRef] [Green Version]
- Bolland, H.R.; Gutierrez, J.; Flechtmann, C.H.W. World catalogue of the spider mite family (Acari: Tetranychidae); Brill Academic Publishers: Leiden, The Netherlands, 1998. [Google Scholar]
- Gross, M.R. Alternative reproductive strategies and tactics: Diversity within sexes. Trends Ecol. Evol. 1996, 11, 92–98. [Google Scholar] [CrossRef]
- Oliveira, R.F.; Taborsky, M.; Brockmann, H.J. Alternative Reproductive Tactics: An Integrative Approach; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Emlen, D.J. The roles of genes and the environment in the expression and evolution of alternative reproductive tactics. In Alternative Reproductive Tactics: An Integrative Approach; Oliveira, R.F., Taborsky, M., Brockmann, H.J., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 85–108. [Google Scholar]
- Andersson, M.; Åhlund, M.; Waldeck, P. Brood parasitism, relatedness and sociality: A kinship role in female reproductive tactics. Biol. Rev. 2018, 94, 307–327. [Google Scholar] [CrossRef]
- Foitzik, S.; Heinze, J.; Oberstadt, B.; Herbers, J.M. Mate guarding and alternative reproductive tactics in the ant Hypoponera opacior. Anim. Behav. 2002, 63, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Cook, J.M.; Reuter, C.; Moore, J.C.; West, S.A. Fighting in fig wasps: Do males avoid killing brothers or do they never meet them? Ecol. Èntomol. 2015, 40, 741–747. [Google Scholar] [CrossRef]
- Helle, W. Fertilization in the two-spotted spider mite (Tetranychus urticae: Acari). Èntomol. Exp. Appl. 1967, 10, 103–110. [Google Scholar] [CrossRef]
- Potter, D.A.; Wrensch, D.L.; Johnston, D.E. Guarding, aggressive behavior, and mating success in male twospotted spider mites. Ann. Èntomol. Soc. Am. 1976, 69, 707–711. [Google Scholar] [CrossRef]
- Potter, D.A.; Wrensch, D.L.; Johnston, D.E.; Galbally, I.E. Aggression and mating success in male spider mites. Science 1976, 193, 160–161. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.A.; Wrensch, D.L. Interrupted matings and the effectiveness of second inseminations in the twospotted spider mite. Ann. Èntomol. Soc. Am. 1978, 71, 882–885. [Google Scholar] [CrossRef]
- Sato, Y.; Sabelis, M.W.; Egas, M.; Faraji, F. Alternative phenotypes of male mating behaviour in the two-spotted spider mite. Exp. Appl. Acarol. 2013, 61, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Sabelis, M.W.; Egas, M. Alternative male mating behaviour in the two-spotted spider mite: Dependence on age and density. Anim. Behav. 2014, 92, 125–131. [Google Scholar] [CrossRef]
- Schausberger, P.; Sato, Y. Parental effects of male alternative reproductive tactics (ARTs) on ARTs of haploid sons. Funct. Ecol. 2019, 33, 1684–1694. [Google Scholar] [CrossRef]
- Jiang, Y.; Bolnick, D.I.; Kirkpatrick, M. Assortative mating in animals. Am. Nat. 2013, 181, E125–E138. [Google Scholar] [CrossRef] [Green Version]
- Baniel, A. Assortative Mating. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T.K., Eds.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Clotuche, G.; Deneubourg, J.-L.; Mailleux, A.-C.; Detrain, C.; Hance, T. Discrimination through silk recognition: The case of the two-spotted spider mite Tetranychus urticae. Comptes Rendus Biol. 2012, 335, 535–540. [Google Scholar] [CrossRef]
- Hamilton, W.D. The genetical evolution of social behavior. I. J. Theor. Biol. 2017, 7, 23–43. [Google Scholar] [CrossRef]
- Hamilton, W.D. The genetical evolution of social behavior. II. J. Theor. Biol. 2017, 7, 44–90. [Google Scholar] [CrossRef]
- Kokko, H.; Ots, I. When not to avoid inbreeding. Evolution 2006, 60, 467–475. [Google Scholar] [CrossRef]
- Puurtinen, M. Mate choice for optimal (k)inbreeding. Evolution 2011, 65, 1501–1505. [Google Scholar] [CrossRef]
- Charlesworth, D. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 1987, 18, 237–268. [Google Scholar] [CrossRef]
- Vala, F.; Breeuwer, J.; Sabelis, M. Sorting out the effects of Wolbachia, genotype and inbreeding on life-history traits of a spider mite. Exp. Appl. Acarol. 2003, 29, 253–264. [Google Scholar] [CrossRef]
- Hamilton, W.D. Extraordinary Sex Ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Schausberger, P.; Gotoh, T.; Sato, Y. Spider mite mothers adjust reproduction and sons’ alternative reproductive tactics to immigrating alien conspecifics. R. Soc. Open Sci. 2019, 6, 191201. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, Z.-Q. Does size matter? Fecundity and longevity of spider mites (Tetranychus urticae) in relation to mating and food availability. Syst. Appl. Acarol. 2018, 23, 1796–1808. [Google Scholar] [CrossRef]
- Alstad, D. Basic Populus Models of Ecology; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Gutierrez, J. Systematics. In Spider Mites, Their Biology, Natural Enemies and Control; Helle, W., Sabelis, M.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1A, pp. 75–90. [Google Scholar]
- Royalty, R.N.; Phelan, P.L.; Hall, F.R. Arrestment of male twospotted spider mite caused by female sex pheromone. J. Chem. Ecol. 1992, 18, 137–153. [Google Scholar] [CrossRef]
- Royalty, R.N.; Phelan, P.L.; Hall, F.R. Comparative effects of form, colour, and pheromone of twospotted spider mite quiescent deutonymphs on male guarding behavior. Physiol. Entomol. 1993, 18, 303–316. [Google Scholar] [CrossRef]
- Mateo, J.M. Recognition systems and biological organization: The perception component of social recognition. Ann. Zool. Fenn. 2004, 41, 729–745. [Google Scholar]
- Wilson, J.R.; Kuehn, R.E.; Beach, F.A. Modification in the sexual behavior of male rats produced by changing the stimulus female. J. Comp. Physiol. Psychol. 1963, 56, 636–644. [Google Scholar] [CrossRef]
- Dewsbury, D.A. Effects of novelty on copulatory behavior: The Coolidge effect and related phenomena. Psychol. Bull. 1981, 89, 464–482. [Google Scholar] [CrossRef]
- Oku, K. Males of the two-spotted spider mite attempt to copulate with mated females: Effects of double mating on fitness of either sex. Exp. Appl. Acarol. 2009, 50, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macke, E.; Magalhães, S.; Bach, F.; Olivieri, I. Experimental evolution of reduced sex Rratio adjustment under local mate competition. Science 2011, 334, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkpatrick, M.; Barton, N.H. The strength of indirect selection on female mating preferences. Proc. Natl. Acad. Sci. USA 1997, 94, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonduriansky, R. The evolution of male mate choice in insects: A synthesis of ideas and evidence. Biol. Rev. 2001, 76, 305–339. [Google Scholar] [CrossRef] [Green Version]
- Bateson, M.; Healy, S.D. Comparative evaluation and its implications for mate choice. Trends Ecol. Evol. 2005, 20, 659–664. [Google Scholar] [CrossRef]
- Jennions, M.D.; Petrie, M. Variation in mate choice and mating preferences: A review of causes and consequences. Biol. Rev. Camb. Philos. Soc. 1997, 72, 283–327. [Google Scholar] [CrossRef]
- Rutstein, A.N.; Brazill-Boast, J.; Griffith, S.C. Evaluating mate choice in the zebra finch. Anim. Behav. 2007, 74, 1277–1284. [Google Scholar] [CrossRef]
- Dougherty, L.R.; Shuker, D.M. The effect of experimental design on the measurement of mate choice: A meta-analysis. Behav. Ecol. 2014, 26, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Bretman, A.; Fricke, C.; Chapman, T. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B Boil. Sci. 2009, 276, 1705–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, B.B.M.; McCarthy, M. Prudent male mate choice under perceived sperm competition risk in the eastern mosquito fish. Behav. Ecol. 2009, 20, 278–282. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schausberger, P.; Sato, Y. Kin-Mediated Male Choice and Alternative Reproductive Tactics in Spider Mites. Biology 2020, 9, 360. https://doi.org/10.3390/biology9110360
Schausberger P, Sato Y. Kin-Mediated Male Choice and Alternative Reproductive Tactics in Spider Mites. Biology. 2020; 9(11):360. https://doi.org/10.3390/biology9110360
Chicago/Turabian StyleSchausberger, Peter, and Yukie Sato. 2020. "Kin-Mediated Male Choice and Alternative Reproductive Tactics in Spider Mites" Biology 9, no. 11: 360. https://doi.org/10.3390/biology9110360
APA StyleSchausberger, P., & Sato, Y. (2020). Kin-Mediated Male Choice and Alternative Reproductive Tactics in Spider Mites. Biology, 9(11), 360. https://doi.org/10.3390/biology9110360





