Abstract
Food technology, health, nutrition, dermatology, and aesthetics have focused on colorless carotenoids. Carotenoids are readily bioavailable and have demonstrated various health-promoting actions. This article reviews the recent literature concerning carotenoids with the aim to systematize the scattered knowledge on carotenoids and aesthetics. The applications of carotenoids in health-promoting and nutrient products and their potential health effects are discussed. The carotenoids, particularly phytoene and phytofluene, have the unique ability to absorb ultraviolet radiation. Their distinct structures and properties, oxidation sensitivity, stiffness, aggregation tendency, and even fluorescence in the case of phytofluene, contribute to their potential benefits. A diet rich in carotenoid-containing products can positively impact skin health, overall well-being, and the prevention of various diseases. Future studies should focus on generating more data about phytoene and phytofluene levels in the skin to accurately assess skin carotenoid status. This expanding area of research holds promise for the development of novel applications in the fields of health and cosmetics.
1. Introduction
The skin plays a vital role as a natural barrier, shielding us from various external elements such as radiation, xenobiotics, micro-organisms, etc. However, it can also impede fundamental processes [1,2,3]. Skin damage can give rise to a range of disorders that can potentially develop into diseases, such as infections or even skin cancer. These conditions have a detrimental impact on overall well-being and health. While a precise and universally agreed-upon definition is lacking, the term “fructose” typically pertains to the ingestion of substances present in food items, includingvitamins, peptides, polysaccharides, polyphenols, coenzyme Q10, polyunsaturated fatty acids, and carotenoids [4,5]. Carotenoids are naturally occurring compounds that can be obtained through the diet from food sources, added as additives, or taken in the form of supplements, among other products. Certain carotenoids are consistently present in human plasma, milk, and various tissues, including the skin [6]. Carotenoids serve not only as natural pigments but also hold significance in the diet due to their ability to function as provitamin A. Among the carotenoid family, phytoene and phytofluene are colorless carotenoids that have received limited attention in dermoaesthetics, cosmetics, and dermatology studies. However, recent comprehensive reviews have highlighted their importance as major dietary carotenoids found in tomatoes, carrots, citrus fruits, and their derivatives. These colorless carotenoids are readily bioavailable, being present as significant carotenoids in plasma, human milk, and the skin. Furthermore, they contribute to various health effects [7].
2. UV Radiation Types and Consequences of Exposure
The skin serves multiple essential functions in the human body. It acts as a protective epithelium, shielding the body from external elements. Additionally, it functions as a connective tissue, providing support, nourishment, and metabolic processes for organic and metal components. The skin also plays a role in the sense of touch and serves as an indicator of overall health. Furthermore, it also serves as a vital organ for environmental communication [8]. The dermis, a layer within the skin, provides mechanical strength and elasticity, while the subcutis acts as insulation and mechanical protection, contributing to thermoregulation [9].
Protection against UV light is crucial to prevent the harmful effects of exposure to sunlight or artificial sources of UV light. These exposures, often associated with tanning, can have detrimental consequences. Various disorders can be attributed to UV exposure, including sunburn, immunosuppression, photocarcinogenesis (the development of skin cancer due to UV radiation), and photoaging (Figure 1). Photoaging is mainly characterized by the breakdown of collagen, leading to the formation of wrinkles. Additionally, unwanted aesthetic signs of photoaging include telangiectasia (visible blood vessels) and hyperpigmentation [10]. Thus, safeguarding the skin from UV radiation is essential to minimize these undesirable effects from both health and aesthetic perspectives. The skin’s barrier function is primarily attributed to the stratum corneum, which acts as a waterproof and relatively impermeable barrier. It plays a crucial role in preventing the penetration of various xenobiotics into the body [11]. The skin employs several mechanisms to protect against ultraviolet radiation (UVR). These include thickening of the epidermis, DNA repair mechanisms, programmed cell death, antioxidant enzymes, and skin pigmentation [12]. In certain cases, dietary components, including carotenoids, may also contribute to the protective role against UVR [13].
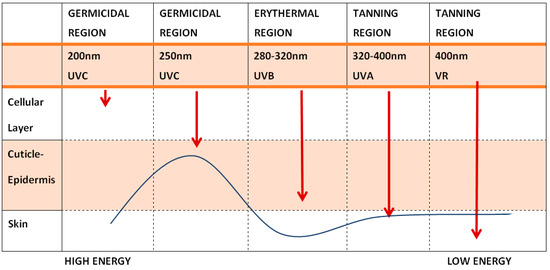
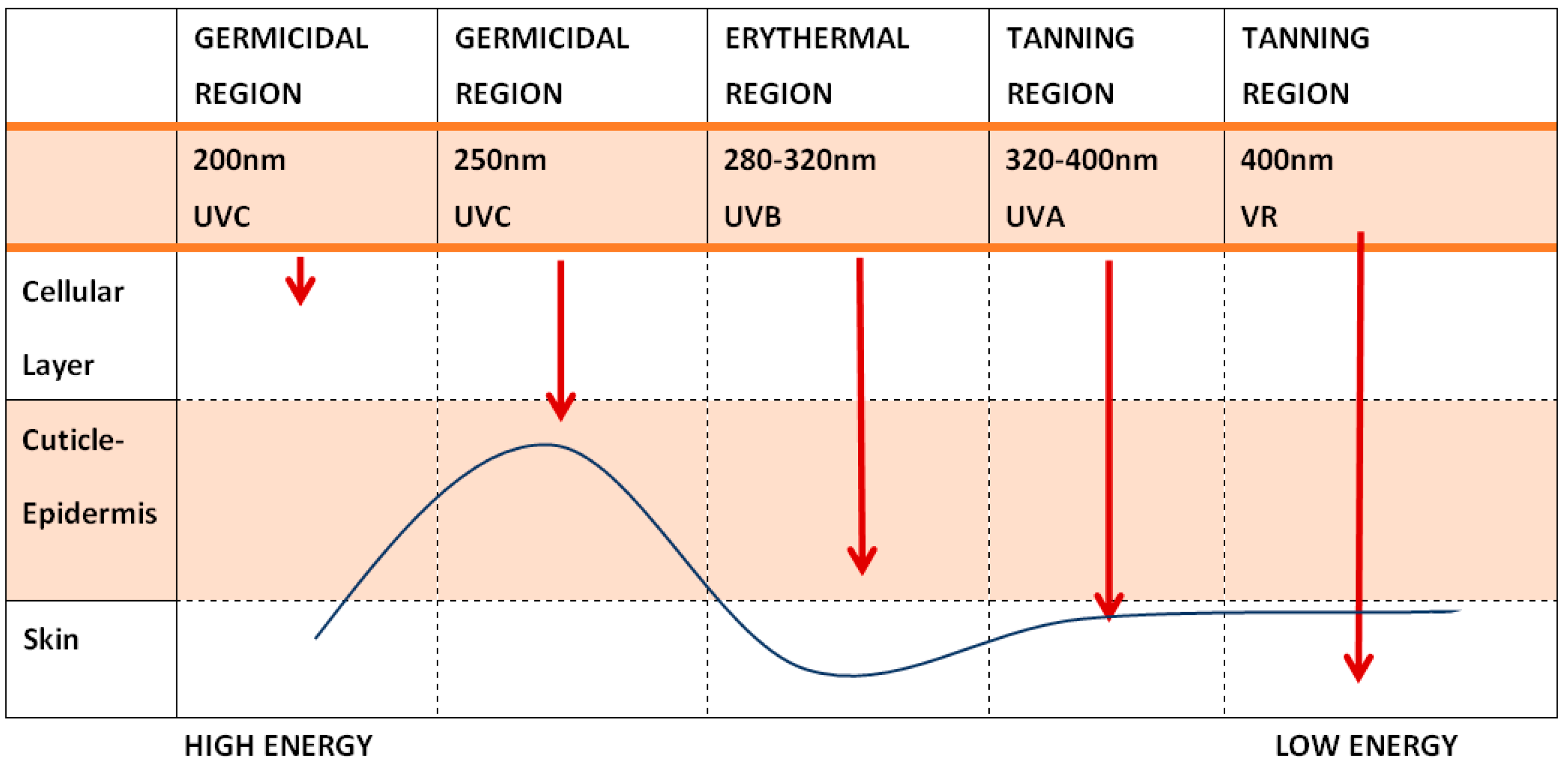
Figure 1.
Depth Penetration of Radiation on the Skin—The longer the wavelength (and the lower the energy of the radiation), the deeper the penetration (modified from Dr. F. Biskanaki).
3. Skin Disorders
It is estimated that there are over 1000 known skin disorders, including infections, drug reactions, psoriasis, eczema, urticaria, acne vulgaris, pityriasisrubra pilaris, Darier’s disease, ichthyosis, lichen ruber, and various types of skin cancers such as basal cell carcinoma, squamous cell carcinoma, and melanoma, among others [14,15]. These conditions contribute significantly to the workload of general practitioners, with approximately one-fifth of all patient referrals being related to skin pathologies. Consequently, skin disorders impose a substantial economic burden on individuals and healthcare services [16,17]. Moreover, certain skin disorders are directly linked to exposure to ultraviolet radiation (UVR). These include photosensitivity disorders, photocarcinogenesis, sunburn, photoaging, and photoimmune modulation [18,19].
One example of a photosensitivity disorder is erythropoietic protoporphyria. This rare inherited hematological disease results in elevated protoporphyrin levels in the plasma, red blood cells, skin, and feces. Protoporphyrin is an endogenous photosensitizer, meaning it becomes activated upon exposure to light [20,21]. This activation can ultimately lead to reactive oxygen species (ROS) forming, causing cellular damage and resulting in clinical symptoms of photosensitivity. Individuals with erythropoietic protoporphyria experience itching, burning, and pain in the skin exposed to sunlight, even after just a few minutes of exposure. This is followed by edema (swelling), erythema (redness), and purpura (purple discoloration) [22,23]. Along with porphyrins, other endogenous compounds such as flavins or amino acids can also act as sensitizing molecules in photosensitivity disorders [24,25].
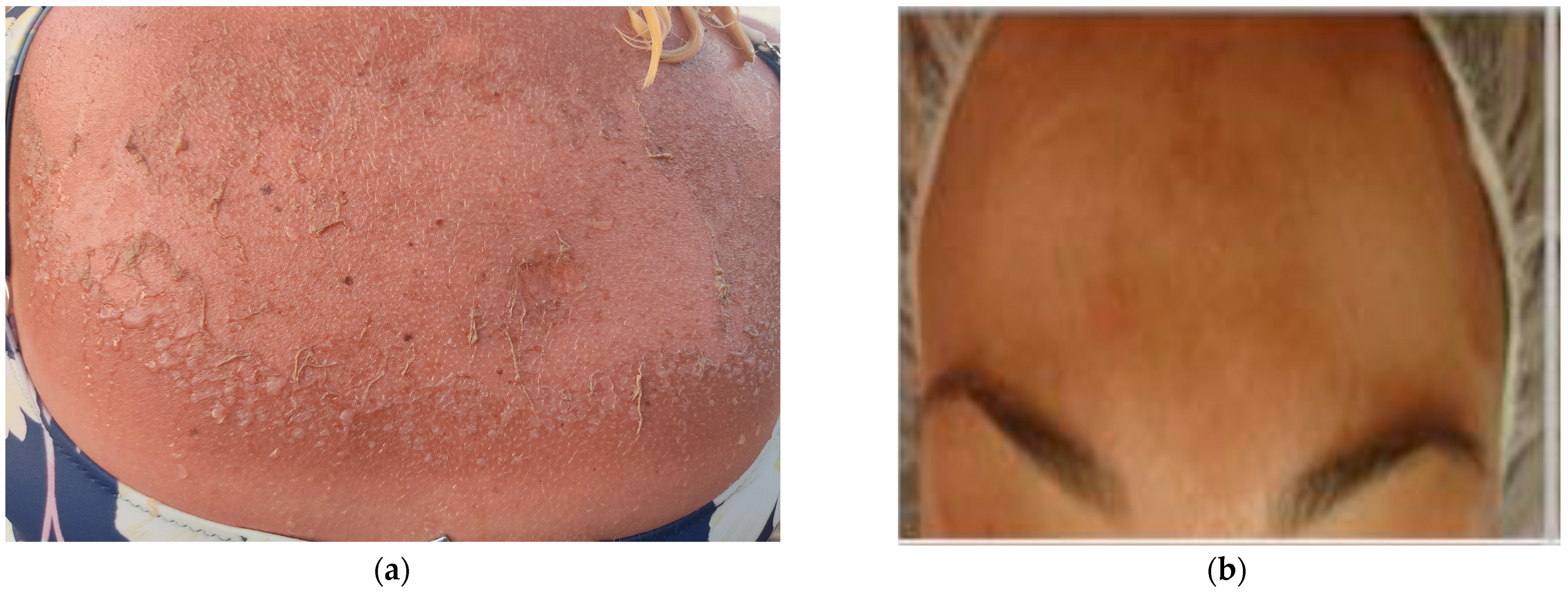
Sunburn, also known as erythema solare, is an acute skin inflammatory reaction due to excessive exposure to natural or artificial UV radiation (see Figure 2a). The extent of sunburn depends on the wavelength of UV radiation, with its effectiveness decreasing as the wavelengths become longer. It has been estimated that it takes approximately 1000 times more UVA than UVB radiation to produce the same erythemal (skin reddening) response. As a result, it is commonly believed that UVB radiation is primarily responsible for causing sunburn. UVB radiation triggers cytokine-mediated processes and activates neuroactive and vasoactive mediators in the skin, leading to inflammatory responses [26,27,28,29].
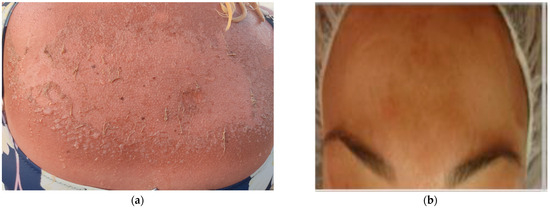
Figure 2.
(a) Severe sunburn in a 45-year-old woman (archival material from Dr. P. Kalofiri). (b) Hyperpigmentation—Melasma and wrinkles at the age of 30 after exposure to solar radiation (archival material from Dr. F. Biskanaki).
The typical symptom of sunburn is the reddening of the skin and dilation of cutaneous blood vessels. In more severe cases, blisters and the ablation of the epidermis may also occur (Figure 2a). It is worth noting that sunburn tends to last longer, up to several days, in older individuals and those with fairer skin. Sunburn cells refer to keratinocytes that undergo apoptosis, a form of programmed cell death [30,31].
The minimal erythema dose (MED) represents the minimum amount of UVB radiation required to cause sunburn and varies significantly depending on an individual’s skin type. Different skin types have different sensitivities to UV radiation so the MED can vary among individuals [32,33,34].
There is compelling evidence suggesting that excessive exposure to ultraviolet radiation (UVR) induces various immunological changes within the immune system, a phenomenon known as photoimmune modulation [35,36]. Both UVA and UVB radiation have been shown to have local and systemic immunosuppressive effects. For instance, following UVR exposure, Langerhans cells-antigen-presenting dendritic cells found in the epidermis and produced in the bone marrow-undergo functional and morphological changes that ultimately lead to their depletion. Additionally, UVR exposure may result in T-cell tolerance, affecting the immune response [37,38].
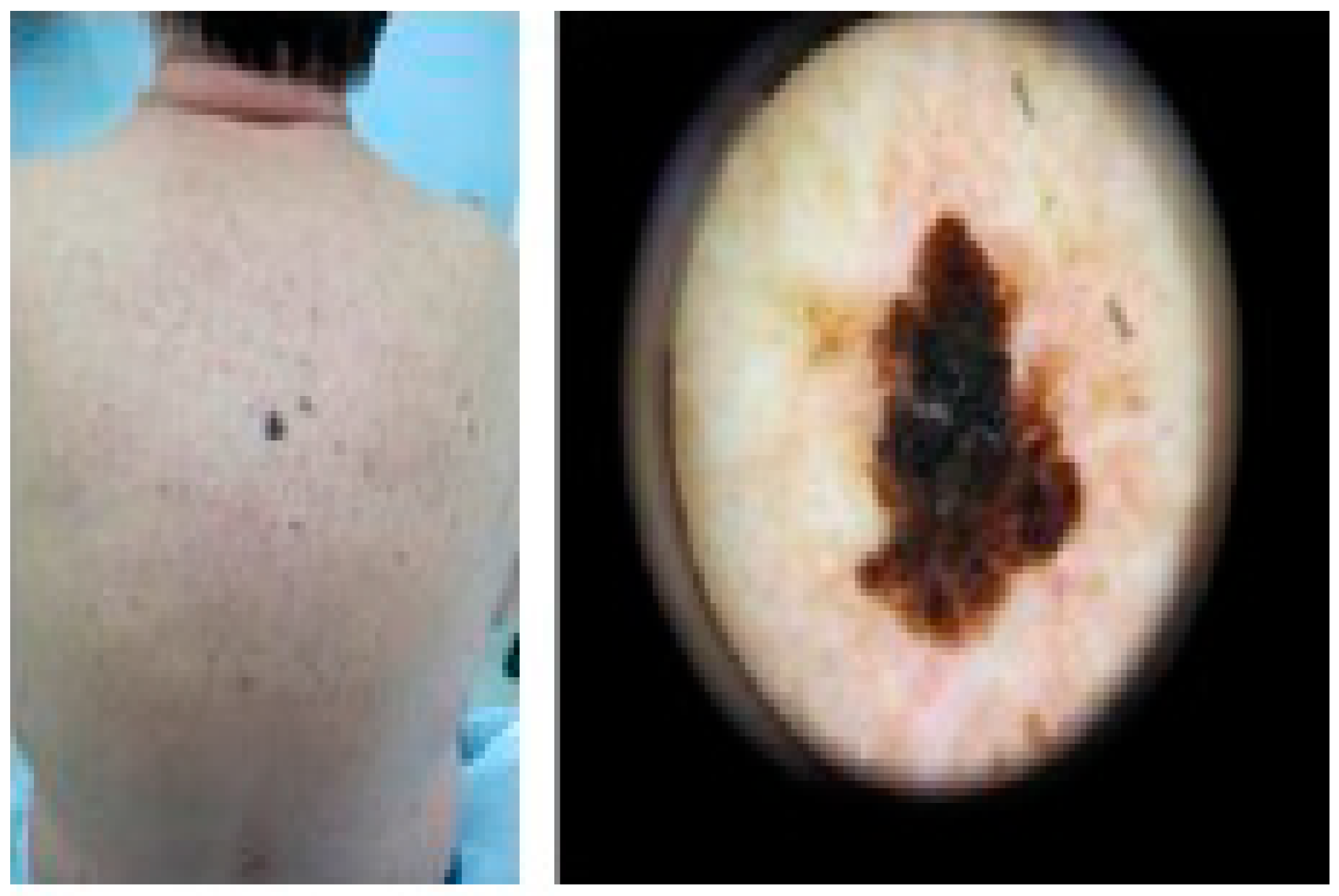
Photocarcinogenesis refers to the process initiated by exposure to solar or artificial light, ultimately leading to the development of skin cancer [39]. UVB radiation is believed to be directly absorbed into DNA, causing structural damage to the DNA bases. On the other hand, UVA radiation is considered to induce indirect DNA damage by generating reactive oxygen species (ROS), resulting in single-strand breaks and DNA-protein crosslinks [40,41]. These mechanisms contribute to the increased risk of skin cancer associated with prolonged or excessive exposure to UVR [39] (see Figure 3). It is important to note that these processes are complex and involve multiple factors, and further research is ongoing to better understand the detailed mechanisms of photoimmune modulation and photocarcinogenesis.
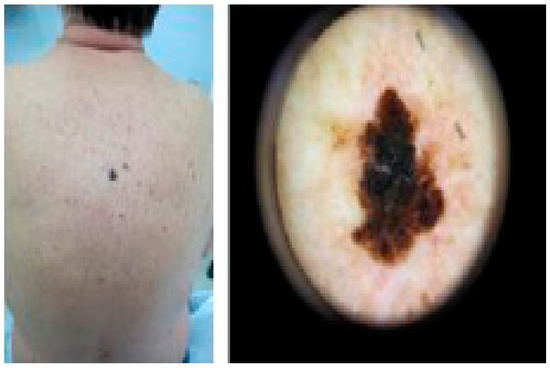
Figure 3.
Melanoma-Male back (archival material from Dr. E. Rallis).
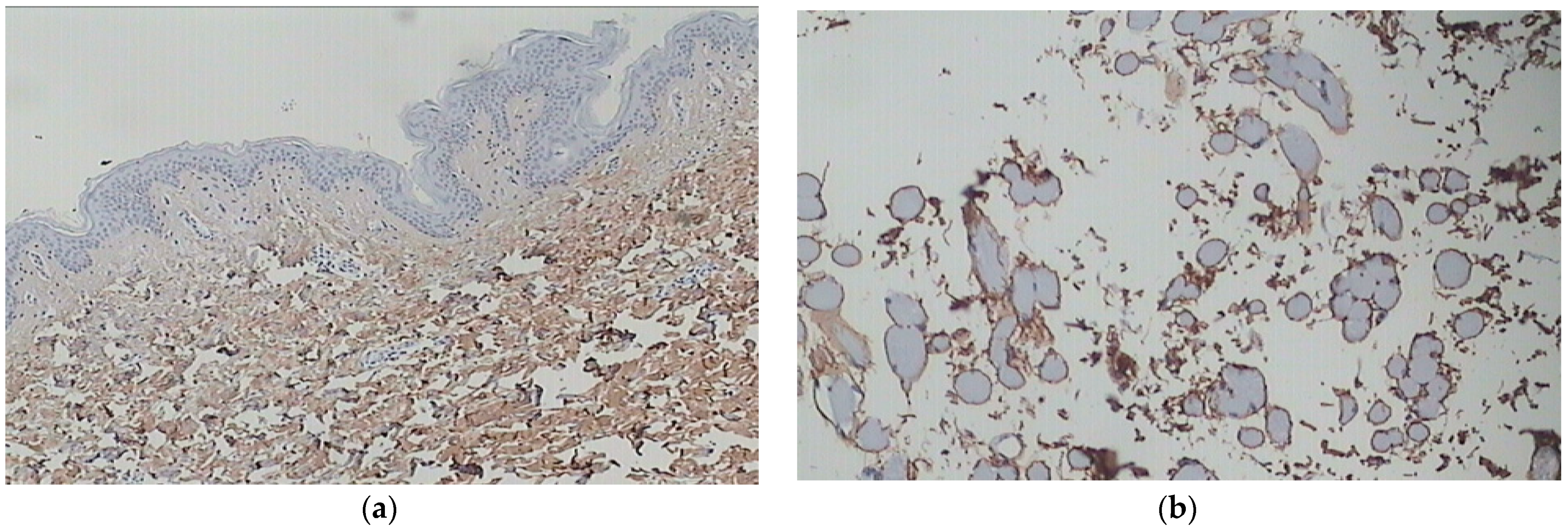
The decline in the skin’s structure and function can be categorized into two main types: intrinsic or chronological aging, which occurs naturally over time and extrinsic aging, which is attributed to external factors. Extrinsic aging encompasses various factors, such as chronic exposure to high levels of ultraviolet radiation (UV) or photoaging and other agents, including smoking, pollution, sleep deprivation, and poor nutrition [42,43]. Chronological aging and photoaging exhibit some histological differences and can sometimes be clinically distinguishable. Skin aging, whether intrinsic or extrinsic, impacts numerous skin characteristics, processes, and functions. These include diminished regenerative capability, changes in pigmentation, compromised thermoregulation, and a decrease in the strength and elasticity provided by the collagen-rich extracellular matrix, among others (Figure 2b). The cumulative effect of these changes results in increased skin fragility and susceptibility to various diseases [44,45]. Photoaging specifically refers to skin aging caused by chronic exposure to UV radiation (Figure 2b). It is characterized by specific features such as pigmented lesions, including actinic lentigines or “age spots”, ephelides or “freckles”, as well as pigmented solar and seborrheic keratosis. Telangiectasia, which refers to the visible dilation of small blood vessels, is another characteristic feature of photoaging. These manifestations of photoaging are a result of long-term exposure to UV radiation and contribute to the overall appearance and condition of aged skin [46,47] (See Figure 4a,b).
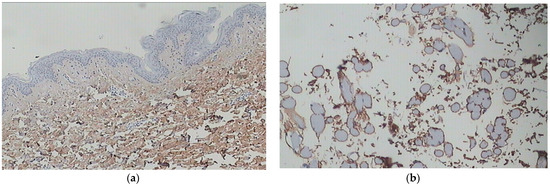
Figure 4.
(a) Youthful, healthy skin (at the age of 25) after staining with COL I antibodies. The collagen fibers appear fine and loosely arranged in the upper dermis, while they are thicker in the lower dermis. There is a uniform distribution of collagen type I. (b) Irregular distribution of collagen type I without homogeneity in photo-exposed skin (age 55) (archival material from Dr. F. Biskanaki).
4. Dietary Carotenoids
The impact of nutrition on the skin has long been a subject of interest for scientists, physicians, pharmacists, and other professionals [48]. Various common food components are frequently utilized in skincare products and formulations. These include vitamins, peptides, polysaccharides, polyphenols, coenzyme Q10, polyunsaturated fatty acids, and carotenoids. These ingredients are often incorporated due to their potential benefits for skin health and appearance [49].
Carotenoids are a class of isoprenoid compounds that are widely distributed in nature. They possess diverse structures, physicochemical properties, and activities, which have evolved to fulfill crucial roles in various biological processes [50]. Carotenoids are involved in essential functions such as photosynthesis, inter-species communication through color signaling, nutrition, and overall health. The primary sources of carotenoids through diet are fruits and vegetables [51]. These natural sources provide an array of carotenoids that contribute to their vibrant colors and offer potential health benefits when consumed. Indeed, carotenoids can be found in various diet components beyond fruits and vegetables. They are also present in herbs, legumes, cereals [52], algae [53,54], foods of animal origin (such as egg yolk, mammal’s milk and tissues, and seafood) [55,56], food additives (such as colorants) [57,58], and as dietary supplements. Among the numerous carotenoids, the main ones found in the diet include lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene. These carotenoids contribute to the coloration of foods and offer potential health benefits when incorporated into the diet. In human fluids and tissues, the most frequently found carotenoids are lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, lycopene, phytoene, and phytofluene [59]. They are present at levels ranging from 0 to 2 mol/L in plasma and 0 to 1 nmol/g in tissues [60,61]. Provitamin A carotenoids, such as α-carotene and β-carotene, are critical in combating vitamin A deficiency. This global health issuehas various manifestations, including dry eye, impaired growth, compromised immunity, and increased child mortality rates [62,63].
Furthermore, accumulating evidence over the past three decades suggests that carotenoids play a role in reducing the risk of developing non-communicable diseases. These diseases encompass various types of cancers, cardiovascular diseases, bone disorders, skin conditions, neurological disorders, metabolic disorders, and eye disorders. Carotenoids have demonstrated the potential to promote overall health and prevent these conditions’ onset [64,65,66].
Phytoene and phytofluene are unique among carotenoids due to their colorless nature. However, they hold great significance from a biosynthetic standpoint as they serve as precursors for the synthesis of other carotenoids. As a result, extensive research has been conducted to understand their biosynthesis. Within the isoprenoid pathway, the condensation of two C20 molecules represents the pivotal step in carotenoid biosynthesis, leading to the formation of (15Z)-phytoene. This geometric isomer is the predominant form of phytoene found in most organisms capable of synthesizing carotenoids [67]. Phytoene and phytofluene have received relatively less attention in the fields of food science and technology, nutrition, health, and cosmetics compared to other frequent carotenoids present in human fluids and tissues, such as lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene. This disparity is attributed mainly to a significant lack of analytical data available for these colorless carotenoids compared to their more pigmented counterparts. Their lack of color poses challenges in their detection and quantification, contributing to the relative neglect of phytoene and phytofluene in research and applications within these fields [68]. Phytoene and phytofluene have the potential to contribute to the health benefits typically associated with lycopene. These carotenoids may play a role in various health-promoting biological activities, individually or in combination with other compounds. This includes their potential involvement in protection against oxidative stress [69], inflammation [70], and even exhibiting anticarcinogenic properties [71].
5. Carotenoids in the Skin
The distribution of carotenoids within the skin is not consistent across all layers and anatomical regions. Typically, the highest concentrations of carotenoids are observed in the stratum corneum, particularly in close proximity to the skin surface [72]. Oxyhemoglobin and deoxyhemoglobin play a significant role in the papillary dermis, which is abundant in blood vessels. In terms of melanin, there are two primary types: eumelanin, which is a dark brown-black insoluble polymer and pheomelanin, which is a lighter red-yellow sulfur-containing polymer. Two main pathways have been identified regarding the delivery and distribution of carotenoids within the skin layers. The first is diffusion from adipose tissue, blood, and lymphatic vessels, allowing carotenoids to reach the skin layers. The second pathway involves the secretion of carotenoids through sweat and/or sebaceous glands onto the skin surface, followed by subsequent penetration into the deeper layers of the skin [73]. Based on the observation that a higher concentration of carotenoids is detected in the outer part of the stratum corneum in untreated skin, it has been suggested that carotenoids are delivered to this location through secretions from eccrine sweat glands and/or sebaceous glands, similar to the mechanism observed with vitamin E. This hypothesis implies that carotenoids, like vitamin E, may be excreted or released by these glands and subsequently distributed to the outer layers of the stratum corneum, contributing to the skin’s overall antioxidant and protective properties. However, further research is needed to fully understand the precise mechanisms of carotenoid delivery and distribution in the skin [74]. Studies have shown that when individuals consume carotenoid-rich products, an increase in the dermal levels of carotenoids can be observed within a relatively short period, typically ranging from 1 to 3 days.
On the other hand, the turnover or renewal process of the stratum corneum, which involves the shedding of old skin cells and the generation of new ones, takes a longer duration of approximately 2 to 3 weeks. This observation suggests that the increase in dermal carotenoid levels occurs more rapidly than the turnover of the stratum corneum. Therefore, the carotenoids consumed through diet or supplementation may be transported and accumulated in the skin layers, including the dermis, before they are gradually incorporated into the newly formed stratum corneum during the natural skin renewal process [75].
6. Factors Acting Skin Carotenoid Levels
Individual differences in skin carotenoid levels are significant and can be attributed to various factors influencing carotenoid bioavailability. These include dietary patterns, lifestyle choices, and genetic factors [76]. The levels of antioxidants in the skin, including carotenoids, are influenced by several variables such as diet, specific skin location or type, individual characteristics like gender and age, overall health status, and stress factors. Environmental factors and lifestyle choices can also impact the skin’s antioxidant levels [77]. It is worth noting that dermal carotenoid levels can be increased relatively quickly through dietary means. By consuming carotenoid-rich foods or supplements, individuals can observe an increase in the levels of carotenoids in their skin within a short period [78]. This highlights the dynamic relationship between dietary intake and the deposition of carotenoids in the skin. Carotenoid distribution in various skin areas exhibits noticeable differences. Studies have reported that the highest levels of carotenoids can be detected in specific regions, such as the forehead and palms of the hands.
Conversely, lower concentrations of carotenoids are typically found in areas like the back of the hands, the inside of the arm, or the dorsal area. These variations in carotenoid levels across different skin areas may be influenced by differencesin sebaceous gland activity, blood flow, or local metabolic processes. Additionally, variations in sun exposure, skin thickness, or the presence of hair follicles in different body regions may also contribute to the observed differences in carotenoid distribution [79].
The distribution of carotenoids within the layers of the skin is not uniform, with the higher levels of carotenoids are typically found in proximity to the skin surface, at a depth of approximately 4 to 8 mm. As the depth increases, carotenoid levels gradually decrease, extending at least up to a depth of 30 mm [80]. Controlled exposure to sunlight has been shown to impact circulating and skin carotenoid levels in human volunteers. It has been observed that exposure to sunlight leads to noticeable decreases in carotenoid levels. Additionally, exposure to specific types of light, such as visible blue-violet and infra-red light, has also resulted in decreased skin carotenoid levels. This decrease is likely attributed to the production of reactive oxygen species (ROS) induced by these types of light [81,82].
7. Mechanisms of Skin Protection by Carotenoids
There is extensive evidence supporting the role of carotenoids in reducing the risk of various diseases, including cancer, cardiovascular diseases, and metabolic disorders [83]. Especially provitamin A carotenoids offer benefits to the skin through their conversion into retinoic acid, which plays vital roles in skin health and function, similar to other retinoids. These compounds are believed to be involved in processes such as keratinocyte proliferation, epidermal differentiation and keratinization, inflammation and oxidative stress reduction, and even enhancing the penetration of topically administered agents, among other functions [84]. Dietary interventions involving carotenoid-rich products have been reported to lead to perceived changes in skin color. Among the carotenoids, astaxanthin has shown potential benefits for the skin, such as protection against erythema (skin redness) and reduced wrinkling. Astaxanthin is a potent antioxidant with anti-inflammatory properties that may contribute to these skin-related benefits [85].
Although not one of the major dietary carotenoids, canthaxanthin has been approved as a food additive in many countries. It has shown usefulness in treating erythropoietic protoporphyria, a genetic disorder characterized by sun sensitivity. However, prolongedand excessive use of canthaxanthin has raised concerns due to its potential accumulation in the retina. As a result, caution is advised regarding its general use as a dietary supplement or additive [86]. The presence of dietary antioxidants and other beneficial diet-sourced compounds is considered a natural strategy to enhance the skin’s baseline defenses against photodamage and other aggressors that can impact its health and appearance. β-carotene, for example, has been shown to offer protection against photodamage caused by visible and infrared radiation. It may also act as an effective antioxidant in sunscreens, helping mitigate UV radiation’s damaging effects on the skin [87,88].
8. Inhibition of Lipid Peroxidation
Carotenoids significantly combat oxidative stress by quenching singlet oxygen and scavenging free radicals, which helps protect cells and tissues from damage. They exhibit these antioxidant properties not only in solution but also in various biological systems such as membranes and cells. However, it is important to note that carotenoids can interact with other compounds, including both antioxidants and non-antioxidants. Under certain conditions, such as high concentrations, specific oxygen tensions, exposure to radiation, or interactions with particular molecules, carotenoids may act as pro-oxidants [89]. This means that they can promote oxidative reactions instead of inhibiting them. The prooxidant effects of carotenoids can have different outcomes, depending on the context.
In some cases, their prooxidant behavior could be harmful, leading to increased oxidative damage and potential health risks. On the other hand, evidence suggests that the prooxidant effects of carotenoids can be beneficial under certain conditions. The complex behavior of carotenoids as both antioxidants and pro-oxidants highlights the importance of understanding their interactions in specific contexts [90].
9. Visible Light-Absorbing Colored Carotenoids-Photoprotection
Beta-carotene, a widely distributed carotenoid found in various foods, including carrots, palm oil, mango, sweet potato, apricot, and green vegetables, has long been recognized for its beneficial effects in the treatment of erythropoietic protoporphyria. This rare inherited hematological disorder is characterized by elevated levels of protoporphyrins in the plasma, red blood cells, skin, and feces [91]. Protoporphyrin serves as an endogenous photosensitizer, meaning that it becomes excited when exposed to ultraviolet radiation (UVR) and can transfer this energy to molecular oxygen in its ground state. This process generates singlet oxygen, a reactive oxygen species (ROS) that can interact with various molecules such as DNA, proteins, and lipids, resulting in cellular damage and clinical symptoms associated with photosensitivity. Patients with erythropoietic protoporphyria typically experience itching, burning, and pain in sun-exposed skin within minutes of exposure to sunlight. These symptoms are followed by edema, erythema (redness), and purpura (purple discoloration). Elevated levels of protoporphyrins and the subsequent production of singlet oxygen contribute to the onset of these symptoms [92].
Lutein is a dietary carotenoid widely distributed and can be found in various green vegetables, a diverse range of fruits, and egg yolk. It is known for its yellow pigment and is commonly consumed through a balanced diet. Lutein plays a significant role in eye health, particularly in the macula, where it acts as a filter for blue light and provides antioxidant protection. On the other hand, lycopene is an acyclic carotenoid primarily found in certain tomato varieties, watermelons, guava, papaya, apricots, and grapefruits. It is responsible for the red color in these foods and is a potent antioxidant. Lycopene has gained attention for its potential health benefits, particularly concerning cardiovascular health and prostate cancer prevention [93,94]. In many in vivo studies, lycopene extracts derived from tomatoes are commonly used. These extracts often include other accompanying compounds such as vitamin E and the colorless carotenoids phytoene and phytofluene. These additional compounds may act synergistically with lycopene, potentially enhancing its biological effects [95].
10. Carotenoids in Dermoaesthetics
Humans have used carotenoids topically for cosmetic purposes long before their scientific discovery. Carotenoid-containing natural substances, such as plant extracts or oils rich in carotenoids, have been applied to the skin for their potential benefits, including skin conditioning, moisturizing, and providing a subtle color or glow. In the 1970s, carotenoids, particularly canthaxanthin, gained popularity as tanning agents in the form of oral supplements [55,96]. These supplements were marketed to promote a tan-like appearance without sun exposure. However, concerns were raised regarding the safety of canthaxanthin supplements when taken at high doses. It was observed that continuous intake of high doses, typically exceeding 30 mg/day, could lead to the formation of canthaxanthin crystals in the eyes [97]. These crystals could cause adverse effects on vision. However, it’s important to note that these crystals were reversible and could disappear when the canthaxanthin intake was discontinued [98].
Regulatory authorities such as the European Food Safety Authority (EFSA) have established guidelines and recommended lower doses for canthaxanthin intake to address safety concerns. The EFSA’s Panel on Food Additives and Nutrient Sources Added to Food (ANS) has set an acceptable daily intake (ADI) for canthaxanthin at 0.03 mg/kg body weight per day. This recommendation aligns with earlier recommendations made by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the Scientific Committee on Food (SCF) [99,100].
11. Conclusions
Conclusively, a diet rich in carotenoid-containing products and the avoidance of stress factors can positively impact skin health, overall well-being, and the prevention of various diseases. Carotenoids are crucial in promoting healthy dietary patterns and reducing the risk of serious illnesses, including cancer, cardiovascular disease, eye disorders, osteoporosis, and metabolic diseases. While colored carotenoids have received more attention in research and applications, the colorless carotenes phytoene and phytofluene have been overlooked due to their lack of color, making their detection more challenging in the past. However, emerging evidence suggests that phytoene and phytofluene may be involved in the health benefits traditionally attributed to colored carotenoids like lycopene, as they often occur together in foods. These unique carotenoids have distinct characteristics, including their ability to absorb UV radiation. Future studies should focus on generating more data about phytoene and phytofluene levels in the skin to accurately assess skin carotenoid status. Currently, the emphasis has been mainly on colored carotenoids. Furthermore, mechanistic studies are needed to understand the specific benefits of colorless carotenoids in the skin, such as their potential for skin whitening and improving aging signs. This expanding area of research holds promise for the development of novel applications in the fields of health and cosmetics.
Author Contributions
Conceptualization, F.B. and E.R.; methodology P.K.; software E.R.; validation, P.K. and F.B.; formal analysis, F.B. and P.K.; investigation, N.T. and E.S.; resources, V.K.; data curation E.A. and F.B.; writing—original draft preparation, F.B.; writing—review and editing, E.R. and V.K.; visualization, E.S.; supervision, E.R. and V.K.; and project administration; P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2017, 45, 347–351. [Google Scholar]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for skin care: A comprehensive review of human clinical studies. Nutrients 2018, 10, 403. [Google Scholar] [PubMed]
- Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar]
- Igor, V.E.; Werner, G. Optical detection methods for carotenoids in human skin. Arch. Biochem. Biophys. 2015, 572, 101–111. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef]
- Biskanaki, F. Relationship between Cosmetic and Aesthetic Non-Invasive Machines with Health and the Environment. In Proceedings of the 10th Panhellenic Conference Life Sciences in the 21st Century, Athens, Greece, 18–20 November 2016; Panhellenic Association of Bioscientists: Athens, Greece, 2016; p. 65. [Google Scholar]
- Lai-Cheong, E.J.; McGrath, A.J. Structure and function of skin, hair and nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Han, S.S.; Park, I.; Chang, S.E.; Lim, W.; Kim, M.S.; Park, G.H.; Chae, J.B.; Huh, C.H.; Na, J.I. Augmented Intelligence Dermatology: Deep Neural Networks Empower Medical Professionals in Diagnosing Skin Cancer and Predicting Treatment Options for 134 Skin Disorders. J. Investig. Dermatol. 2020, 140, 1753–1761. [Google Scholar] [CrossRef]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M.; Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) Project Team. Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J. Investig. Dermatol. 2020, 133, 377–385. [Google Scholar] [CrossRef]
- Rencz, F.; Gulácsi, L.; Péntek, M.; Baji, P.; Brodszky, V.; Gyula, R.; Szegedi, A. Work productivity loss and cost-effectiveness of etanercept in moderate to severe psoriasis in Hungary. Acta Dermato-Venereol. 2018, 98, 560–566. [Google Scholar]
- Sohn, A.; Qureshi, A.A. The economic burden of skin disease in the United States. J. Am. Acad. Dermatol. 2003, 48, 592–599. [Google Scholar] [CrossRef]
- deGruijl, F.R.; Rebel, H.G. Photocarcinogenesis: Mechanisms, models, and human relevance. Photochem. Photobiol. Sci. 2021, 20, 39–59. [Google Scholar]
- Wang, S.Q.; Dusza, S.W.; Scope, A.; Braun, R.P.; Marghoob, A.A. The biology of sunburn. J. Am. Acad. Dermatol. 2018, 84, 928–946. [Google Scholar]
- Balwani, M.; Desnick, R.J.; Anderson, K.E.; Bloomer, J.; Elias, S.; Gochuico, B.R.; Bissell, D.M. Disease burden and patient-reported outcomes in patients with Erythropoietic Protoporphyria (EPP). Orphanet J. Rare Dis. 2020, 15, 1–13. [Google Scholar]
- Wahlin, S.; Asplund, J.; Von Euler, M.; Sandberg, S.; Harper, P.; Andersson, C. Clinical characterization of erythropoietic protoporphyria in Sweden: A population-based study. J. Am. Acad. Dermatol. 2020, 82, 1197–1204. [Google Scholar]
- Warren, M.J.; Elder, G.H. Porphyria and its impact on the skin. Exp. Dermatol. 2020, 29, 644–653. [Google Scholar]
- Lenglet, H.; Sommer, J.R.; Urech, A.; Gouya, L.; Marzin, K.; Minder, E.I.; Frank, J. Skin manifestations and associated morbidity in inherited porphyrias: Experience from the Swiss Porphyria Centre. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 416–425. [Google Scholar]
- Lim, H.W.; Collins, S.A.; Resnik, S. Photosensitivity reactions and disorders. In Fitzpatrick’s Dermatology, 9th ed.; Mc Graw-Hill Education: USA, 2020; pp. 349–357. [Google Scholar]
- Young, A.R. Acute and chronic effects of ultraviolet radiation: Molecular mechanisms and cellular responses. Physiol. Rev. 2018, 98, 767–815. [Google Scholar]
- Zhang, L.; Lu, S.; Wei, C.; Quan, T. UVB-induced skin pigmentation: An update. Int. J. Mol. Sci. 2020, 21, 5116. [Google Scholar]
- Fisher, D.E.; James, W.D. The effects of ultraviolet radiation on the skin. In Fitzpatrick’s Dermatology, 9th ed.; Mc Graw-Hill Education: USA, 2020; pp. 346–348. [Google Scholar]
- Heckman, C.J.; Darlow, S.; Cohen-Filipic, J.; Kloss, J.D.; Manne, S.L.; Munshi, T.; Perlis, C.S. Psychosocial Correlates of Sunburn among Young Adult Women. Int. J. Environ. Res. Public Health 2012, 9, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Shipman, W.D.; Chyou, S.; Ramanathan, A.; Izmirly, P.M.; Sharma, S.; Pannellini, T.; Dasoveanu, D.C.; Qing, X.; Magro, C.M.; Granstein, R.D.; et al. A protective Langerhans cell–keratinocyte axis that is dysfunctional in photosensitivity. Sci. Transl. Med. 2018, 10, eaap9527. [Google Scholar] [CrossRef]
- Hassan, S.; Purdie, K.J.; Wang, J.; Harwood, C.A.; Proby, C.M.; Pourreyron, C.; Mladkova, N.; Nagano, A.; Dhayade, S.; Athineos, D.; et al. A Unique Panel of Patient-Derived Cutaneous Squamous Cell Carcinoma Cell Lines Provides a Preclinical Pathway for Therapeutic Testing. Int. J. Mol. Sci. 2019, 20, 3428. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Fukunaga, A.; Washio, K.; Taguchi, K.; Oda, Y.; Ogura, K.; Nishigori, C. Anti-Inflammatory Role of Langerhans Cells and Apoptotic Keratinocytes in Ultraviolet-B–Induced Cutaneous Inflammation. J. Immunol. 2017, 199, 2937–2947. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nishioka, K. Action spectrum and minimal erythema dose (MED) for ultraviolet B radiation-induced erythema in human skin: A review. J. Dermatol. Sci. 2021, 102, 77–81. [Google Scholar]
- Lehmann, M.; Pfahlberg, A.B.; Sandmann, H.; Uter, W.; Gefeller, O. Public Health Messages Associated with Low UV Index Values Need Reconsideration. Int. J. Environ. Res. Public Health 2019, 16, 2067. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Grandi, C.; D’Ovidio, M.C. Balance between Health Risks and Benefits for Outdoor Workers Exposed to Solar Radiation: An Overview on the Role of Near Infrared Radiation Alone and in Combination with Other Solar Spectral Bands. Int. J. Environ. Res. Public Health 2020, 17, 1357. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef]
- Tewari, A.; Sarkany, R. Ultraviolet radiation and cutaneous infection. Br. J. Dermatol. 2018, 178, 449–460. [Google Scholar]
- Tran, A.Q.; Hoeffler, J.P.; Chen, J.G. Artificial light at night and cancer: Global study investigates connection. Environ. Health Perspect. 2018, 126, 047002. [Google Scholar]
- Biskanaki, F.; Rallis, E.; Skouras, G.; Stofas, A.; Thymara, E.; Kavantzas, N.; Lazaris, A.C.; Kefala, V. Impact of Solar Ultraviolet Radiation in the Expression of Type I Collagen in the Dermis. Cosmetics 2021, 8, 46. [Google Scholar] [CrossRef]
- Dizdaroglu, M. Oxidatively induced DNA damage: Mechanisms, repair and disease. Cancer Lett. 2012, 327, 26–47. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N. Topical cosmeceuticals for photoaging and photodamaged skin. Dermatol. Clin. 2020, 38, 167–179. [Google Scholar]
- Lan, C.-C.E.; Hung, Y.-T.; Fang, A.-H.; Ching-Shuang, W. Effects of irradiance on UVA-induced skin aging. J. Dermatol. Sci. 2019, 94, 220–228. [Google Scholar] [CrossRef]
- Biskanaki, F.; Kefala, V.; Lazaris, A.C.; Rallis, E. Aging and the Impact of Solar Ultraviolet Radiation on the Expression of Type I and Type VI Collagen. Cosmetics 2023, 10, 48. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Molecular Mechanisms of Changes in Homeostasis of the Dermal Extracellular Matrix: Both Involutional and Mediated by Ultraviolet Radiation. Int. J. Mol. Sci. 2022, 23, 6655. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef] [PubMed]
- Biskanaki, F.; Rallis, E.; Skouras, G.; Kapranou, A.; Papadopoulos, V.; Protopapa, E.; Revelos, K.; Diamantopoulou, K.; Kefala, V.; Lazaris, A.C. Non-Melanoma Skin Cancer (NMSC) and Collagen Type VI: Histological study of the Damaging Effect of Solar Radiation on the Dermis. J. Community Med. Public Health Rep. 2021, 4. [Google Scholar] [CrossRef]
- Darvin, M.E.; Lademann, J.; von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutri-cosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plantcarotenoids: Recent advances and future perspectives. MolHorticulture 2022, 2, 3. [Google Scholar] [CrossRef]
- Manfred, E.; Adrian, W. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bi-oactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Fan, D.; Banskota, A.H.; Stefanova, R.; Khan, W.; Hafting, J.; Craigie, J.; Critchley, A.T.; Prithiviraj, B. Bioactive components of the edible strain of red alga, Chondruscrispus, enhance oxidative stress tolerance in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1180–1190. [Google Scholar] [CrossRef]
- Darvin, M.E.; Jung, S.; Schanzer, S.; Richter, H.; Kurth, E.; Thiede, G.; Meinke, M.C.; Lademann, J. Influence of the Systemic Application of Blue–Green Spirulina platensis Algae on the Cutaneous Carotenoids and Elastic Fibers in Vivo. Cosmetics 2015, 2, 302–312. [Google Scholar] [CrossRef]
- Rasmussen, H.M.; Muzhingi, T.; Eggert, E.M.R.; Johnson, E. Lutein, zeaxanthin, meso-zeaxanthin content in egg yolk and their absence in fish and seafood. J. Food Compos. Anal. 2012, 27, 139–144. [Google Scholar] [CrossRef]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam.Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016, 52, 1–15. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Rollo, M.; Williams, R.; Wood, L.; Garg, M.; Jensen, M.; Collins, C. A Systematic Review of Technology-Based Dietary Intake Assessment Validation Studies That Include Carotenoid Biomarkers. Nutrients 2017, 9, 140. [Google Scholar] [CrossRef]
- Pezdirc, K.; Hutchesson, M.J.; Williams, R.L.; Rollo, M.E.; Burrows, T.L.; Wood, L.G.; Oldmeadow, C.; Collins, C.E. Consuming High-Carotenoid Fruit and Vegetables Influences Skin Yellowness and Plasma Carotenoids in Young Women: A Single-Blind Randomized Crossover Trial. J. Acad. Nutr. Diet. 2016, 116, 1257–1265. [Google Scholar] [CrossRef]
- Van Hoang, D.; Pham, N.; Lee, A.; Tran, D.; Binns, C. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef]
- Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Mares, J. Lutein and Zeaxanthin Isomers in Eye Health and Disease. Annu. Rev. Nutr. 2016, 36, 571–602. [Google Scholar] [CrossRef]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.-F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic aspects of carotenoid health benefits—Where are we now? Nutr. Res. Rev. 2021, 34, 276. [Google Scholar] [CrossRef] [PubMed]
- Paula, M.-B.; Marielle, M.; Charles, D.; Charlotte, H.; Marion, N.; Patrick, B.; Antonio, J.M.-M.; Emmanuelle, R. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene. Food Chem. 2019, 300, 125232. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients reside in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and the skin: Physiology and pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Hakkur, M.; ElHachlafi, N.; Charfi, S.; Balahbib, A.; Guaouguaou, F.-E.; Rebezov, M.; Maksimiuk, N.; Shariati, M.A.; et al. Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci. Technol. 2021, 118, 519–538. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Loh, W.W.; Sutanto, C.N.; Yao, Y.; Kim, J.E. Skin Carotenoid Status and Plasma Carotenoids: Biomarkers of Dietary Carotenoids, Fruits and Vegetables for Middle-Aged and Older Singaporean Adults. Br. J. Nutr. 2021, 126, 1398. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. Carrot Man: A Case of Excessive Beta-Carotene Ingestion. Int. J. Eat. Disord. 2012, 45, 816–818. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Martini, D.; Negrini, L.; Marino, M.; Riso, P.; Del Bo, C.; Porrini, M. What Is the Current Direction of the Research on Carotenoids and Human Health? An Overview of Registered Clinical Trials. Nutrients 2022, 14, 1191. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 2015, 15, 15–19. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Zerres, S.; Stahl, W. Carotenoids in human skin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158588. [Google Scholar] [CrossRef]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Nam, G.-W. Sunscreen Boosting Effect by Solid Lipid Nanoparticles-Loaded Fucoxanthin Formulation. Cosmetics 2020, 7, 14. [Google Scholar] [CrossRef]
- Terao, J.; Minami, Y.; Bando, N. Singlet molecular oxygen quenching activity of carotenoids: Relevance to protection of the skin from photoaging. J. Clin. Biochem. Nutr. 2011, 48, 57–62. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, V.A.; Tyutereva, E.V.; Voitsekhovskaja, O.V. Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles. Int. J. Mol. Sci. 2020, 21, 3237. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. Carotenoides: Estructura, propiedades y funciones. In Carotenoidesenagroalimentación y Salud; Meléndez-Martínez, A., Ed.; Editorial Terracota: Ciudad de Mexico, Mexico, 2017. [Google Scholar]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Kurzawa, M.; Wilczyńska, E.; Brudzyńska, P.; Sionkowska, A. Total Phenolic Content, Antioxidant Capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), Carrot (Daucuscarota), Tomato (Solanum lycopersicum), and Hop (Humuluslupulus) Extracts. Cosmetics 2022, 9, 134. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Farrona, S.; Ventura, M.R.; Abranches, R. Canthaxanthin, a Red-Hot Carotenoid: Applications, Synthesis, and Biosynthetic Evolution. Plants 2020, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.; Barredo, J.L. Carotenoids Production: A Healthy and Profitable Industry. In Microbial Carotenoids; Barreiro, C., Barredo, J.L., Eds.; Humana Press: New York, NY, USA, 2018; pp. 7–22. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the re-evaluation of canthaxanthin (E 161 g) as a food additive. EFSA J. 2010, 8, 1852–1893. [Google Scholar] [CrossRef]
- Damaziak, K.; Marzec, A.; Riedel, J.; Szeliga, J.; Koczywąs, E.; Cisneros, F.; Michalczuk, M.; Łukasiewicz, M.; Gozdowski, D.; Siennicka, A.; et al. Effect of dietary canthaxanthin and iodine on the production performance and egg quality of laying hens. Poult. Sci. 2018, 97, 4008–4019. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).