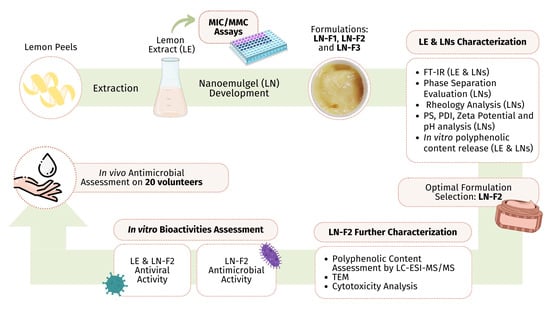
Formulation and Characterization of Non-Toxic, Antimicrobial, and Alcohol-Free Hand Sanitizer Nanoemulgel Based on Lemon Peel Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Materials and Sample Preparation
2.3. Microbial Strains’ Growth Conditions
2.4. Cell Culture Growth Conditions
2.5. Lemon Peel Extraction Methodology
2.6. Antimicrobial Activity of the Lemon Peel Extract (LE)
2.7. Preparation of Lemon-Extract-Based Nanoemulgel (LN) Formulations
2.8. Fourier Transform Infrared (FTIR) Spectroscopy Characterization of LE and LN Formulations
2.9. Organoleptic Properties and Phase Separation Evaluation of LN Formulations
2.10. Rheology Analysis of LN Formulations
2.11. Particle Size, Polydispersity Index, Zeta Potential, and pH Analysis of LN Formulations
2.12. In Vitro Polyphenolic Content Release of LE and LN Formulations
2.13. Identification and Quantification of LN-F2 Phenolic Content by LC-ESI-MS/MS
2.14. LN-F2 Transmission Electron Microscopy (TEM) Characterization
2.15. LE and LN-F2 Cytotoxicity Evaluation by Inverted Light Microscopy Analysis
2.16. LE and LN-F2 Bioactivity Assessment
2.16.1. In Vitro Antiviral Activity
Viruses’ Propagation Conditions
Viruses’ Quantification
2.16.2. In Vitro and In Vivo LN-F2 Antimicrobial Activity
Zone of Inhibition (ZI) Analysis by Well and Disk Diffusion Assays
In Vivo Assessment
2.17. Statistical Analysis
3. Results and Discussion
3.1. Lemon Peel Extract Antimicrobial Activity
3.2. Development of LE-Based Nanoemulgel Formulations
3.3. LE and LN Formulations’ Physicochemical Analysis
3.3.1. LE and LN Formulations’ FTIR Analysis
3.3.2. LN Formulations’ Organoleptic Properties, Phase Separation, and Rheology Analysis
3.3.3. LN Formulations’ Particle Size, Polydispersity Index, Zeta Potential, and pH Assessment
3.4. In Vitro Release of the LE and LN Formulations’ Polyphenolic Content
3.5. LN Optimal Formulation Selection and Characterization
3.5.1. Phenolic Content Analysis
3.5.2. TEM Characterization
3.5.3. Cytotoxicity Assessment on MA-104 Cells
3.6. Bioactivity Analyses
3.6.1. LE and LN-F2 Antiviral Activity
3.6.2. LN-F2 In Vitro Antimicrobial Activity
3.6.3. LN-F2 In Vivo Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golin, A.P.; Choi, D.; Ghahary, A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control 2020, 48, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Khadka, P.; Das, S.C. Alcohol-based hand sanitizer—composition, proper use and precautions. Germs 2021, 11, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Hamad Vuai, S.A.; Sahini, M.G.; Sule, K.S.; Ripanda, A.S.; Mwanga, H.M. A comparative in-vitro study on antimicrobial efficacy of on-market alcohol-based hand washing sanitizers towards combating microbes and its application in combating COVID-19 global outbreak. Heliyon 2022, 8, e11689. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.M.; Mohammed, R.S.; Abdelsalam, E.; Ashour, W.E.; Magalhães, D.; Pintado, M.; El Habbasha, E.S. Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value. Horticulturae 2024, 10, 180. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Magalhães, D.; Campos, D.A.; Porretta, S.; Dellapina, G.; Poli, G.; Istanbullu, Y.; Demir, S.; San Martín, Á.M.; García-Gómez, P.; et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods 2022, 11, 3859. [Google Scholar] [CrossRef] [PubMed]
- Vilas Boas, A.; Gómez-García, R.; Campos, D.; Correia, M.; Pintado, M. Integrated Biorefinery Strategy for Orange Juice By-products Valorization: A Sustainable Protocol to Obtain Bioactive Compounds. In Food Waste Conversion; Methods and Protocols in Food Science; Humana: New York, NY, USA, 2023; pp. 113–124. [Google Scholar]
- Shri Balakrishna, A.; Saradindu, G.; Giriraj, Y.; Kavita, S.; Sirsendu, G.; Sushil, J. Formulation, Evaluation and Antibacterial Efficiency of water-based herbal Hand Sanitizer Gel. bioRxiv 2018, 373928. [Google Scholar] [CrossRef]
- Rodrigues, C.V.; Pintado, M. Hesperidin from Orange Peel as a Promising Skincare Bioactive: An Overview. Int. J. Mol. Sci. 2024, 25, 1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Benohoud, M.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. Green extraction of polyphenols from citrus peel by-products and their antifungal activity against Aspergillus flavus. Food Chem. X 2021, 12, 100144. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Bashir, M.; Ahmad, J.; Asif, M.; Khan, S.U.; Irfan, M.; Ibrahim, A.Y.; Asghar, S.; Khan, I.U.; Iqbal, M.S.; Haseeb, A.; et al. Nanoemulgel, an Innovative Carrier for Diflunisal Topical Delivery with Profound Anti-Inflammatory Effect: In vitro and in vivo Evaluation. Int. J. Nanomed. 2021, 16, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Asfour, M.H.; Mohsen, A.M. Formulation and evaluation of pH-sensitive rutin nanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2018, 9, 17–26. [Google Scholar] [CrossRef]
- Elhabak, M.; Ibrahim, S.; Abouelatta, S.M. Topical delivery of l-ascorbic acid spanlastics for stability enhancement and treatment of UVB induced damaged skin. Drug Deliv. 2021, 28, 445–453. [Google Scholar] [CrossRef]
- Chellapa, P.; Eid, A.M.; Elmarzugi, N. Preparation and characterization of virgin coconut oil nanoemulgel. J. Chem. Pharm. Res. 2015, 7, 787–793. [Google Scholar]
- Srivastava, M.; Kohli, K.; Ali, M. Formulation development of novel in situ nanoemulgel (NEG) of ketoprofen for the treatment of periodontitis. Drug Deliv. 2016, 23, 154–166. [Google Scholar] [CrossRef]
- Sohail, M.; Naveed, A.; Abdul, R.; Khan, H.M.S.; Khan, H. An approach to enhanced stability: Formulation and characterization of Solanum lycopersicum derived lycopene based topical emulgel. Saudi Pharm. J. 2018, 26, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.M.; Abd El-Alim, S.H.; Asfour, M.H.; Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.-S.; Awad, G. Transdermal fennel essential oil nanoemulsions with promising hepatic dysfunction healing effect: In vitro and in vivo study. Pharm. Dev. Technol. 2019, 24, 729–738. [Google Scholar] [CrossRef]
- Abd El-Alim, S.H.; Salama, A.; Darwish, A.B. Provesicular elastic carriers of Simvastatin for enhanced wound healing activity: An in-vitro/in-vivo study. Int. J. Pharm. 2020, 585, 119470. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Jin, T.Z.; Chen, W.; He, Q.; Zou, Z.; Zhao, H.; Ye, X.; Guo, M. Preparation and characterization of gellan gum-chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocoll. 2021, 114, 106570. [Google Scholar] [CrossRef]
- Ammar, N.M.; Hassan, H.A.; Mohammed, M.A.; Serag, A.; Abd El-Alim, S.H.; Elmotasem, H.; El Raey, M.; El Gendy, A.N.; Sobeh, M.; Abdel-Hamid, A.-H.Z. Metabolomic profiling to reveal the therapeutic potency of Posidonia oceanica nanoparticles in diabetic rats. RSC Adv. 2021, 11, 8398–8410. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zeng, L.; Wang, C.; Yang, Y.; Zhou, W.; Li, F.; Tan, Z. Assessment of the cytotoxicity of ionic liquids on Spodoptera frugiperda 9 (Sf-9) cell lines via in vitro assays. J. Hazard. Mater. 2018, 348, 1–9. [Google Scholar] [CrossRef] [PubMed]
- England, P.H. Detection and Enumeration of Bacteria in Swabs and Other Environmental Samples, 4th ed.; Volume National Infection Service, Food, Water and Environmental Microbiology Standard Method FNES4 (E1): London, UK, 2017; p. 22. [Google Scholar]
- Aa, L.; Is, H.; Jh, D.; Jfr, L. Bacterial contamination of the hands of food handlers as indicator of hand washing efficacy in some convenient food industries. Pak. J. Med. Sci. 2014, 30, 755–758. [Google Scholar] [PubMed]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Elwakeel, K.Z.; El-Liethy, M.A.; Ahmed, M.S.; Ezzat, S.M.; Kamel, M.M. Facile synthesis of magnetic disinfectant immobilized with silver ions for water pathogenic microorganism’s deactivation. Environ. Sci. Pollut. Res. Int. 2018, 25, 22797–22809. [Google Scholar] [CrossRef] [PubMed]
- Magréault, S.; Jauréguy, F.; Carbonnelle, E.; Zahar, J.R. When and How to Use MIC in Clinical Practice? Antibiotics 2022, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Otang, W.M.; Afolayan, A.J. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape, South Africa. S. Afr. J. Bot. 2016, 102, 46–49. [Google Scholar] [CrossRef]
- Henderson, A.H.; Fachrial, E.; Lister, I.N.E. Antimicrobial Activity of Lemon (Citrus limon) Peel Extract Against Escherichia coli. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 39, 268–273. [Google Scholar]
- John, S.; Monica, S.; Priyadarshini, S.; Sivaraj, C.; Arumugam, P. Antioxidant and antimicrobial activity of lemon peel. Int. J. Pharm. Sci. Rev. Res. 2017, 46, 115–118. [Google Scholar]
- Saleem, M.; Durani, A.I.; Asari, A.; Ahmed, M.; Ahmad, M.; Yousaf, N.; Muddassar, M. Investigation of antioxidant and antibacterial effects of citrus fruits peels extracts using different extracting agents: Phytochemical analysis with in silico studies. Heliyon 2023, 9, e15433. [Google Scholar] [CrossRef]
- Shanmugam, R.; Lakki Reddy Venkata, B.R.; Geetha, R.V. Broad spectrum antibacterial silver nanoparticle green synthesis: Characterization, and mechanism of action. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–444. [Google Scholar]
- Karaman, R.; Jubeh, B.; Breijyeh, Z. Resistance of Gram-Positive Bacteria to Current Antibacterial Agents and Overcoming Approaches. Molecules 2020, 25, 2888. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Khalil, M.I.; Al-Zaban, M.I. Novel Biogenic Silver Nanoparticle-Induced Reactive Oxygen Species Inhibit the Biofilm Formation and Virulence Activities of Methicillin-Resistant Staphylococcus aureus (MRSA) Strain. Front. Bioeng. Biotechnol. 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhao, M.; McClements, D. Improving the stability of wheat protein-stabilized emulsions: Effect of pectin and xanthan gum addition. Food Hydrocoll. 2015, 43, 377–387. [Google Scholar] [CrossRef]
- Mulia, K.; Ramadhan, R.M.A.; Krisanti, E.A.J.M.W.C. Formulation and characterization of nanoemulgel mangosteen extract in virgin coconut oil for topical formulation. MATEC Web. Conf. 2018, 156, 01013. [Google Scholar] [CrossRef][Green Version]
- Matman, N.; Min Oo, Y.; Amnuaikit, T.; Somnuk, K. Continuous production of nanoemulsion for skincare product using a 3D-printed rotor-stator hydrodynamic cavitation reactor. Ultrason. Sonochemistry 2022, 83, 105926. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M. Preparation and characterization of phospholipid complexes of naringenin for effective drug delivery. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 253–260. [Google Scholar] [CrossRef]
- Thombare, N.; Mahto, A.; Singh, D.; Roy Chowdhury, A.; Ansari, M. Comparative FTIR Characterization of Various Natural Gums: A Criterion for Their Identification. J. Polym. Environ. 2023, 31, 3372–3380. [Google Scholar] [CrossRef]
- Rehman, M.; Rasul, A.; Khan, M.; Hanif, M.; Naaem Aamir, M.; Khan, H.m.; Hameed, M.; Akram, M. Development of niosomal formulations loaded with cyclosporine A and evaluation of its compatibility. Trop. J. Pharm. Res. 2018, 17, 1457–1464. [Google Scholar] [CrossRef]
- Kondiah, P.P.D.; Rants’o, T.A.; Mdanda, S.; Mohlomi, L.M.; Choonara, Y.E. A Poly (Caprolactone)-Cellulose Nanocomposite Hydrogel for Transdermal Delivery of Hydrocortisone in Treating Psoriasis Vulgaris. Polymers 2022, 14, 2633. [Google Scholar] [CrossRef]
- Kanaze, F.; Kokkalou, E.; Niopas, I.; Georgarakis, M.; Stergiou, A.; Bikiaris, D. Thermalanalysis study of flavonoid solid dispersions having enhanced solubility. J. Therm. Anal. Calorim. 2006, 83, 283–290. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent insights into Nanoemulsions: Their preparation, properties and applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef] [PubMed]
- Verdú-Soriano, J.; Casado-Díaz, A.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Moreno-Moreno, P.; Dorado, G.; Quesada-Gómez, J.M.; Rodríguez-Mañas, L.; Lázaro-Martínez, J.L. Hard-to-Heal Wound Healing: Superiority of Hydrogel EHO-85 (Containing Olea europaea Leaf Extract) vs. a Standard Hydrogel. A Randomized Controlled Trial. Gels 2023, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Issa, L.; Al-kharouf, O.; Jaber, R.; Hreash, F. Development of Coriandrum sativum Oil Nanoemulgel and Evaluation of Its Antimicrobial and Anticancer Activity. BioMed Res. Int. 2021, 2021, 5247816. [Google Scholar] [CrossRef] [PubMed]
- Chookiat, S.; Theansungnoen, T.; Kiattisin, K.; Intharuksa, A. Nanoemulsions Containing Mucuna pruriens (L.) DC. Seed Extract for Cosmetic Applications. Cosmetics 2024, 11, 29. [Google Scholar] [CrossRef]
- Jusril, N.A.; Abu Bakar, S.I.; Khalil, K.A.; Md Saad, W.M.; Wen, N.K.; Adenan, M.I. Development and Optimization of Nanoemulsion from Ethanolic Extract of Centella asiatica (NanoSECA) Using D-Optimal Mixture Design to Improve Blood-Brain Barrier Permeability. Evid.-Based Complement. Altern. Med. 2022, 2022, 3483511. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Jiang, Y.; Liu, X. Chapter 12—Nanoemulsions-Based Drug Delivery for Brain Tumors. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Gupta, U., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 327–358. [Google Scholar]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Sungpud, C.; Panpipat, W.; Chaijan, M.; Sae Yoon, A. Techno-biofunctionality of mangostin extract-loaded virgin coconut oil nanoemulsion and nanoemulgel. PLoS ONE 2020, 15, e0227979. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon by-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Gadhave, D.; Tupe, S.; Tagalpallewar, A.; Gorain, B.; Choudhury, H.; Kokare, C. Nose-to-brain delivery of amisulpride-loaded lipid-based poloxamer-gellan gum nanoemulgel: In vitro and in vivo pharmacological studies. Int. J. Pharm. 2021, 607, 121050. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.E.; Shehata, T.M.; Mohamed, M.E.; Younis, N.S.; Elsewedy, H.S. Enhancement of Curcumin Anti-Inflammatory Effect via Formulation into Myrrh Oil-Based Nanoemulgel. Polymers 2021, 13, 577. [Google Scholar] [CrossRef]
- Sultan, M.H.; Javed, S.; Madkhali, O.A.; Alam, M.I.; Almoshari, Y.; Bakkari, M.A.; Sivadasan, D.; Salawi, A.; Jabeen, A.; Ahsan, W. Development and Optimization of Methylcellulose-Based Nanoemulgel Loaded with Nigella sativa Oil for Oral Health Management: Quadratic Model Approach. Molecules 2022, 27, 1796. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; El-Enshasy, H.A.; Aziz, R.; Elmarzugi, N.A. Preparation, characterization and anti-inflammatory activity of Swietenia macrophylla nanoemulgel. J. Nanomed. Nanotechnol. 2014, 5, 1–10. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Diab, K.A. In Vitro Studies on Phytochemical Content, Antioxidant, Anticancer, Immunomodulatory, and Antigenotoxic Activities of Lemon, Grapefruit, and Mandarin Citrus Peels. Asian Pac. J. Cancer Prev. 2016, 17, 3559–3567. [Google Scholar] [PubMed]
- Imran, M.; Basharat, D.; Khalid, S.; Aslam, M.; Syed, F.; Jabeen, S.; Kamran, H.; Muhammad, Z.; Shahid, M.Z.; Tufail, T.; et al. Citrus Peel Polyphenols: Recent Updates and Perspectives. Int. J. Biosci. 2020, 16, 53–70. [Google Scholar]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Saaty, A.H. Grapefruit Seed Extracts’ Antibacterial and Antiviral Activity: Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Impact. Arch. Pharm. Pract. 2022, 13, 69. [Google Scholar] [CrossRef]
- Mejri, H.; Aidi Wannes, W.; Mahjoub, F.H.; Hammami, M.; Dussault, C.; Legault, J.; Saidani-Tounsi, M. Potential bio-functional properties of Citrus aurantium L. leaf: Chemical composition, antiviral activity on herpes simplex virus type-1, antiproliferative effects on human lung and colon cancer cells and oxidative protection. Int. J. Environ. Health Res. 2024, 34, 1113–1123. [Google Scholar] [CrossRef]
- Tang, K.; He, S.; Zhang, X.; Guo, J.; Chen, Q.; Yan, F.; Banadyga, L.; Zhu, W.; Qiu, X.; Guo, Y. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antivir. Res. 2018, 160, 87–93. [Google Scholar] [CrossRef]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Miyake, Y.; Hiramitsu, M. Isolation and extraction of antimicrobial substances against oral bacteria from lemon peel. J. Food Sci. Technol. 2011, 48, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Albak, F.; TekİN, A.R. Development of Functional Chocolate with Spices and Lemon Peel Powder by using Response Surface Method: Development of Functional Chocolate. Akad. Gıda 2014, 12, 19–25. [Google Scholar]
- Çilingir, S.; Goksu, A.; Sabanci, S. Production of Pectin from Lemon Peel Powder Using Ohmic Heating-Assisted Extraction Process. Food Bioprocess Technol. 2021, 14, 1349–1360. [Google Scholar] [CrossRef]
- Šafranko, S.; Šubarić, D.; Jerković, I.; Jokić, S. Citrus By-Products as a Valuable Source of Biologically Active Compounds with Promising Pharmaceutical, Biological and Biomedical Potential. Pharmaceuticals 2023, 16, 1081. [Google Scholar] [CrossRef]
- Yabalak, E.; Erdoğan Eliuz, E.A.; Nazlı, M.D. Evaluation of Citrus reticulata essential oil: Chemical composition and antibacterial effectiveness incorporated gelatin on E. coli and S. aureus. Int. J. Environ. Health Res. 2022, 32, 1261–1270. [Google Scholar] [CrossRef]
- Abirami, S.; Edwin Raj, B.; Soundarya, T.; Kannan, M.; Sugapriya, D.; Al-Dayan, N.; Ahmed Mohammed, A. Exploring antifungal activities of acetone extract of selected Indian medicinal plants against human dermal fungal pathogens. Saudi J. Biol. Sci. 2021, 28, 2180–2187. [Google Scholar] [CrossRef]











| Ingredients (g) | Formulations | |||
|---|---|---|---|---|
| LN-F1 | LN-F2 | LN-F3 | ||
| Phase A | LE | 0.100 | 0.100 | 0.100 |
| Mineral Oil | 2.000 | 2.000 | 2.000 | |
| Span 80 | 2.000 | 2.000 | 2.000 | |
| Cremophor RH 40 | 0.400 | 0.400 | 0.400 | |
| PEG 400 | 0.400 | 0.400 | 0.400 | |
| Phase B | Xanthan Gum | 0.025 | 0.050 | 0.100 |
| Water (dH2O) | 5.075 | 5.050 | 5.000 | |
| Formulation | Particle Size ± SD (nm) | Zeta Potential ± SD (mV) | PDI ± SD | pH ± SD |
|---|---|---|---|---|
| LN-F1 | 123.30 ± 0.18 | −15.90 ± 1.97 | 0.422 ± 0.01 | 5.51 ± 0.24 |
| LN-F2 | 152.80 ± 1.78 | −43.60 ± 2.64 | 0.43 ± 0.06 | 5.59 ± 0.07 |
| LN-F3 | 176.90 ± 4.36 | −56.80 ± 3.77 | 0.46 ± 0.19 | 5.57 ± 0.73 |
| Phenolic Compound | Calibration Curve Equation | R2 | LOD (ng/mL) | LOQ (ng/mL) | Rt (min) | Concentration ± SD (µg/g) |
|---|---|---|---|---|---|---|
| Narirutin | y = −0.002538650 + 0.0256495x | 0.9998 | 10.25 | 20.01 | 10.84 | 195.000 ± 0.670 |
| Naringenin | y = −0.000761480 + 0.0310687x | 0.9996 | 12.31 | 23.57 | 15.02 | 160.320 ± 0.803 |
| Hesperidin | y = 0.000321812 + 0.00008838x | 0.9997 | 15.41 | 30.19 | 11.10 | 74.000 ± 0.410 |
| Chlorogenic acid | y = −0.000162510 + 0.0082195x | 0.9993 | 21.84 | 30.84 | 8.13 | 9.530 ± 0.510 |
| Diosmin | y = −0.040602700 + 0.1132100x | 0.9997 | 15.10 | 30.62 | 11.03 | 4.830 ± 0.030 |
| Coumaric acid | y = −0.000916311 + 0.0643206x | 0.9995 | 15.03 | 30.58 | 9.75 | 2. 540 ± 0.010 |
| Rutin | y = −0.002097290 + 0.0613716x | 0.9998 | 15.06 | 30.37 | 9.91 | 1.490 ± 0.009 |
| Ellagic acid | y = −0.000728175 + 0.0480413x | 0.9996 | 15.14 | 30.89 | 10.12 | 1.200 ± 0.002 |
| Ferulic acid | y = −0.000938050 + 0.0189100x | 0.9987 | 14.98 | 30.97 | 10.38 | 1.170 ± 0.006 |
| Saponarin | y = 0.001245340 + 0.0568465x | 0.9993 | 15.54 | 30.47 | 8.66 | 0.420 ± 0.004 |
| Hesperetin | y = 0.000213181 + 0.0007883x | 0.9996 | 15.41 | 30.12 | 15.56 | 0.210 ± 0.001 |
| Methyl gallate | y = −0.001515260 + 0.0801915x | 0.9998 | 15.78 | 40.21 | 7.65 | 0.120 ± 0.001 |
| Volunteer ID | Age | Gender | Total Bacterial Counts (CFU/cm2) | R% | Total Fungal Counts (CFU/cm2) | R% | ||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | |||||
| 1 | 39 | M | 1200 | 100 | 91.66 | 95 | 3 | 94.96 |
| 2 | 30 | M | 850 | 20 | 97.47 | 20 | 0 | 100.00 |
| 3 | 44 | M | 190 | 10 | 94.73 | 10 | 0 | 100.00 |
| 4 | 43 | M | 130 | 0 | 100.00 | 5 | 0 | 100.00 |
| 5 | 25 | F | 200 | 5 | 97.50 | 0 | 0 | N/A |
| 6 | 35 | M | 1100 | 120 | 89.09 | 100 | 9 | 91.00 |
| 7 | 40 | M | 500 | 30 | 94.00 | 150 | 0 | 100.00 |
| 8 | 41 | M | 90 | 0 | 100.00 | 12 | 0 | 100.00 |
| 9 | 39 | F | 420 | 30 | 92.85 | 190 | 4 | 97.89 |
| 10 | 26 | M | 1000 | 105 | 98.50 | 580 | 60 | 89.65 |
| 11 | 32 | M | 1500 | 250 | 83.33 | 120 | 10 | 91.66 |
| 12 | 38 | F | 750 | 45 | 94.00 | 35 | 0 | 100.00 |
| 13 | 48 | M | 60 | 0 | 100.00 | 0 | 0 | N/A |
| 14 | 67 | M | 250 | 10 | 96.00 | 18 | 0 | 100.00 |
| 15 | 65 | M | 690 | 55 | 92.02 | 90 | 5 | 94.44 |
| 16 | 44 | M | 500 | 40 | 92.00 | 150 | 10 | 93.33 |
| 17 | 30 | M | 990 | 30 | 96.96 | 80 | 3 | 96.25 |
| 18 | 39 | M | 250 | 20 | 92.00 | 30 | 0 | 100.00 |
| 19 | 43 | M | 120 | 0 | 100.00 | 0 | 0 | N/A |
| 20 | 30 | M | 50 | 0 | 100.00 | 20 | 0 | 100.00 |
| Average ± SD | - | - | - | - | 95.11 ± 4.41 | - | - | 97.01 ± 3.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, F.M.; Shalaby, E.S.; El-Liethy, M.A.; Abd-Elmaksoud, S.; Mohammed, R.S.; Shalaby, S.I.; Rodrigues, C.V.; Pintado, M.; Habbasha, E.S.E. Formulation and Characterization of Non-Toxic, Antimicrobial, and Alcohol-Free Hand Sanitizer Nanoemulgel Based on Lemon Peel Extract. Cosmetics 2024, 11, 59. https://doi.org/10.3390/cosmetics11020059
Ibrahim FM, Shalaby ES, El-Liethy MA, Abd-Elmaksoud S, Mohammed RS, Shalaby SI, Rodrigues CV, Pintado M, Habbasha ESE. Formulation and Characterization of Non-Toxic, Antimicrobial, and Alcohol-Free Hand Sanitizer Nanoemulgel Based on Lemon Peel Extract. Cosmetics. 2024; 11(2):59. https://doi.org/10.3390/cosmetics11020059
Chicago/Turabian StyleIbrahim, Faten Mohamed, Eman Samy Shalaby, Mohamed Azab El-Liethy, Sherif Abd-Elmaksoud, Reda Sayed Mohammed, Said I. Shalaby, Cristina V. Rodrigues, Manuela Pintado, and El Sayed El Habbasha. 2024. "Formulation and Characterization of Non-Toxic, Antimicrobial, and Alcohol-Free Hand Sanitizer Nanoemulgel Based on Lemon Peel Extract" Cosmetics 11, no. 2: 59. https://doi.org/10.3390/cosmetics11020059
APA StyleIbrahim, F. M., Shalaby, E. S., El-Liethy, M. A., Abd-Elmaksoud, S., Mohammed, R. S., Shalaby, S. I., Rodrigues, C. V., Pintado, M., & Habbasha, E. S. E. (2024). Formulation and Characterization of Non-Toxic, Antimicrobial, and Alcohol-Free Hand Sanitizer Nanoemulgel Based on Lemon Peel Extract. Cosmetics, 11(2), 59. https://doi.org/10.3390/cosmetics11020059