Abstract
In skin aging, from intrinsic factors or exposure to ultraviolet (UV) radiation, there is loss of structural fibrillar collagen and regulatory heat shock proteins. Phenolic compounds, with hydroxyl groups attached to an aromatic ring, have antioxidative and anti-inflammatory properties. Nicotinamide is an amide derivative of niacin or vitamin B3, with an amide linked to an aromatic ring, with UV absorptive, antioxidant, anti-inflammatory and anti-cell death/apoptosis properties. The goal of this research was to investigate the anti-skin aging mechanism of nicotinamide and its derivatives, 2,6-dihydroxynicotinamide, 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxypicolinamide (collectively niacin derivatives), through the stimulation of fibrillar collagens (type I, III and V, at protein and/or promoter levels) and the expression of heat shock proteins (HSP)-27, 47, 70, and 90 in non-irradiated or UVA radiated dermal fibroblasts; and from its direct antioxidant activity. UVA radiation inhibited the expression of types I and III collagen, and HSP-47 in dermal fibroblasts. The niacin derivatives significantly and similarly stimulated the expression of types I (transcriptionally), III and V collagens in non-irradiated, and UVA radiated fibroblasts indicating predominant effects. The 2,6-dihydroxynicotinamide had greater stimulatory effect on types I and III collagen in the non-irradiated, and UVA radiated fibroblasts, as well as greater direct antioxidant activity than the other niacin derivatives. The niacin derivatives, with a few exceptions, stimulated the expression of HSP-27, 47, 70 and 90 in non-irradiated, and UVA radiated fibroblasts. However, they had varied effects on the expression of the different HSPs in non-irradiated, and UVA radiated fibroblasts indicating non-predominant, albeit stimulatory, effect. Overall, nicotinamide and its derivatives have anti skin aging potential through the stimulation of fibrillar collagen and HSPs.
1. Introduction
The structural integrity of the extracellular matrix (ECM) is essential to skin health [1,2,3,4,5,6,7,8]. The fibrillar collagen (types I, III, and V) is the predominant structural protein of the ECM. The dermal fibroblasts are the primary synthesizers of fibrillar collagen [1,2,3,4,8]. The structural integrity of the fibrillar collagen is compromised with aging, from intrinsic factors and exposure to solar ultraviolet (UV)A radiation (penetrating the dermis) and UVB radiation (penetrating the epidermis) [1,2,3,4,5,6,7,8,9]. These skin aging factors cause oxidative stress and inflammation, and overwhelm the intrinsic cellular capacity to counteract the increased formation of reactive oxygen species (ROS) and inflammatory mediators. The oxidative stress and inflammation alter the cellular signal transduction pathways, including the mitogen activated protein kinase (MAPK) and nuclear factor-kB (NF-kB) pathways, and thereby the expression of the ECM genes as well as the structure of the ECM proteins [1,2,3,4,5,6,7,8]. The compromise to the fibrillar collagen, from its reduced expression and increased degradation, manifests as wrinkles, loose and aged skin [1,2,3,4,5,6,7,8].
The chaperone proteins that facilitate the formation of the ECM and the prevention of molecular oxidative damage are the heat shock proteins (HSP). The HSPs facilitate proper protein folding and prevent their degradation. HSP-27 increases cellular antioxidant score by increasing cellular reduced glutathione, and reducing oxidized proteins [10]. HSP-70, which is reduced with cellular aging, provides anti-inflammatory and skin protective properties [11,12]. HSP-47, a collagen specific chaperone protein, is co-stimulated with fibrillar collagen by ECM strengthening agents, such as copper, in dermal fibroblasts [8]. HSP-90 facilitates the migration of dermal fibroblasts, and the maturation of the ECM, imperative to wound healing [13].
Polyphenols with their anti-oxidative and anti-inflammatory properties have been identified to beneficially regulate the ECM, and thereby prevent skin aging [1,2,3,4,5,6,7]. The structure of the phenolic components includes at least one aromatic ring with one or more hydroxyl groups [5]. The activity of the phenolic compounds is dependent on the number and location of these hydroxyl groups [5,14,15,16]. Nicotinamide is an amide derivative of niacin or vitamin B3, with an amide linked to an aromatic ring. It has UV absorptive, antioxidant and anti-inflammatory properties, and in addition promotes cellular metabolism and survival [17,18,19,20,21,22,23,24,25,26,27]. Nicotinamide inhibits MAPK, NF-kB, and the inflammatory mediators (interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and prostaglandins), and prevents solar-simulated UV induced immunosuppression, and thereby carcinogenesis, in vivo [17,18,19]. Nicotinamide facilitates “cellular longevity” by stimulating protein kinase B (PKB) pathway that inhibits mediators of apoptosis, prevents UV radiation induced inhibition of ATP and glycolysis in keratinocytes, and arsenic/UV radiation induced oxidative DNA damage in the form of 8-oxo-7,8-dihydro-2'-deoxyguanosine and pyrimidine dimers [20,21,22]. Nicotinamide reduces hyperpigmentation, fine lines and wrinkles, and improves retinoic acid biosynthesis and epidermal barrier function, independently or in combination with 0.02% tretinoin [23,24,25,26,27]. The mechanism of anti-skin aging effect of nicotinamide through the stimulation of expression of fibrillar collagen, and promotion of cellular survival/health through the stimulation of HSPs in dermal fibroblasts has not been reported.
The goal of this research was to determine the beneficial regulation of the fibrillar collagen (types I, III, V) and HSPs (27, 47, 70, 90) by nicotinamide, and three of its derivatives, 2,6-dihydroxynicotinamide, 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxypicolinamide (collectively niacin derivatives) in non-irradiated or UVA-radiated dermal fibroblasts; as well as the direct antioxidant activity of the nicotinamide derivatives. We have reported that nicotinamide exhibits direct antioxidant activity in millimolar concentrations, up to 50% of control [6]. The hypothesis of this research was that these niacin derivatives would stimulate fibrillar collagen and HSPs, and exhibit direct antioxidant property, with the hydroxyl derivatives providing greater effectiveness.
2. Results
2.1. Stimulation of Type I Collagen, Type I Collagen Promoter, Type III Collagen, and Type V Collagen Protein by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in Non-Irradiated Fibroblasts
The niacin derivatives significantly stimulated the expression of type I collagen at 0.1% and 1% (except 3-hydroxypicolinamide at 0.1%), type I collagen promoter activity at 0.01%, 0.1% and 1%, and types III and V collagen at 1% in non-irradiated fibroblasts, in comparison with control (p < 0.05) (Figure 1). In addition, 2,6-dihydroxynicotinamide significantly stimulated expression of type III collagen at 0.1% and type V collagen at 0.01%, and 0.1% (p < 0.05) (Figure 1c,d). The niacin derivatives had similar activities, except for the significantly greater stimulation of types I and III collagen by 2,6-dihydroxynicotinamide.
Relative to the expression of type I collagen protein (0.7 μg/mL as 100%) in non-irradiated control cells (0% niacin derivative), 0.1% and 1% of each of the niacin derivatives stimulated type I collagen protein levels as follows: nicotinamide to 206%, and 250% of non-irradiated control; 2,6-dihydroxynicotinamide to 483%, and 339% of non-irradiated control; and 2,4,5,6-tetrahydroxynicotinamide to 251%, and 242% of non-irradiated control (p < 0.05) (Figure 1a). The 3-hydroxypicolinamide stimulated type I collagen at 1% to 271% of non-irradiated control (p < 0.05) (Figure 1a). Relative to the type I collagen promoter activity (750 pg/mL as 100%) in non-irradiated control cells (0% niacin derivative), 0.01%, 0.1%, and 1% of each of the niacin derivatives stimulated type I collagen promoter activity as follows: nicotinamide to 271%, 308%, and 159% of non-irradiated control; 2,6-dihydroxynicotinamide to 216%, 521%, and 248% of non-irradiated control; 2,4,5,6-tetrahydroxynicotinamide to 145%, 201%, and 206% of non-irradiated control; and 3-hydroxypicolinamide to 149%, 259%, and 418% of non-irradiated control (p < 0.05) (Figure 1b). Relative to the expression of type III collagen protein (0.3 μg/mL as 100%) in non-irradiated control cells (0% niacin derivative), 1% of each of the niacin derivatives stimulated type III collagen levels as follows: nicotinamide to 166% of non-irradiated control; 2,6-dihydroxynicotinamide to 638% of non-irradiated control; 2,4,5,6-tetrahydroxynicotinamide to 218% of non-irradiated control, 3-hydroxylpicolinamide to 277% of non-irradiated control (p < 0.05) (Figure 1c). In addition, 0.1% 2,6-dihydroxynicotinamide stimulated type III collagen levels to 510% of control (p < 0.05) (Figure 1c). Relative to the expression of type V collagen protein (0.15 μg/mL as 100%) in non-irradiated control cells (0% niacin derivative), 1% of each of the niacin derivatives stimulated type V collagen levels as follows: nicotinamide to 167% of non-irradiated control; 2,6-dihydroxynicotinamide to 121% of non-irradiated control; 2,4,5,6-tetrahydroxynicotinamide to 136% of non-irradiated control, 3-hydroxylpicolinamide to 251% of non-irradiated control (p < 0.05) (Figure 1d). In addition, 0.01% and 0.1% 2,6-dihydroxynicotinamide stimulated type V collagen levels to 152%, and 244% (p < 0.05) (Figure 1d).
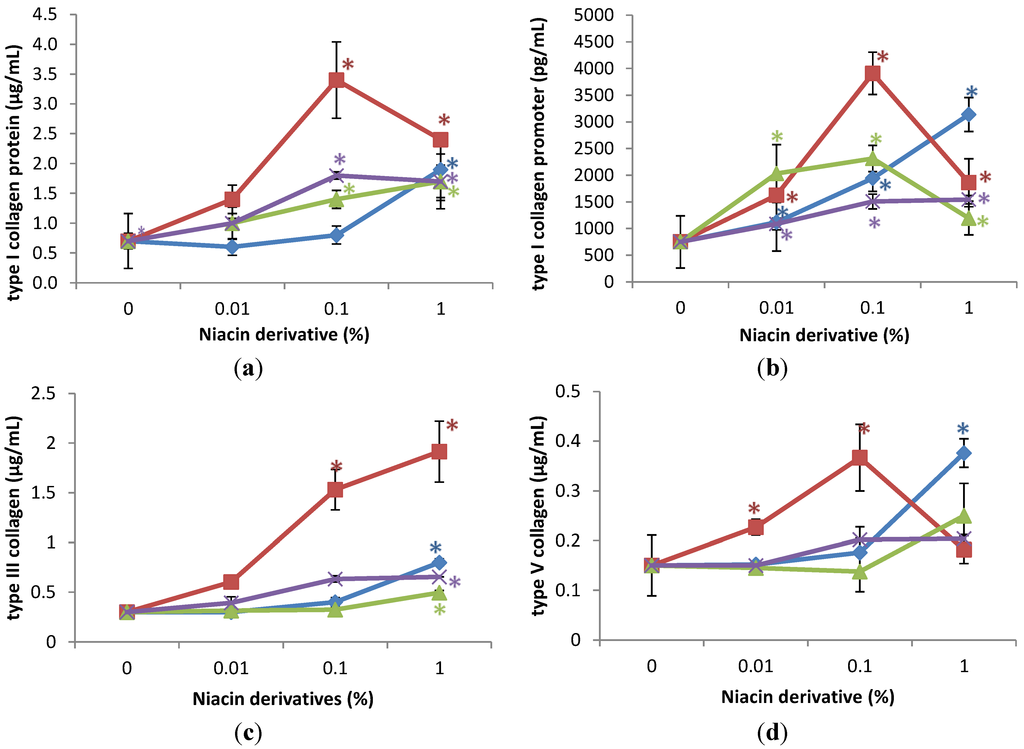
Figure 1.
Stimulation of type I collagen protein (a); type I collagen promoter activity (b); type III collagen protein (c); and type V collagen protein (d) by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in non-irradiated dermal fibroblasts; * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
Figure 1.
Stimulation of type I collagen protein (a); type I collagen promoter activity (b); type III collagen protein (c); and type V collagen protein (d) by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in non-irradiated dermal fibroblasts; * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
2.2. Stimulation of Expression of Type I Collagen, Type I Collagen Promoter, Type III Collagen, and Type V Collagen Protein by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in UVA Radiated Fibroblasts
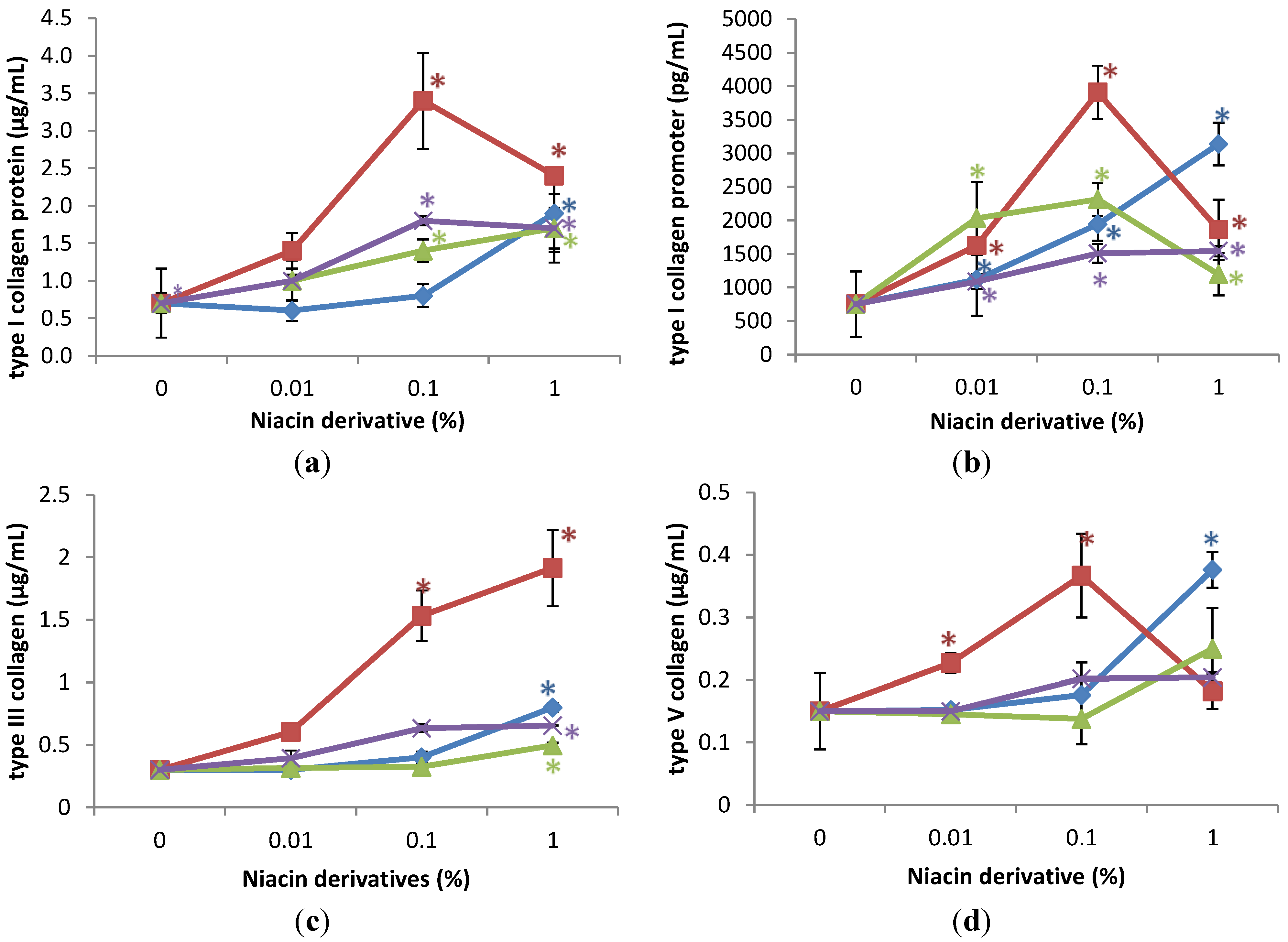
UVA-radiation inhibited type I collagen protein levels to 60% of non-irradiated control cells (control), type I collagen promoter activity to 50% of control, and type III collagen protein levels to 75% of control; and did not significantly alter the expression of type V collagen.
The niacin derivatives significantly stimulated the expression of type I collagen at 0.1% and 1%, type I collagen promoter activity at 0.01%, 0.1% and 1%, and types III and V collagen at 1% in UVA-radiated dermal fibroblasts, in comparison with UVA radiated control (0% niacin derivative) (p < 0.05) (Figure 2). In addition, 2,6-dihydroxynicotinamide and 3-hydroxypicolinamide significantly stimulated expression of types III and V collagen at 0.1% (p < 0.05) (Figure 2c,d). The niacin derivatives had similar activities, except for the significantly greater stimulation of types I and III collagen by 2,6-dihydroxynicotinamide.
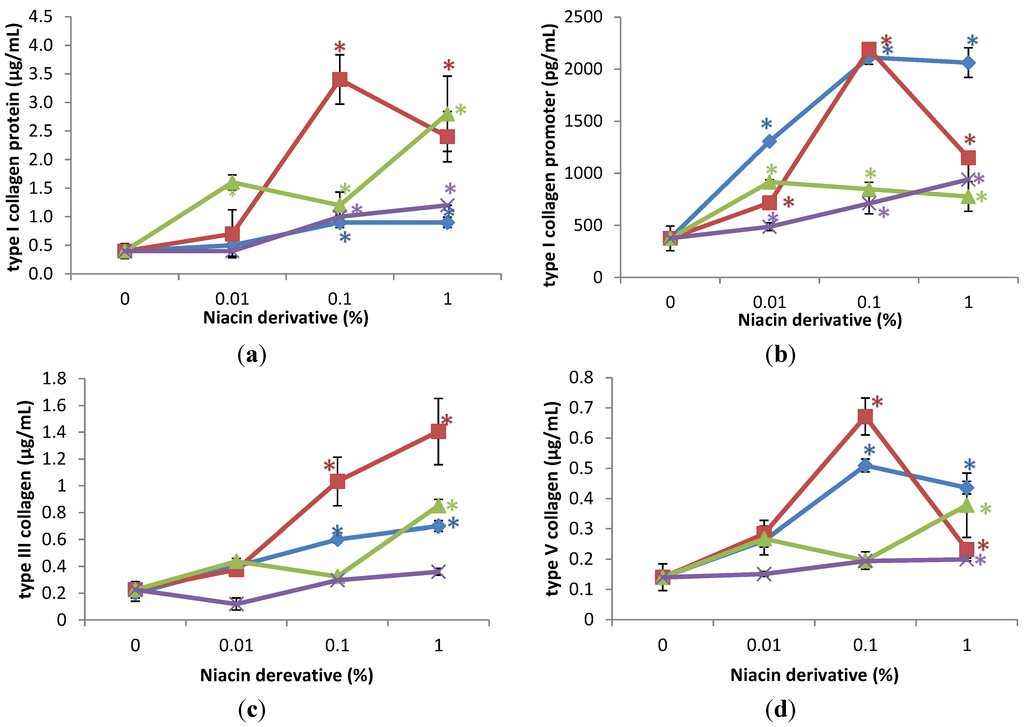
Figure 2.
Stimulation of type I collagen protein (a); type I collagen promoter activity (b); type III collagen protein (c); and type V collagen protein (d) by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in UVA-radiated dermal fibroblasts; * p < 0.05, relative to UVA radiated control cells, error bars (a–d) represent standard deviation, n = 4.
Figure 2.
Stimulation of type I collagen protein (a); type I collagen promoter activity (b); type III collagen protein (c); and type V collagen protein (d) by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in UVA-radiated dermal fibroblasts; * p < 0.05, relative to UVA radiated control cells, error bars (a–d) represent standard deviation, n = 4.
Relative to the expression of type I collagen protein (0.42 μg/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 0.1% and 1% of each of the niacin derivatives stimulated type I collagen levels as follows: nicotinamide to 289%, and 658% of UVA-radiated control; 2,6-dihydroxynicotinamide to 595%, and 521% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide to 243%, and 287% of UVA-radiated control; and 3-hydroxypicolinamide to 210%, and 210% of UVA-radiated control (p < 0.05) (Figure 2a). Relative to the activity of type I collagen promoter (375 pg/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 0.01%, 0.1%, and 1% of each of the niacin derivatives stimulated type I collagen promoter activity as follows: nicotinamide to 244%, 226%, and 207% of UVA-radiated control; 2,6-dihydroxynicotinamide to 191%, 584%, and 306% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide stimulated type I collagen promoter levels to 130%, 190%, and 250%; and 3-hydroxypicolinamide to 348%, 564%, and 550% of UVA-radiated control (p < 0.05) (Figure 2b). Relative to the expression of type III collagen protein (0.225 μg/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 1% of each of the niacin derivatives stimulated type III collagen levels as follows: nicotinamide to 378% of UVA-radiated control; 2,6-dihydroxynicotinamide to 625% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide to 160% of UVA-radiated control, and 3-hydroxylpicolinamide to 316% of control of UVA-radiated control (p < 0.05) (Figure 2c). In addition, 2,6-dihydroxynicotinamide and 3-hydroxypicolinamide at 0.1% stimulated type III collagen levels to 459%, and 281% of UVA-radiated control (p < 0.05) (Figure 2c). Relative to the expression of type V collagen protein (0.15 μg/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 1% of each of the niacin derivatives stimulated type V collagen levels as follows: nicotinamide to 269% of UVA-radiated control; 2,6-dihydroxynicotinamide to 165% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide to 142% of UVA-radiated control, and 3-hydroxylpicolinamide to 310% of control of UVA-radiated control (p < 0.05) (Figure 2d). In addition, 2,6-dihydroxynicotinamide and 3-hydroxypicolinamide at 0.1% stimulated type V collagen levels to 477%, and 362% of UVA-radiated control (p < 0.05) (Figure 2d).
2.3. Stimulation of Heat Shock Protein-27, Heat Shock Protein-47, Heat Shock Protein-70, and Heat Shock Protein-90 by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide in Non-Irradiated Fibroblasts
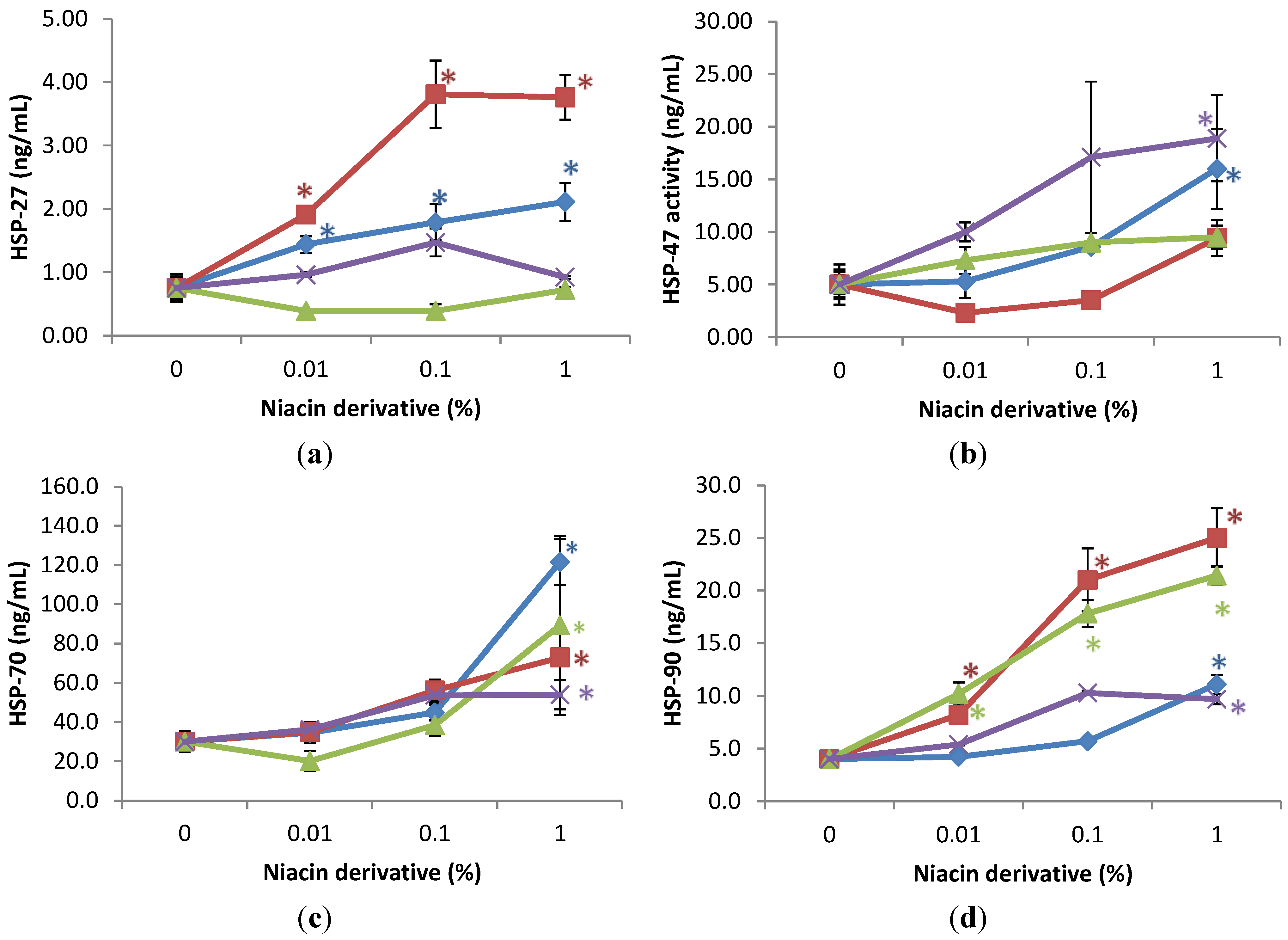
The expression of HSPs was significantly stimulated by the niacin derivatives in the non-irradiated fibroblasts, in comparison with control, as follows: HSP-27 by 2,6-dihydroxynicotinamide and 3-hydroxypicolinamide 0.01%, 0.1% and 1%; HSP-47 by 2,4,5,6-tetra hydroxynicotinamide and 3-hydroxypicolinamide at 1%; HSP-70 by each of the niacin derivatives at 1%, and HSP-90 by each of the niacin derivatives at 1% and in addition by nicotinamide and 2,6-dihydroxynicotinamide at 0.1% (p < 0.05) (Figure 3). The 2,6-dihydroxynicotinamide exhibited greater stimulatory effect, than the other niacin derivatives, on the expression of HSP-27, and the 2,6-dihydroxynicotinamide and nicotinamide exhibited greater stimulatory effect on HSP-90.
Relative to the expression of HSP-27 (0.7 ng/mL as 100%) in non-irradiated control cells (0% niacin derivative), 0.01%, 0.1%, and 1% of each of the niacin derivatives stimulated HSP-27 as follows: 2,6-dihydroxynicotinamide to 254%, 508%, and 501% of non-irradiated control; and 3-hydroxypicolinamide to 192%, 239%, and 281% of non-irradiated control (p < 0.05) (Figure 3a). Relative to the expression of HSP-47 (5 ng/mL as 100%) in non-irradiated control cells (0% niacin derivative), 1% of each of the niacin derivatives stimulated HSP-47 as follows: 2,4,5,6-tetrahydroxynicotinamide to 379% of non-irradiated control; and 3-hydroxypicolinamide to 252% of non-irradiated control (p < 0.05) (Figure 3b). Relative to the expression of HSP-70 (30 ng/mL as 100%) in non-irradiated control cells (0 niacin derivative), 1% of each of the niacin derivatives stimulated HSP-70 levels as follows: nicotinamide to 405% of non-irradiated control; 2,6-dihydroxynicotinamide to 297% of non-irradiated control; 2,4,5,6-tetrahydroxynicotinamide to 180% of non-irradiated control, 3-hydroxylpicolinamide to 251% of non-irradiated control (p < 0.05) (Figure 3c). Relative to the expression of HSP-90 (4 ng/mL as 100%) in non-irradiated control cells (0 niacin derivative), 1% of each of the niacin derivatives stimulated HSP-90 levels as follows: nicotinamide to 418% of non-irradiated control; 2,6-dihydroxynicotinamide to 630% of non-irradiated control; 2,4,5,6-tetrahydroxynicotinamide to 243% of non-irradiated control, 3-hydroxylpicolinamide to 277% of non-irradiated control (p < 0.05) (Figure 3d). In addition, 0.01% and 1% 2,6-dihydroxynicotinamide stimulated HSP-90 to 204%; and 630% of control (p < 0.05) (Figure 3d).
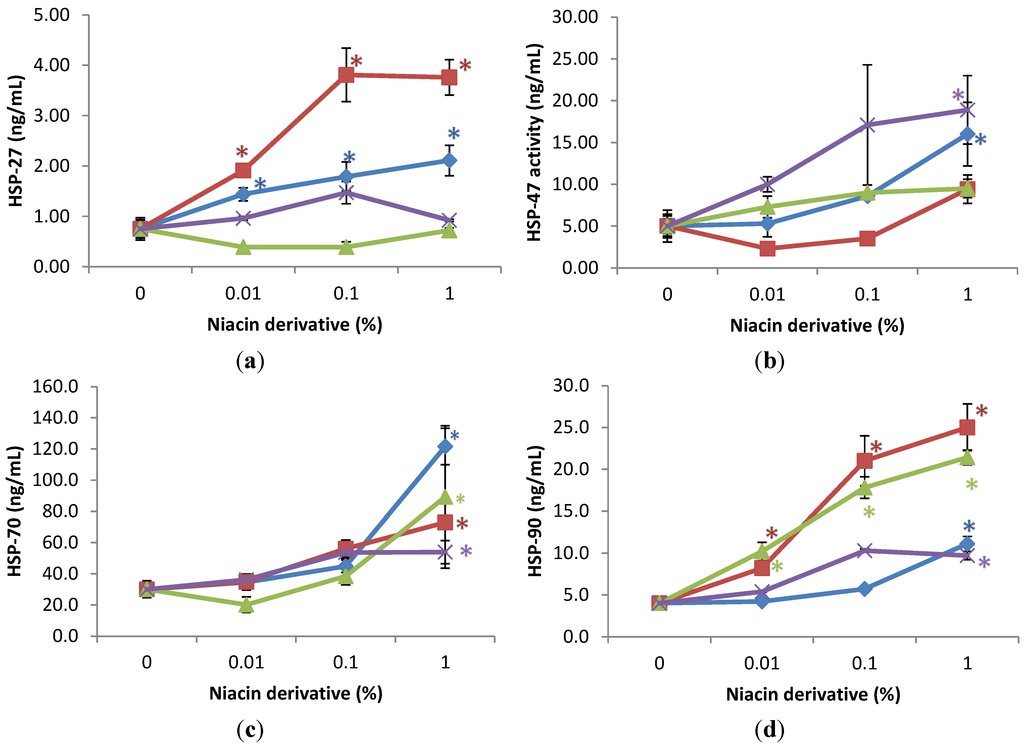
Figure 3.
Stimulation of HSP-27 (a); HSP-47 (b); HSP-70 (c); and HSP-90 (d) proteins by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in dermal fibroblasts; * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
Figure 3.
Stimulation of HSP-27 (a); HSP-47 (b); HSP-70 (c); and HSP-90 (d) proteins by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in dermal fibroblasts; * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
2.4. Stimulation of Heat Shock Protein-27, Heat Shock Protein-47, Heat Shock Protein-70, and Heat Shock Protein-90 by Nicotinamide, 2,6-Dihydroxynicotinamide, 2,4,5,6-Tetrahydroxynicotinamide, and 3-Hydroxypicolinamide Nicotinamide in UVA Radiated Fibroblasts
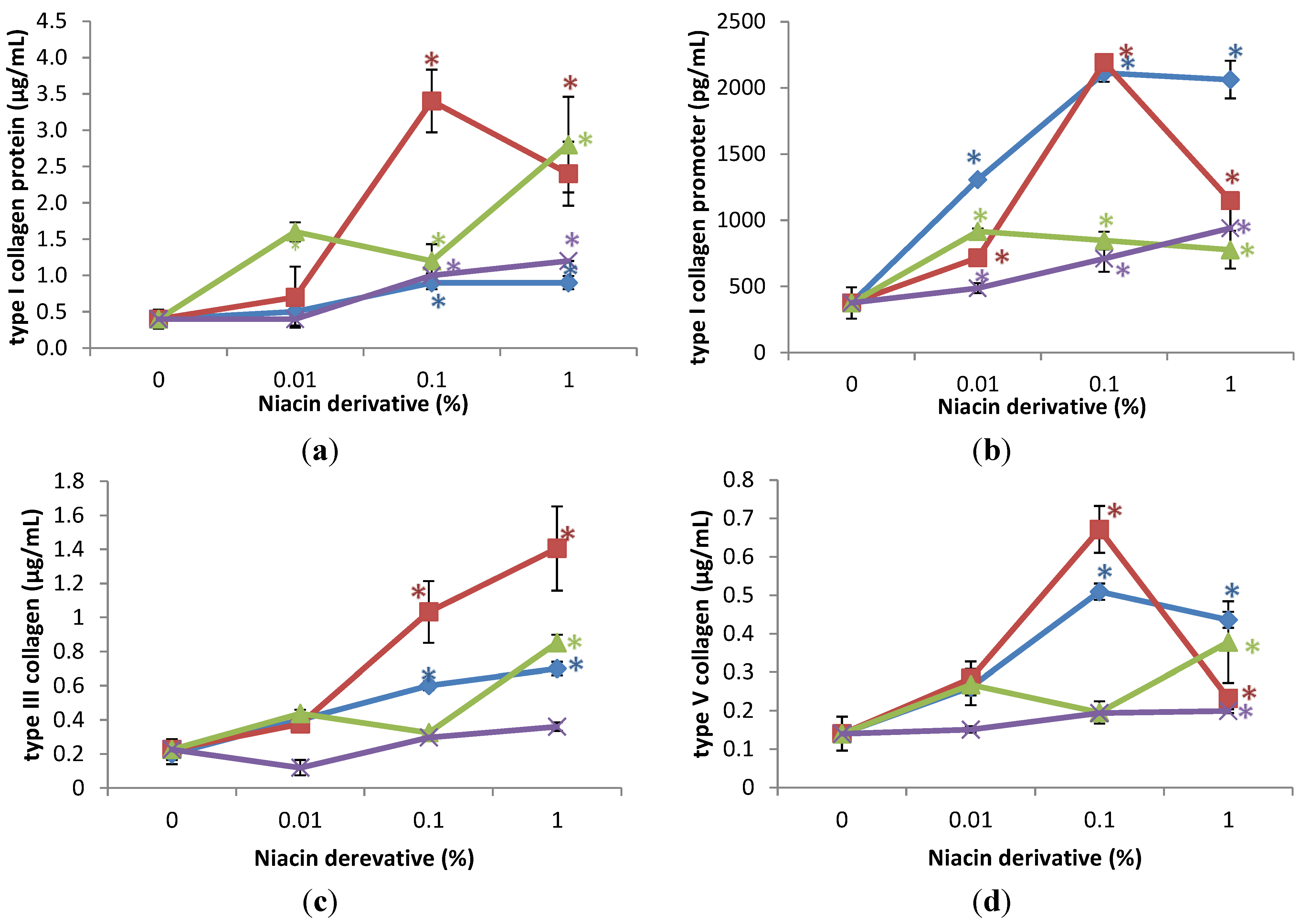
UVA-radiation did not significantly alter the expression of HSPs, except for the inhibition of HSP-47 to 56% of non-irradiated control cells (control).
The niacin derivatives significantly stimulated the expression of HSP-27 and HSP-47 at 1% (except for HSP-27 by 2,4,5,6-tetrahydroxynicotinamide), HSP-70 and HSP-90 at 0.01 and 1% (except for HSP-90 by 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxylpicolinamide) in UVA-radiated dermal fibroblasts, in comparison with UVA radiated control (0% niacin derivative) (p < 0.05) (Figure 4). In addition, nicotinamide significantly stimulated HSP-27 at 0.01 and 1%, and HSP-47 at 0.1% in the UVA-radiated dermal fibroblasts (p < 0.05) (Figure 4a,b).
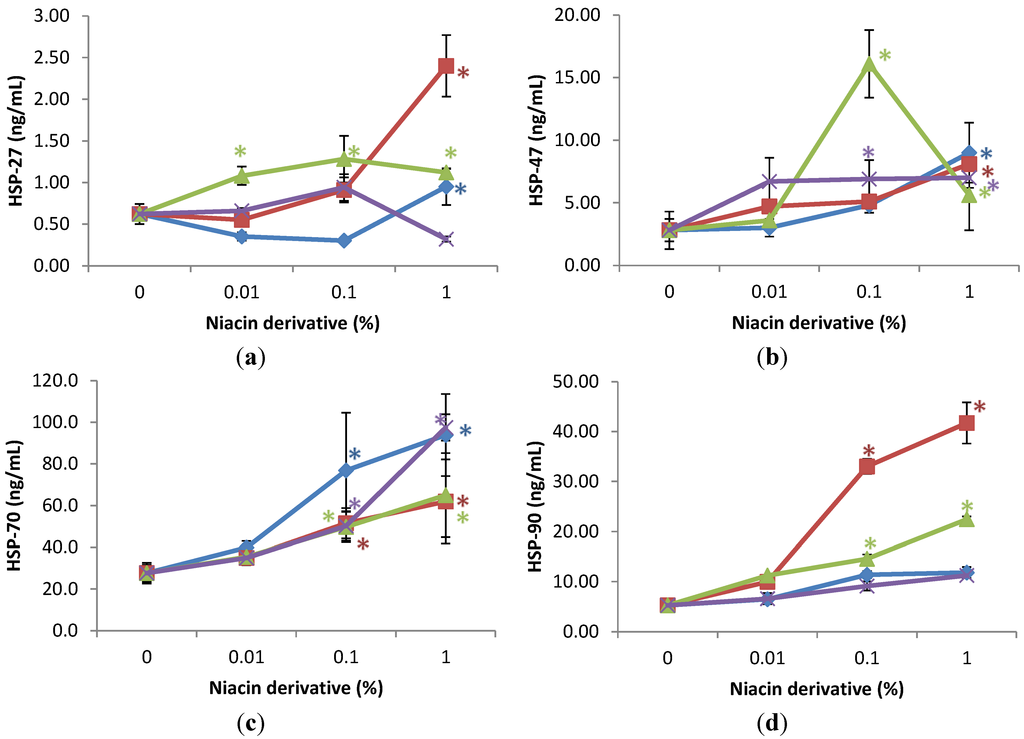
Figure 4.
Stimulation of HSP-27 (a); HSP-47 (b); HSP-70 (c); and HSP-90 (d) proteins by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in UVA-radiated dermal fibroblasts; * p < 0.05, relative to UVA radiated control cells, error bars (a–d) represent standard deviation, n = 4.
Figure 4.
Stimulation of HSP-27 (a); HSP-47 (b); HSP-70 (c); and HSP-90 (d) proteins by nicotinamide (green line), 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line) in UVA-radiated dermal fibroblasts; * p < 0.05, relative to UVA radiated control cells, error bars (a–d) represent standard deviation, n = 4.
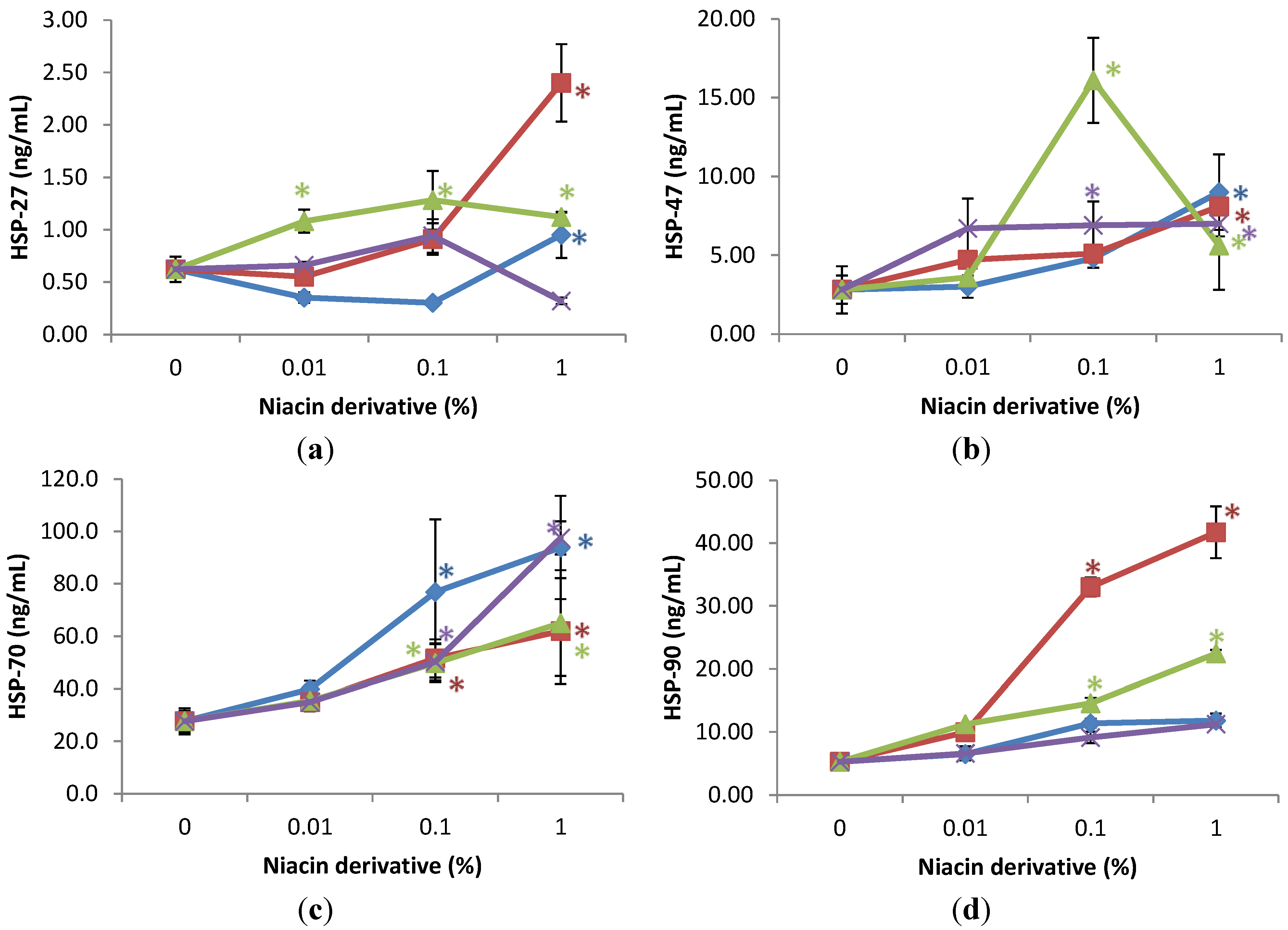
Relative to the expression of HSP-27 (0.6 ng/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 1% of each niacin derivatives stimulated HSP-27 as follows: nicotinamide to 180% of UVA-radiated control, 2,6-dihydroxynicotinamide to 388% of UVA-radiated control; and 3-hydroxylpicolinamide to 154% of UVA-radiated control (p < 0.05) (Figure 4a). In addition, 0.01% and 0.1% nicotinamide stimulated HSP-27 to 175%; and 207% of control (p < 0.05) (Figure 4a). Relative to the expression of HSP-47 (2.8 ng/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 1% of each niacin derivatives stimulated HSP-47 as follows: nicotinamide to 201% of UVA-radiated control, 2,6-dihydroxynicotinamide to 290% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide to 250% of UVA-radiated control, and picolinamide to 372% of UVA-radiated control (p < 0.05) (Figure 4b). In addition, 0.1% nicotinamide stimulated HSP-47 to 575% of control (p < 0.05) (Figure 4b). Relative to the expression of heat shock protein-70 (27 ng/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 0.1% and 1% of each niacin derivatives stimulated HSP-70 as follows: nicotinamide to 279%, and 339% of UVA-radiated control; 2,6-dihydroxynicotinamide to 180%, and 236% of UVA-radiated control; 2,4,5,6-tetrahydroxynicotinamide to 182%, and 353% of UVA-radiated control; 3-hydroxylpicolinamide to 186%, and 224% of UVA-radiated control (p < 0.05) (Figure 4c). Relative to the expression of HSP-90 (4.5 ng/mL as 100%) in UVA-radiated control cells (0% niacin derivative), 0.1% and 1% nicotinamide and 2,6-dihydroxynicotinamide stimulated HSP-90 as follows: nicotinamide to 231%, and 330% of UVA-radiated control; and 2,6-dihydroxynicotinamide to 630%, and 796% of UVA-radiated control (p < 0.05) (Figure 4d).
2.5. Direct Inhibition of ABTS Oxidation by Nicotinamide and Its Derivatives
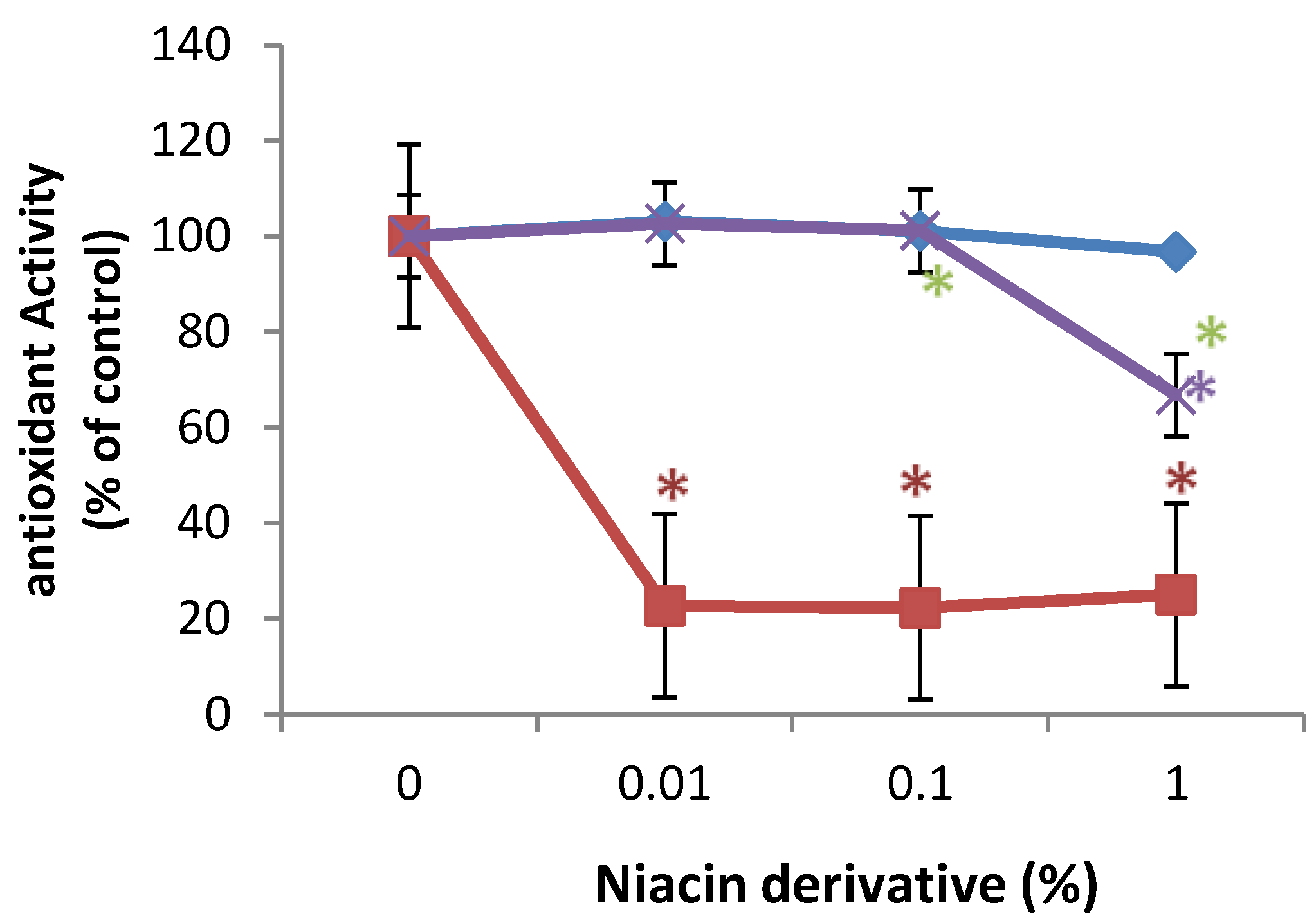
In comparison with control (100%, 0% niacin derivative), 0.01%, 0.1%, and 1% of 2,6-dihydroxynicotinamide significantly inhibited 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) oxidation to 20% of control, and 1% of 2,4,5,6-tetrahydroxynicotinamide significantly inhibited ABTS oxidation to 62% of control (p < 0.05) (Figure 5). 3-hydroxylpicolinamide did not significantly alter ABTS oxidation (Figure 5).
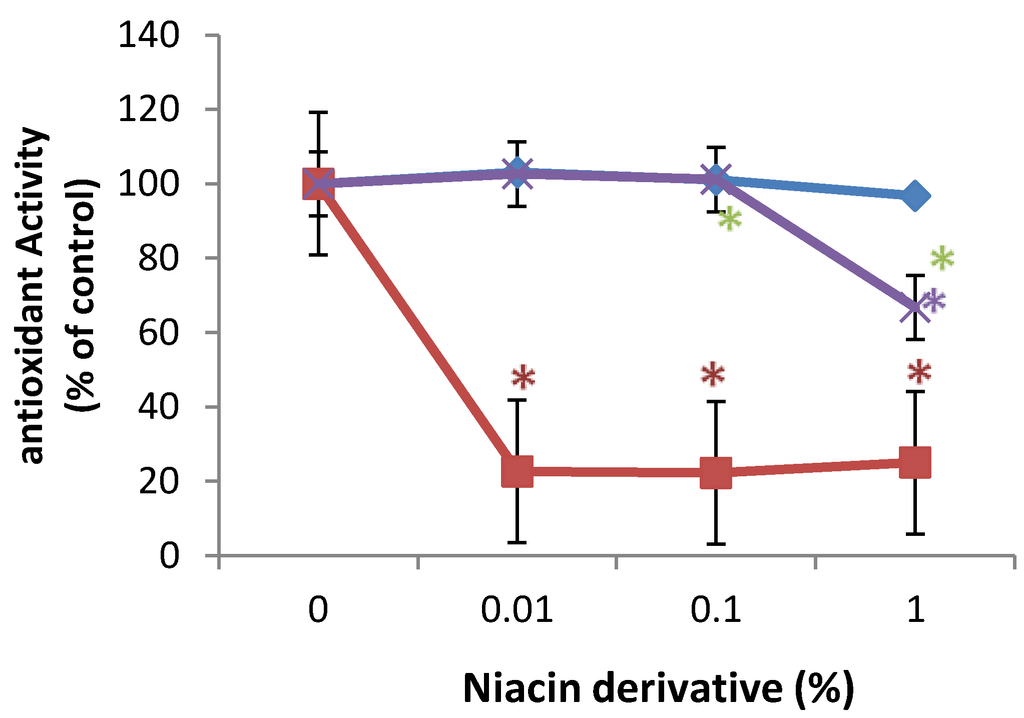
Figure 5.
Direct inhibition of ABTS oxidation by 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line); * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
Figure 5.
Direct inhibition of ABTS oxidation by 2,6-dihydroxynicotinamide (red line), 2,4,5,6-tetrahydroxynicotinamide (violet line), and 3-hydroxypicolinamide (blue line); * p < 0.05, relative to control, error bars (a–d) represent standard deviation, n = 4.
3. Discussion
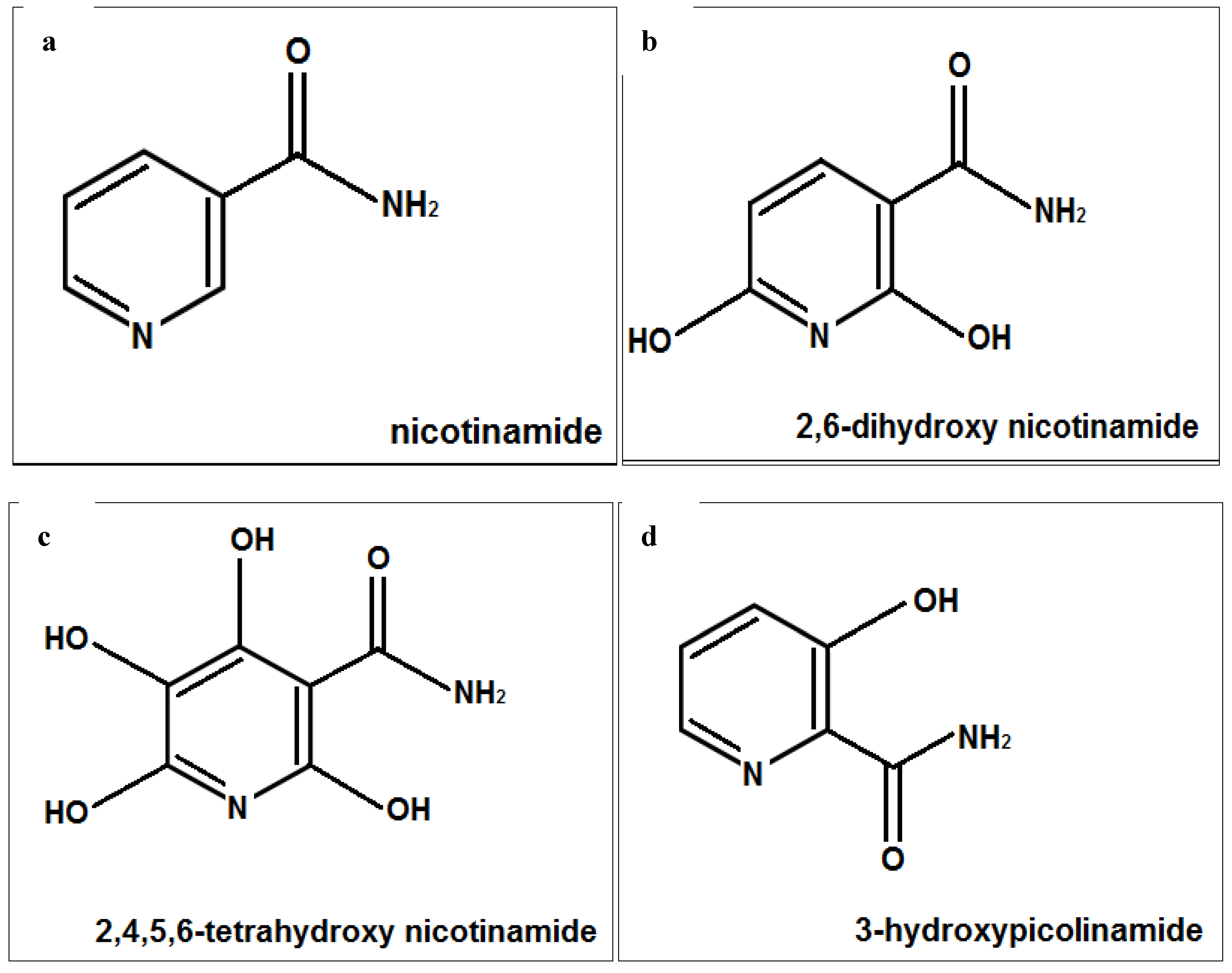
The skin is exposed to aging agents, especially UV radiation, and its antioxidant defense as well as NAD content is reduced with aging [1,2,3,4,5,6,7,8,28]. Nicotinamide has antioxidant and anti-cellular stress properties. It has anti skin aging and wrinkle reducing ability, independently or in combination with retinoids [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The complexes of 3-hydroxypicolinamide have been examined for their photophysical properties [30,31,32]. The structure of nicotinamide and 3-hydroxypicolimanide may contribute to their physical activity, and polyphenols with their hydroxyl groups are known for their antioxidant and anti-skin aging properties [1,2,3,4,5,6,7,8,14,15,16,30,31,32,33]. Intrinsic as well as photoaging is associated with loss of structural collagen, and molecular chaperones [1,2,3,4,5,6,7,8,34]. UVA radiations has been called the “aging ray” and since it penetrate the dermis it has propensity for carcinogenesis, damage to biomolecules, generation of ROS/oxidative stress, immunosuppression, and inflammation [35,36,37,38,39,40]. The hypothesis of this research was that nicotimande and its derivatives, 2,6-dihydroxynicotinamide, 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxypicolinamide (Figure 6), would beneficially regulate structural fibrillar collagen and heat shock proteins in non-irradiated, and UVA radiated dermal fibroblasts, and exhibit direct antioxidant activity.
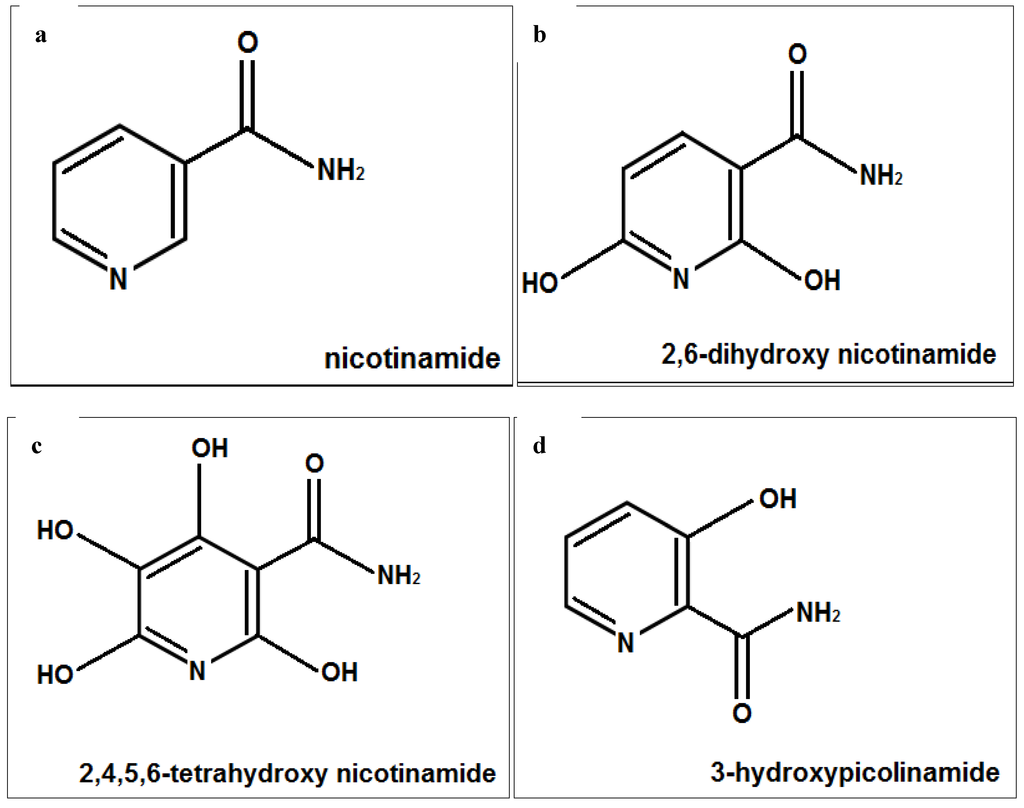
Figure 6.
Structures of nicotinamide (a); 6-dihydroxynicotinamide (b); 2,4,5,6-tetrahydroxynicotinamide (c); and 3-hydroxypicolinamide (d).
Figure 6.
Structures of nicotinamide (a); 6-dihydroxynicotinamide (b); 2,4,5,6-tetrahydroxynicotinamide (c); and 3-hydroxypicolinamide (d).
UV radiation inhibited the expression of types I and III in dermal fibroblasts. The niacin derivatives had similar effects on the expression of fibrillar collagen in non-irradiated and UVA radiated fibroblasts. With few exception, all of the niacin derivatives significantly stimulated the expression of type I collagen at 0.1% and 1%, type I collagen promoter activity at 0.01%, 0.1% and 1%, and types III and V collagen at 1% in non-irradiated, and UVA-radiated dermal fibroblasts, suggesting predominant effects of these niacin derivatives. The 2,6-dihydroxynicotinamide exhibited greater stimulatory effect of type I and III collagen in non-irradiated and UVA radiated fibroblasts, than the other niacin derivatives. The 2,6-dihydroxynicotinamide exhibited greater direct antioxidant activity than the other niacin derivatives, including nicotinamide [6], suggesting that its greater collagen stimulatory effect is from its greater antioxidant activity.
UV radiation inhibited the expression of HSP-47 in dermal fibroblasts. The niacin derivatives had varied effects on the expression of the different HSPs in non-irradiated and UVA radiated fibroblasts. The HSP-27 was stimulated by 2,6-dihydroxynicotinamide and 3-hydroxypicolinamide at 0.01%, 0.1% and 1% in non-irradiated cells, but only at 1% in the UVA-irradiated cells. While nicotinamide did not alter HSP-27 in non-irradiated fibroblasts, it stimulated HSP-27 at 0.01%, 0.1% and 1% in UVA-irradiated fibroblasts. The HSP-47 was stimulated by 1% 2,4,5,6-tetra hydroxynicotinamide and 3-hydroxypicolinamide in non-irradiated fibroblasts, and by 1% of all the niacin derivatives in UVA radiated fibroblasts. The HSP-70 was stimulated by all the niacin derivatives at 1% in non-irradiated fibroblasts, but also at 0.01% in UVA radiated fibroblasts. Nicotinamide and 2,6-dihydroxynicotinamide were stimulatory to the expression of HSP-90 in non-irradiated and UVA radiated fibroblasts, however the effect of nicotinamide was less stimulatory in the UV radiated cells. It is inferred that the effects of the niacin derivatives on the expression of HSPs do not predominant in the UVA exposed fibroblasts.
Niacin/nicotinamide serves as precursor for several cellular coenzymes, essential to metabolism, and functions in cellular housekeeping by counteracting oxidative stress and inflammation [17,18,19,20,21,22,23,24,25,26,27,41,42,43,44,45,46,47,48,49,50]. It has diverse mechanisms for its activities. We report for the first time that nicotinamide as well as its derivatives, 2,6-dihydroxynicotinamide, 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxypicolinamide, have anti-skin aging potential through the stimulation of expression of type I, III and V collagen, and HSP-27, 47, 70 and 90 in non-irradiated, and UVA radiated fibroblasts.
4. Materials and Methods
4.1. Cell Culture and Dosing
Human adult dermal fibroblasts from two donors (Cascade Biologics), respectively, were cultured in complete Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum, 1% penicillin/streptomycin (P/S) and 1% l-glutamine (Sigma, St. Louis, MO, USA), seeded for 24 h, rinsed with sterile Hanks balanced salt solution (Sigma), and dosed with or without UVA-radiation (2.5 J/cm2), as previously described, followed with or without niacin derivatives (0.01%, 0.1%, 1% of respective 50 mg/mL stock solutions) in experimental media (DMEM containing 1X serum replacement and 1% P/S) for 24 h [2,3,4]. The UV radiation was via a four tubes UVA lamp with irradiance of 1 × 10−3 W/cm2 for sum of wavelengths 320–400 nm. Four independent experiments, in replicates of 3–4, were performed with dermal fibroblasts at passages 4–12. The media were examined for types I, III, and V proteins, and the cells for HSP-27, 47, 70 and 90 proteins. The cells were co-transfected with type I collagen promoter-reporter and control plasmids prior to dosing to determine the regulation of promoter activity. The cells were examined for cell viability (CellTiter 96® Aqueous One or MTS assay (tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS) + electron coupling reagent (phenazine ethosulfate; PES)) (Promega, Madison, WI, USA)). The cell viability was not altered at these concentrations of UVA radiation or niacin derivatives.
4.2. Collagen (Types I, III, V), and HSP (27, 47. 70, 90) Protein Levels
One hundred μL aliquots of media or cells from each sample, and respective standards were added to independent wells of 96 well plates for 24 h at 4 °C. The wells were blocked with bovine serum albumin, and then incubated with respective antibodies (Millipore-type I collagen: CC050, AB745, AB758B; type III collagen: CC054, AB747; type V collagen: CC077, AB763P; Enzo Life sciences-HSP-27: ADI-EKS-500; HSP-47: ADI-SPA-470; HSP-70: ADI-EKS-700B; HSP-90: ADI-EKS-895) for 1 h at room temperature (protein detector ELISA kit, 55-81-10, KPL Lab. Inc., Gaithersburg, MD, USA). The plates were washed with wash buffer, incubated with respective secondary antibodies linked to peroxidase for 1 h at room temperature, washed, and subsequently incubated with peroxidase substrate until color development, which was measured spectrophotometrically at 405 nm.
4.3. Type I Collagen Promoter Activity
Fibroblasts were co-transfected with COL1α1 promoter-firefly luciferase plasmid (pGL4 vector) (gift from Dr. Joel Rosenbloom, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA) and thymidine kinase (TK) promoter-hRenilla luciferase plasmid (Promega) (for normalization of transfection efficiency) using Escort (Sigma) for 24 h, prior to dosing with or without UVA-radiation +/− niacin derivatives for 24 h. The cells were measured for luminescence from firefly, and renilla luciferase activities with specific substrates and quantitated using recombinant luciferase as standard (Promega).
4.4. Antioxidant Activity
The direct antioxidant activity of nicotinamide derivatives was determined by incubating them with ABTS® (2,2'-azino-di-(3-ethylbenzthiazoline sulphonate)) and metmyoglobin, and determining the inhibition of the oxidation of ABTS® to ABTS® radical by metmyoglobin by the nicotinamide derivatives, spectrophotometrically at 405 nm (Cayman Chemical Antioxidant Assay kit, Ann Arbor, MI, USA).
4.5. Data Analysis
The significant effects of UVA or niacin derivatives were analyzed relative to respective controls (without niacin derivatives) by ANOVA and student t-tests at 95% confidence interval. The effects of UVA radiation on dermal fibroblasts were statistically analyzed relative to non-irradiated control cells. The significant effects of the niacin derivatives on non-irradiated cells were analyzed relative to non-irradiated control cells (0% niacin derivative). The significant effects of each of the niacin derivatives on UVA-radiated fibroblasts were analyzed relative to UVA radiated control cells (0% niacin derivative).
5. Conclusion
Ultraviolet (UV) radiation, more so UVA, damages the dermal collagen fibers and regulatory heat shock proteins. The effects of UVA radiation are primarily through the induction of oxidative stress and inflammation. Phenolic compounds exhibit antioxidant and anti-inflammatory properties, which are dependent on the number and location of hydroxyl groups. This research examined the potential of niacin derivatives, nicotinamide, 2,6-dihydroxynicotinamide, 2,4,5,6-tetrahydroxynicotinamide, and 3-hydroxypicolinamide, to beneficially regulate types I, III and V collagen, and heat shock proteins 27, 47, 70 and 90 in non-irradiated or UVA-radiated dermal fibroblasts; as well as exhibit direct antioxidant activity. The niacin derivatives, more so 2,6-dihydroxynicotinamide, stimulated collagen fibers and differentially up-regulated heat shock proteins in non-irradiated, and UVA radiated fibroblasts, and exhibited direct antioxidant activity. It is inferred that the niacin derivatives have photoprotective and anti-skin aging potential in cosmetics.
Acknowledgments
The authors thank Marvin Tuason, Halyna Siomyk and Tam Ho for their contribution. Funding was provided by Industrial Farmaceutica Cantabria (IFC), Spain.
Author Contributions
Neena Philips directed the research and wrote the manuscript. Jovinna Chalensouk-Khaosaat provided editorial assistance and literature searches. Salvador Gonzalez provided the project and funding from IFC.
Conflicts of Interest
Salvador Gonzalez serves as a consultant to Industrial Farmaceutica Cantabria (IFC), Spain.
References
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.; Conte, J.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrixmetalloproteinases, and stimulation of biosynthesis of fibrillar collagens, elastin and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar] [PubMed]
- Philips, N.; Smith, J.; Keller, T.; Gonzalez, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Conte, J.; Chen, Y.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and its inhibitors, fibrillar collagens and transforming growth factor-β by P. leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Keller, T.; Hendrix, C.; Hamilton, S.; Arena, R.; Tuason, M.; Gonzalez, S. Regulation of the extracellular matrix remodeling by lutein in dermal fibroblasts, melanoma cells, and ultraviolet radiation exposed fibroblasts. Arch. Dermatol. Res. 2007, 299, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Siomyk, H.; Bynum, D.; Gonzalez, S. Skin cancer, polyphenols, and oxidative stress. In Cancer: Oxidative Stress and Dietary Antioxidants; Academic Press: Waltham, MA, USA, 2014. [Google Scholar]
- Philips, N.; Samuel, M.; Parakandi, H.; Siomyk, H.; Gopal, S.; Jia, H.; Shahin, H. Vitamins in the therapy of inflammatory and oxidative disease. In Frontiers in Clinical Drug Research-Anti Allery Agents; Bentham Science Publishers: Bussum, The Netherlands, 2013; Volume 1. [Google Scholar]
- Philips, N.; Samuel, P.; Siomyk, H.; Parakandi, H.; Jia, H.; Gopal, S.; Shahin, H. Improved cell metabolism and strengthening of the extracellular matrix by nicotinamide, and copper for anti-skin aging. In Skin Aging: New Research; Nova Science: Hauppauge, NY, USA, 2012. [Google Scholar]
- Philips, N.; Samuel, P.; Parakandi, H.; Gopal, S.; Siomyk, H.; Ministro, A.; Thompson, T.; Borkow, G. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-β1 vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect. Tissue Res. 2012, 53, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Khorramizadeh, M.R.; Tredget, E.E.; Telasky, C.; Shen, Q.; Ghahary, A. Aging differentially modulates the expression of collagen and collagenase in dermal fibroblasts. Mol. Cell. Biochem. 1999, 194, 99–108. [Google Scholar] [CrossRef]
- Arrigo, A.P.; Virot, S.; Chaufour, S.; Firdaus, W.; Kretz-Remy, C.; Diaz-Latoud, C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid. Redox Signal. 2005, 7, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Hoshino, T.; Yamashita, Y.; Tanaka, K.; Maji, D.; Sato, K.; Adachi, H.; Sobue, G.; Ihn, H.; Funasaka, Y.; et al. Prevention of UVB radiation-induced epidermal damage by expression of heat shock protein 70. J. Biol. Chem. 2010, 285, 5848–5858. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann-Conrad, A.; Heydari, A.R.; You, S.; Richardson, A. The expression of heat shock protein 70 decreases with cellular senescence in vitro and in cells derived from young and old human subjects. Exp. Cell Res. 1998, 241, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.X.; Tang, J.J.; Luo, H.; Jin, X.L.; Dai, F.; Yang, J.; Qian, Y.P.; Li, X.Z.; Zhou, B. Antioxidant and antiproliferative activities of hydroxyl-substituted Schiff bases. Bioorg. Med. Chem. Lett. 2010, 20, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gazák, R.; Sedmera, P.; Vrbacký, M.; Vostálová, J.; Drahota, Z.; Marhol, P.; Walterová, D.; Kren, V. Molecular mechanisms of silybin and 2,3-dehydrosilybin antiradical activity—Role of individual hydroxyl groups. Free Radic. Biol. Med. 2009, 46, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Moon, K.Y.; Lee, J.; Kim, Y.S. Downregulation of NF-kappaB activation in human keratinocytes by melanogenic inhibitors. J. Dermatol. Sci. 2003, 31, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L.; Patterson, C.R.S.; Stapelberg, M.; Park, J.; Barnetson, R.S.; Halliday, G.M. Ultraviolet radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Investig. Dermatol. 2008, 128, 447–454. [Google Scholar] [PubMed]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Halliday, G.M.; Damian, D.L. Nicotinamide enhances repair of arsenic and UVR-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS ONE 2015, 10, e0117491. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: AB vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E.; Bertona, M.; Altabas, K.; Altabas, V.; Alessandrinin, G. Anti-inflammatory effect of a topical preparation containing nicotinamide, retinol, and 7-dehydrocholesterol in patients with acne: a gene expression study. Clin. Cosmet. Invest. Dermatol. 2012, 5, 33–37. [Google Scholar] [CrossRef]
- Kawada, A.; Konishi, N.; Oiso, N.; Kawara, S.; Date, A. Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J. Dermatol. 2008, 35, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Pinkas-Sarafova, A.; Markova, N.G.; Simon, M. Dynamic changes in nicotinamide pyridine dinucleotide content in normal human epidermal keratinocytes and their effect on retinoic acid biosynthesis. Biochem. Biophys. Res. Commun. 2005, 336, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R. Nicotinamide-containing sunscreens for use in Australasian countries and cancer-provoking conditions. Med. Hypotheses 2003, 60, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Oblong, J.E. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair 2014, 23, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Hillebrand, G.G.; Raleigh, P.; Li, J.; Marmor, M.J.; Bertucci, V.; Grimes, P.E.; Mandy, S.H.; Perez, M.I.; Weinkle, S.H.; et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br. J. Dermatol. 2010, 162, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.L.; Filho, P.C.; Neri, C.R.; Serra, O.A.; de Figueiredo, A.T.; Varela, J.A.; Longo, E. Synthesis and study of the photophysical properties of a new Eu3+ complex with 3-hydroxypicolinamide. J. Fluoresc. 2011, 21, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Imakubo, T.; Ichikawa, M.; Taniguchi, Y. A photoluminescent six-coordinated zinc(II) complex with hydroxides as axial ligands, [Zn(Hhpa)2(OH)2] (Hhpa = 3-hydroxypicolinamide). Dalton Trans. 2006, 21, 881–883. [Google Scholar] [CrossRef]
- Largeron, M.; Fleury, M.B. Acid-base properties of pristinamycin IA and related compounds. J. Pharm. Sci. 1992, 81, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Wessels, Q.; Pretorius, E.; Smith, C.M.; Nel, H. The potential of a niacinamide dominated cosmeceutical formulation on fibroblast activity and wound healing in vitro. Int. Wound J. 2014, 11, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014, 2, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, anti-oxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Bachelor, M.A.; Bowden, G.T. UVA-mediated activation of signaling pathways involved in skin tumor promotion and progression. Semin. Cancer Biol. 2004, 14, 131–138. [Google Scholar] [CrossRef] [PubMed]
- DiGiovanni, J. Multistage carcinogenesis in mouse skin. Pharmacol. Ther. 1992, 54, 63–128. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Photocarcinogenesis: UVA vsx. UVB. Methods Enzymol. 2000, 319, 359–366. [Google Scholar]
- Krutmann, J. The role of UVA rays in skin aging. Eur. J. Dermatol. 2001, 11, 170–171. [Google Scholar] [PubMed]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- DiPalma, J.R.; Thayer, W.S. Use of niacin as a drug. Annu. Rev. Nutr. 1991, 11, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Benavente, C.A.; Jacobson, M.K.; Jacobson, E.L. NAD in skin: Therapeutic approaches for niacin. Curr. Pharm. Des. 2009, 15, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Thappa, D.M. Pellagra and skin. Int. J. Dermatol. 2002, 41, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chong, Z.Z.; Maiese, K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front. Biosci 2004, 9, 2500–2520. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.M.; Rawling, J.M.; Roebuck, B.D.; Kirkland, J.B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 1995, 125, 1455–1461. [Google Scholar] [PubMed]
- Yui, R.; Matsuura, E.T. Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat. Res. 2006, 594, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Wang, D.; Hwang, C.P.; Liu, H.W.; Wei, J.; Lee, R.P.; Chen, H.I. The protective effect of niacinamide on ischemia-reperfusion-induced liver injury. J. Biomed. Sci. 2001, 8, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Traister, A.; Breitman, I.; Bar-Lev, E.; Zvibel, I.; Harel, A.; Halpern, Z.; Oren, R. Nicotinamide induces apoptosis and reduces collagen I and pro-inflammatory cytokines expression in rat hepatic stellate cells. Scand. J. Gastroenterol. 2005, 40, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z. Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003, 24, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.L.; Boyer, J.L. Hepatic toxicity from large doses of vitamin-B3 (Nicotinamide). N. Engl. J. Med. 1973, 289, 1180–1182. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
