Enhancement of Skin Permeation and Penetration of β-Arbutin Fabricated in Chitosan Nanoparticles as the Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-Arbutin Loaded CSNPs
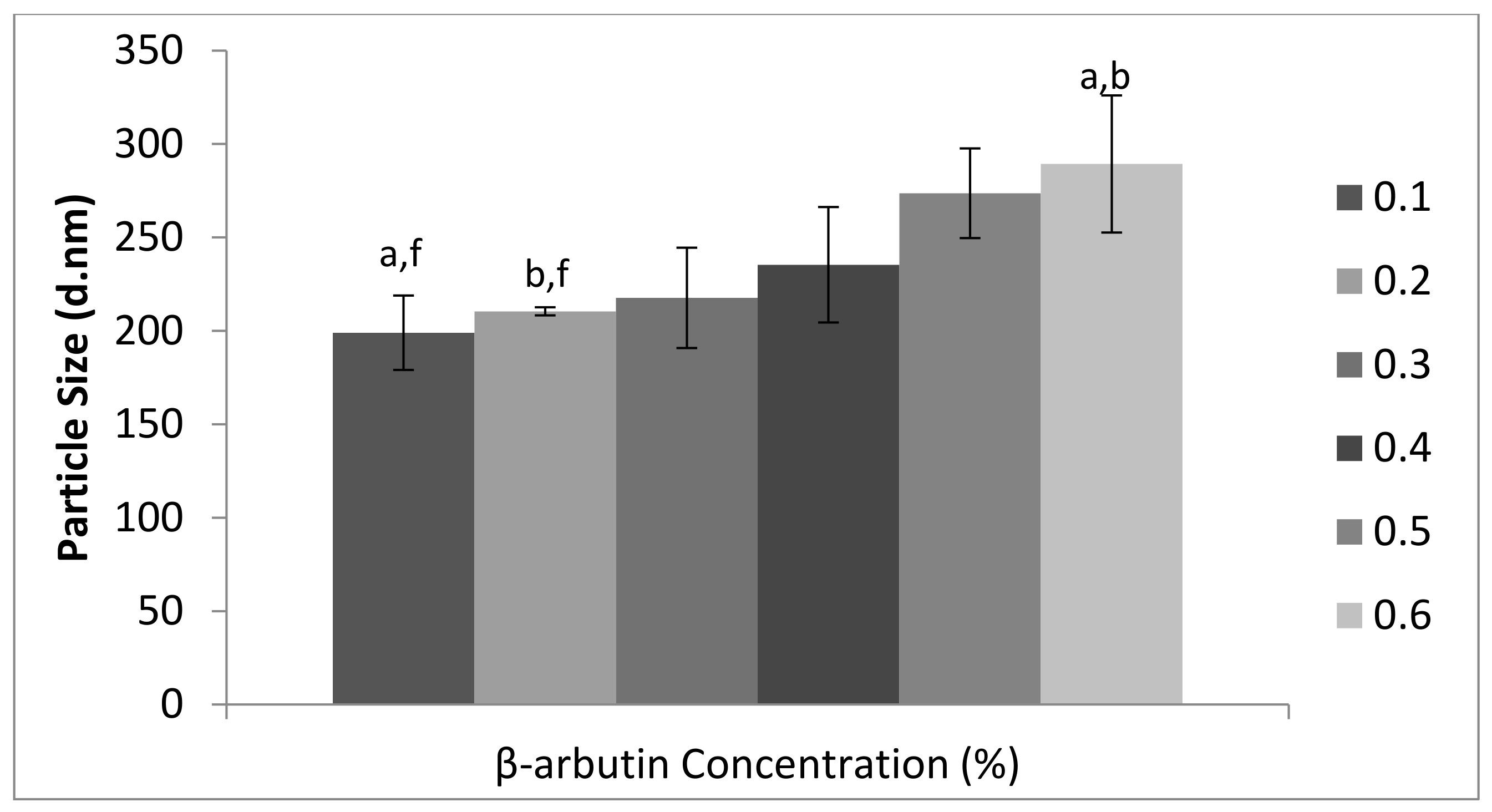
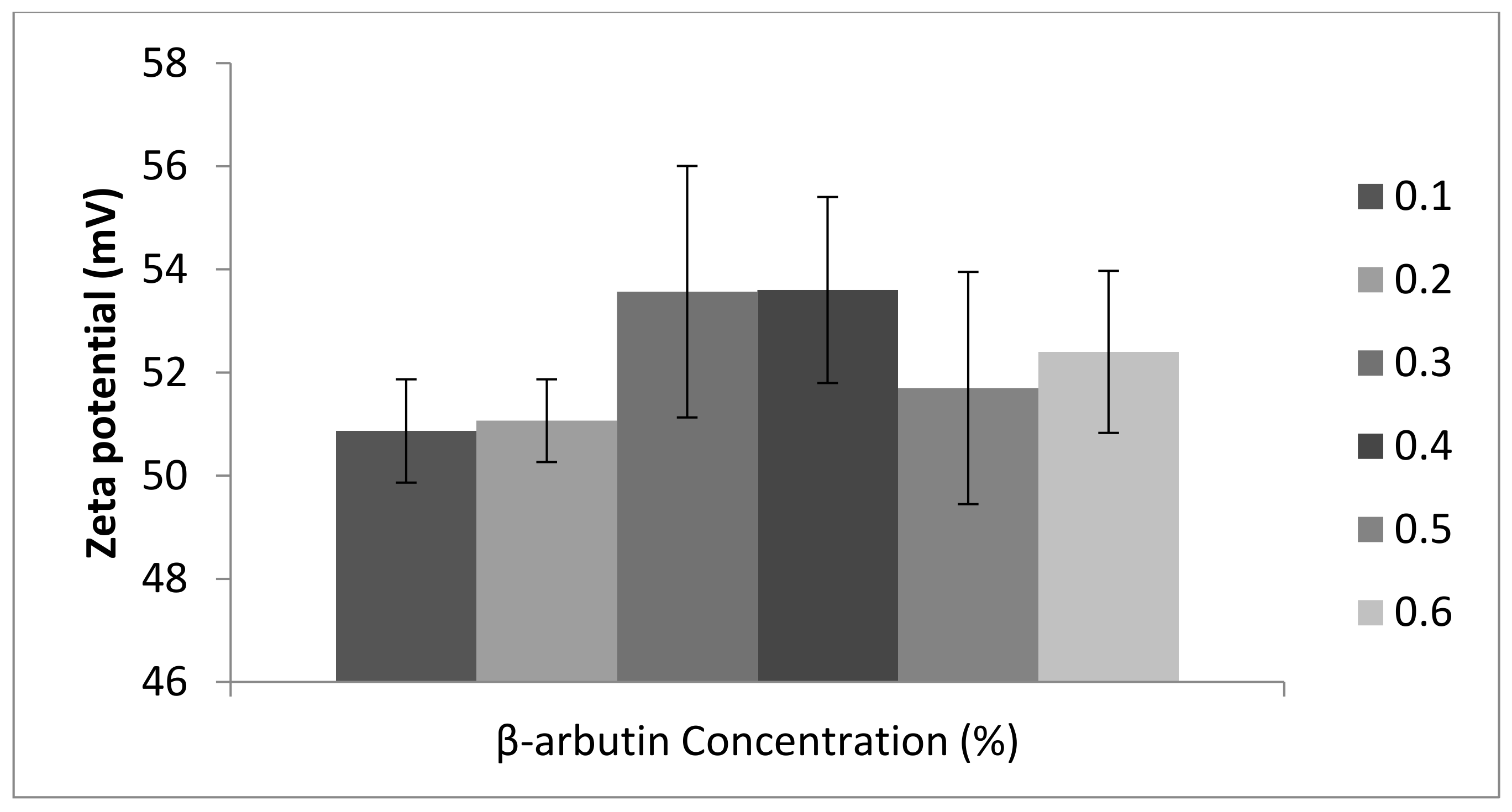
2.3. Evaluation of Polydispersity Index, Particle Size, and Zeta Potential
2.4. Evaluation of Loading Capacity (LC) and Entrapment Efficiency (EE)
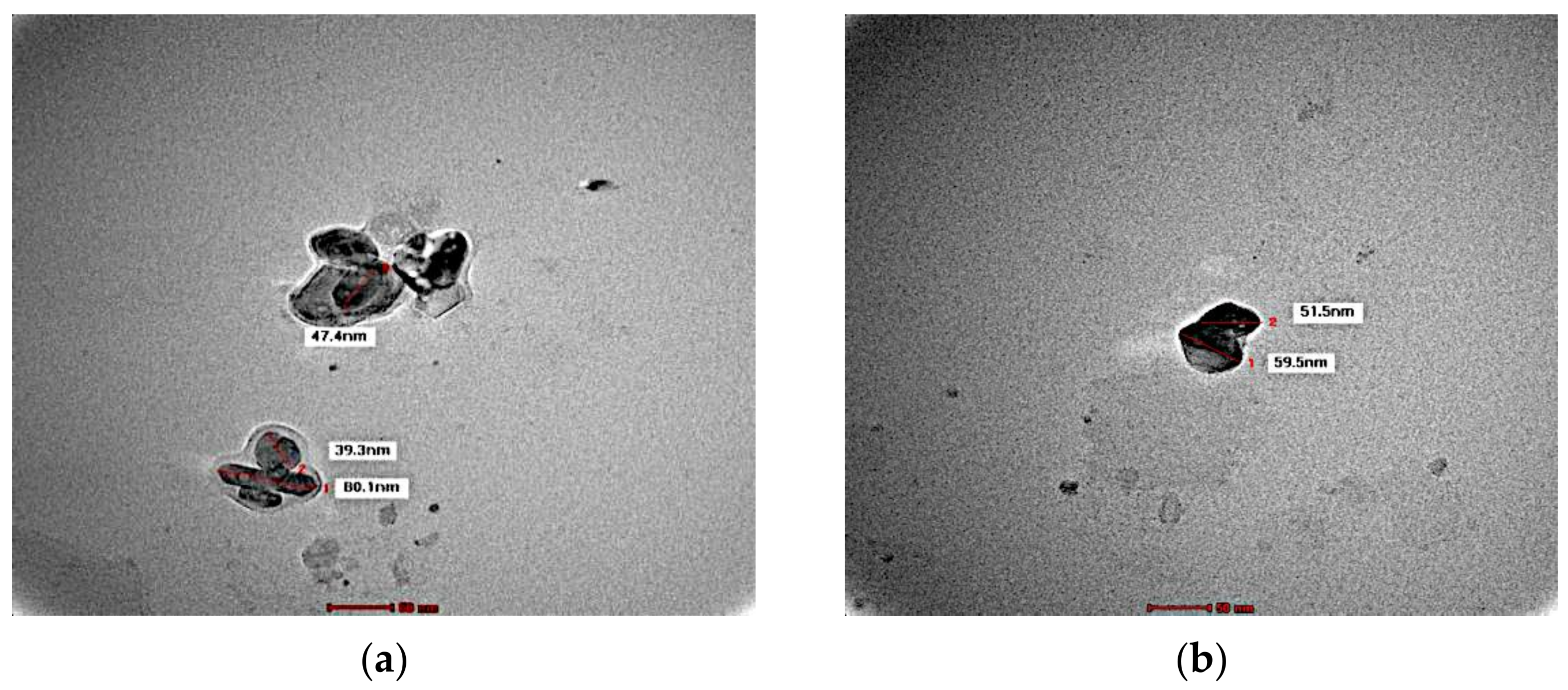
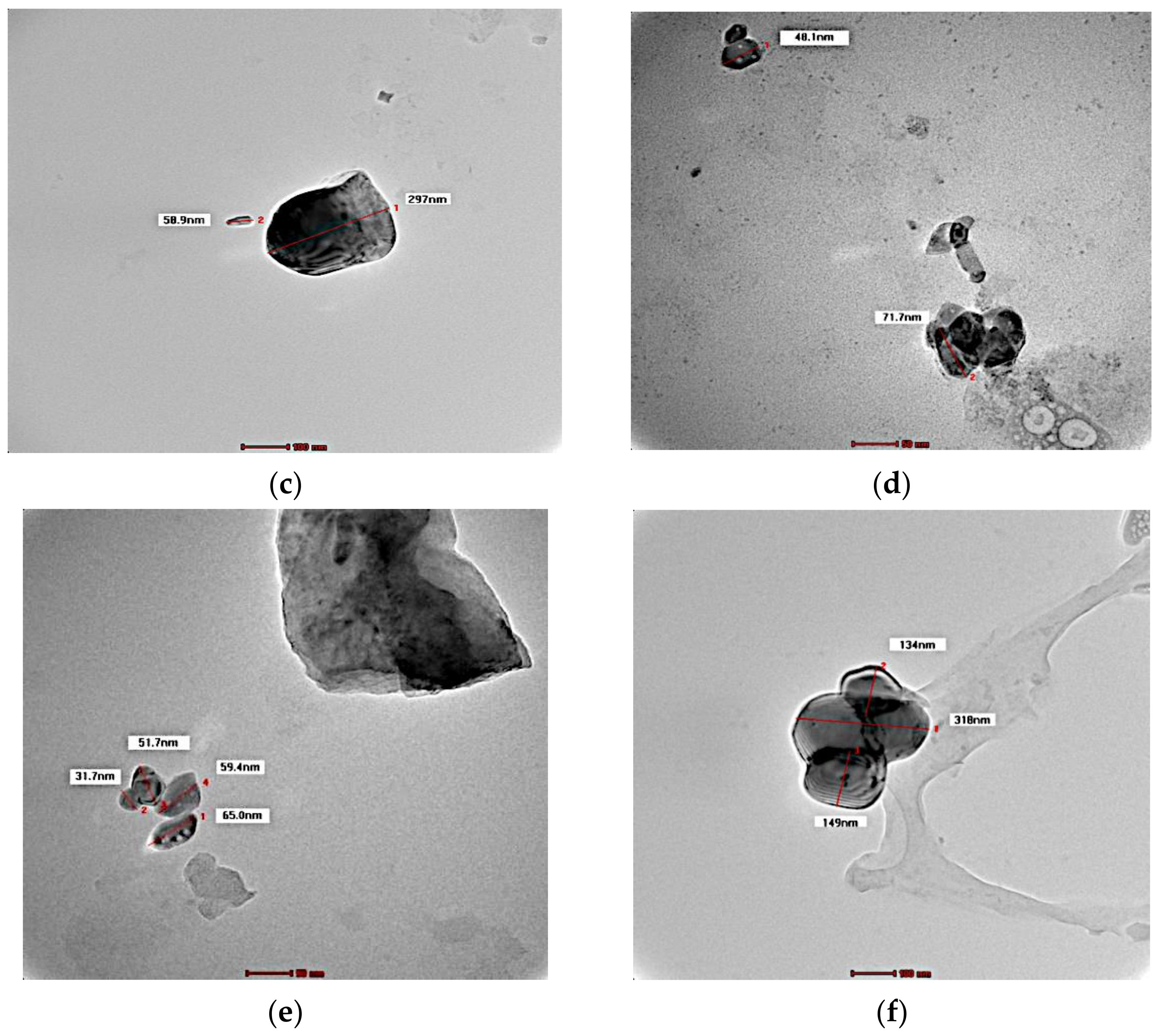
2.5. TEM Analysis
2.6. FTIR Analysis
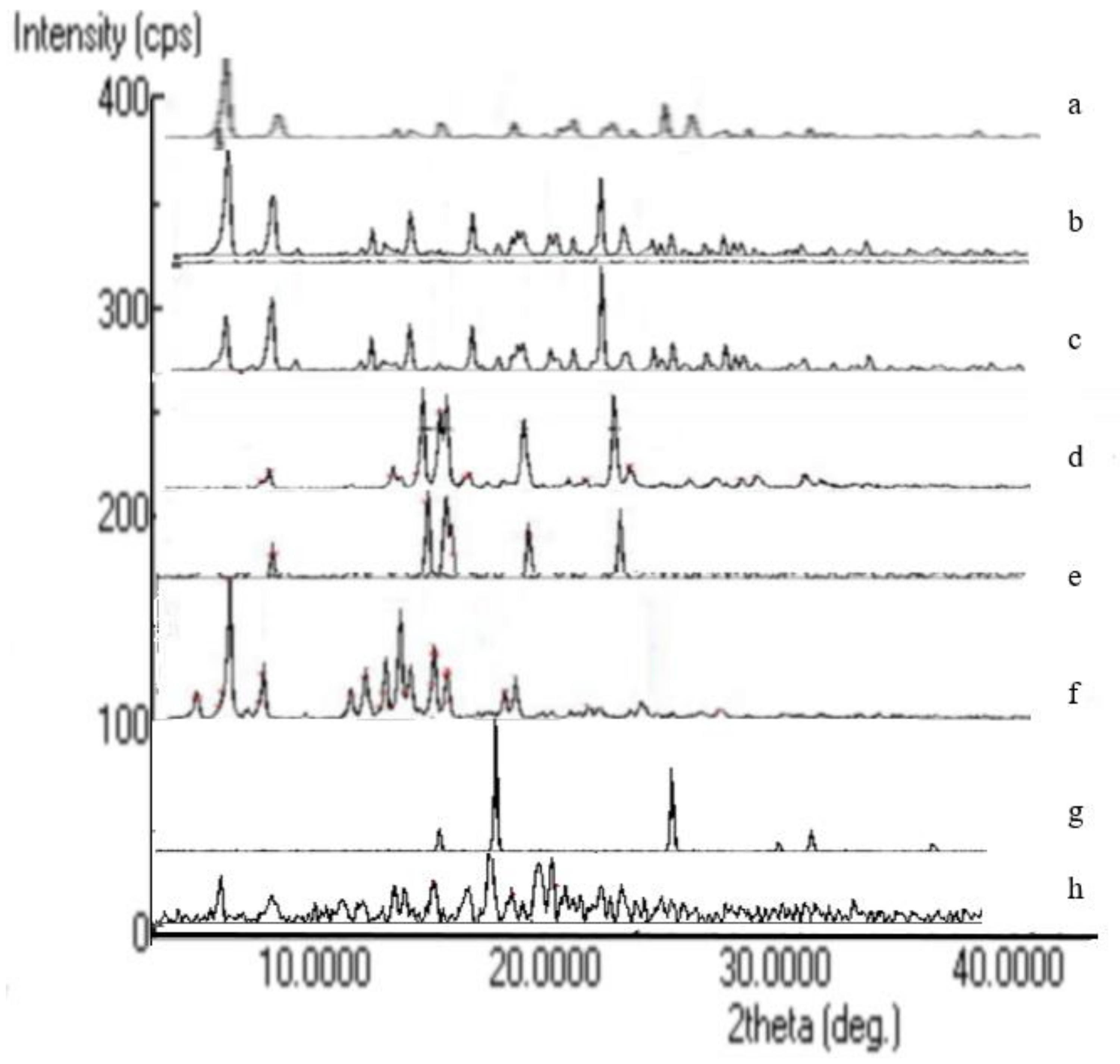
2.7. XRD Analysis
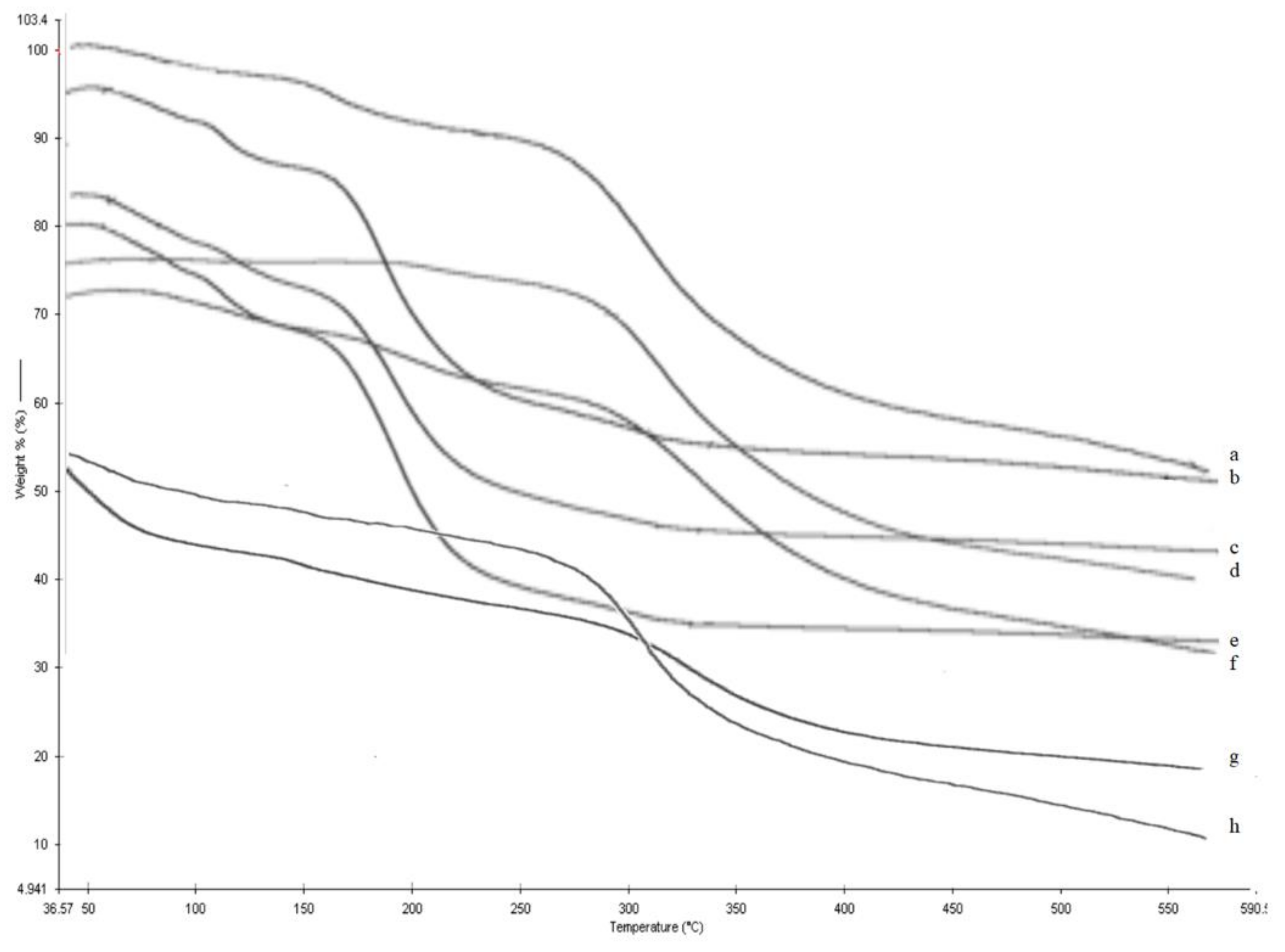
2.8. TGA Analysis
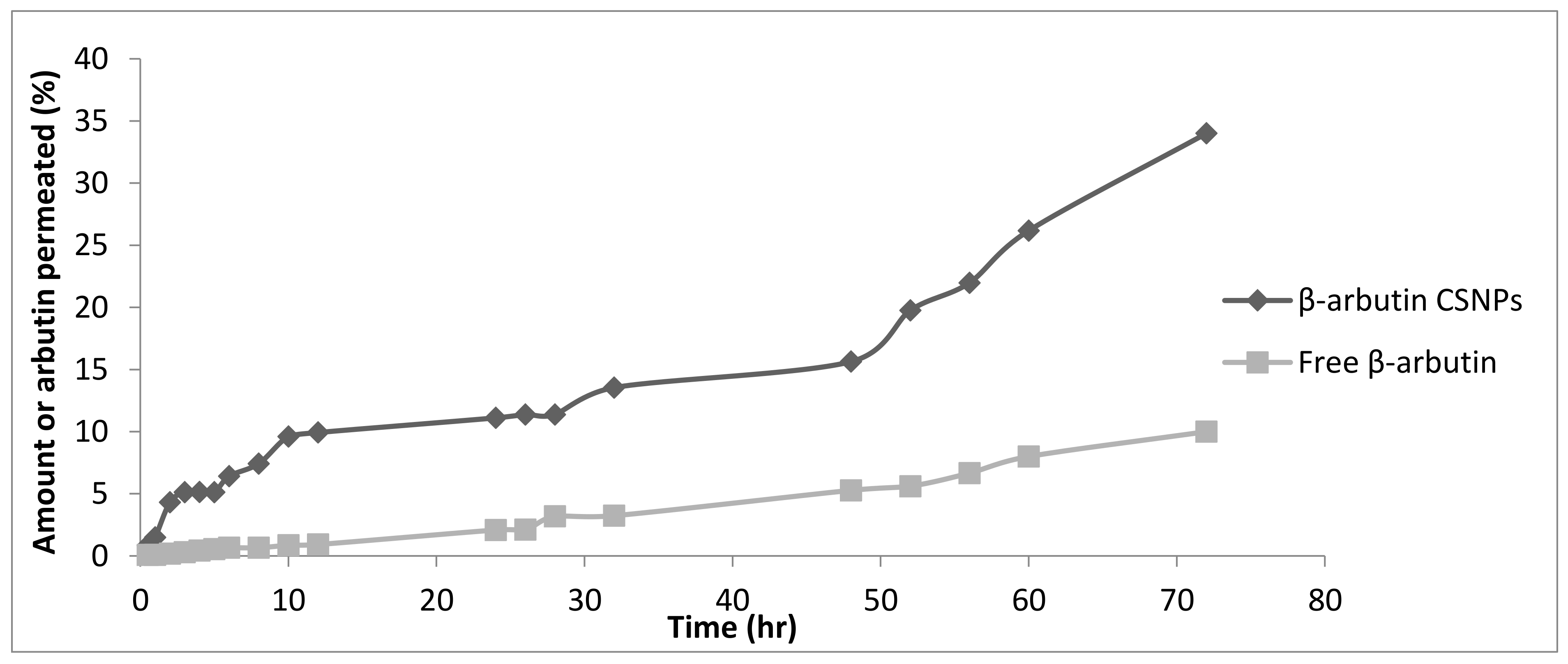
2.9. In Vitro Permeation Study
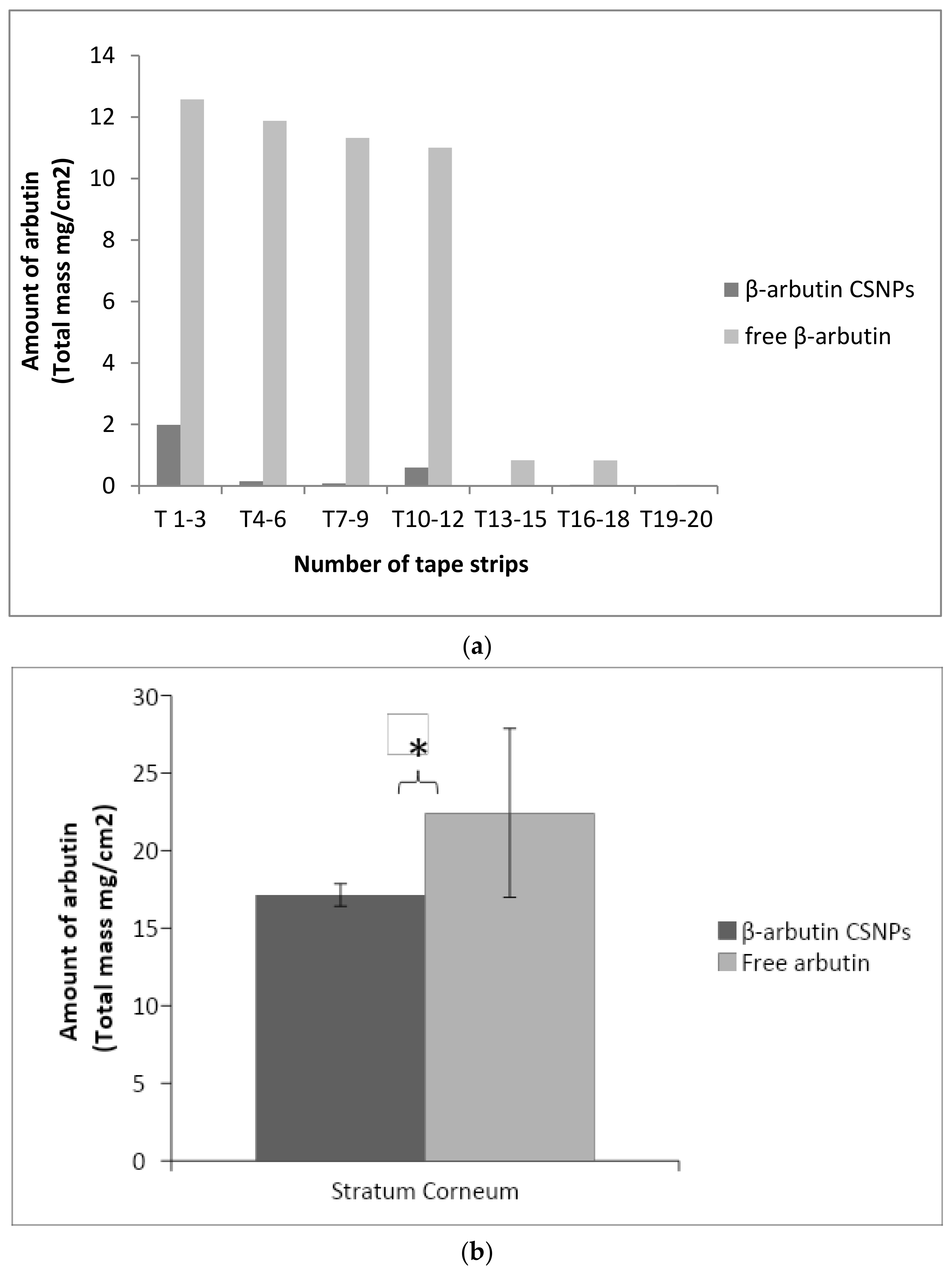
2.10. In Vitro Penetration Study
2.11. RP-HPLC System
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization β-Arbutin CSNPs
3.2. Efficiency (EE) and Loading Capacity (LC) of β-Arbutin CSNPs
3.3. Morphology
3.4. FTIR
3.5. XRD Analysis
3.6. TGA Analysis
3.7. In Vitro Permeation Study
3.8. In Vitro Penetration Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Adhikari, B.; Dhara, S. Development of chitosan & tripolyphosphate fibers through pH dependent ionotropic gelation. J. Carbohydr. Res. 2011, 346, 2582–2588. [Google Scholar]
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Curcumin-loaded N,O-carboxymethyl chitosan nanoparticles for cancer drug delivery. J. Biomater. Sci. 2012, 23, 1381–1400. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.C.; DeLouise, L.A. Nanoparticle-Enabled Transdermal Drug Delivery Systems for Enhanced Dose Control and Tissue Targeting. J. Investig. Dermatol. 2007, 127, 1701–1712. [Google Scholar] [CrossRef] [Green Version]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nano Biotechnol. 2004, 2, 3. [Google Scholar]
- Seo, D.-H.; Jung, J.-H.; Lee, J.-E.; Jeon, E.-J.; Kim, W.; Park, C.-S. Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives. Appl. Biochem. Biotechnol. 2012, 95, 1417–1425. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T. Chitosan nanoparticle as protein delivery carrier: Systematic examination of fabrication conditions for efficient loading and release. J. Colloid. Surface. B 2007, 59, 24–34. [Google Scholar] [CrossRef]
- Yassin, A.E.; Anwer, M.K.; Mowafy, H.A.; El-Bagory, I.M.; Bayomi, M.A.; Alsarra, I.A. Optimization of 5-fluorouracil solid-lipid nanoparticles: A preliminary study to treat colon cancer. Int. J. Med. Sci. 2010, 7, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.M.; Shi, L.E.; Zhang, Z.L.; Chen, J.M.; Shi, D.D.; Yang, J.; Tang, Z.X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Chaudhury, A.; Das, S. Recent Advancement of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents. AAPS PharmSciTech 2011, 12, 10–20. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-a-glycosides and a comparison of their inhibitory effects with those of a-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [Green Version]
- Antonio, R.; Massimiliano, B.; Paolo, B.B.; Bellicha, A.C. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar]
- Ghadiri, M.; Fatemi, S.; Vatanara, A.; Doroud, D.; Najafabadi, A.R.; Darabi, M.; Rahimi, A.A. Loading hydrophilic drug in solid lipid media as nanoparticles: Statistical modeling of entrapment efficiency and particle size. J. Pharma. 2012, 424, 128–137. [Google Scholar] [CrossRef]
- Liu, C.-Q.; Deng, L.; Zhang, P.; Zhang, S.-R.; Liu, L.; Xu, T.; Wang, F.; Tan, T.W. Screening of high α-arbutin producing strains and production of α-arbutin by fermentation. J. Microb. Biotechnol. 2013, 29, 1391–1398. [Google Scholar] [CrossRef]
- Agarwal, M.; Nagar, D.P.; Srivastava, N.; Agarwal, M.K. Chitosan Nanoparticles based Drug Delivery: An Update. Int. J. Adv. Multidiscip. Res. 2015, 2, 1–13. [Google Scholar]
- Frederiksen, K.; Guy, R.H.; Petersson, K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur. J. Pharm. Biopharm. 2015, 91, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.M.; Braga, C.R.; Fook, M.V.; Raposo, C.M.; Carvalho, L.H.; Canedo, E.L. Application of Infrared Spectroscopy to Analysis of Chitosan/Clay Nanocomposites Infrared Spectroscopy-Material Science, Engineering and Technology; IntechOpen Limited: London, UK, 2012; Chapter 3. [Google Scholar]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. Chitosan, and liposomes: A skin penetration study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef]
- Avonto, C.; Wang, Y.H.; Avula, B.; Wang, M.; Rua, D.; Khan, I.A. Investigation of the instability of alpha and beta-arbutin. J. Cosmet. Sci. 2014, 80, PPL23. [Google Scholar] [CrossRef]
- Clara, M.; Ruo, L.; Gianluca, C. Chitosan nanoparticles as therapeutic protein nano carriers: The effect of pH on particle formation and encapsulation efficiency. Polymer. Compos. 2013, 34, 1538–1545. [Google Scholar]
- Eunsun, L.; Sang, J.P.; Jae, H.L.; Min, S.K.; Chun, H.K. Preparation of Chitosan/TPP nanoparticles and their physical and biological properties. J. Pharm. Sci. 2016, 11, 166–167. [Google Scholar]
- Illum, L. Nanoparticulate systems for delivery of drugs: A real improvement over simple systems. J. Pharm. Al Sci. 2007, 96, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Calvo, P.; Alonso, M.J. Polysaccharide colloidal particles as delivery systems macromolecules. Adv. Drug Deliv. Rev. 2001, 47, 83–97. [Google Scholar] [CrossRef]
- Liu, B.R.; Chan, M.H.; Chen, H.H.; Lo, S.Y.; Huang, Y.W.; Lee, H.J. Effects of Surface Charge and Particle Size of Cell-Penetrating Peptide/Nanoparticle Complexes on Cellular Internalization in Cell Membrane; Mandraccia, L., Slavin, G., Eds.; Nova Science Publishers: New York, NY, USA, 2013; Chapter 3. [Google Scholar]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Shu, S.; Zhang, X.; Teng, D.; Wang, Z.; Li, C. Polyelectrolyte nanoparticles based on water-soluble chitosan-poly (Laspartic acid)-polyethylene glycol for controlled protein release. Carbohydr. Res. 2009, 344, 1197–1204. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Callari, M.; Lu, H.; Stenzel, M.H. Direct correlation between zeta potential and cellular uptake of poly (methacrylic acid) post modified with guanidinium functionalities. J. Macro Phys. 2016, 217, 2302–2309. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Ph.D. Thesis, Dublin City University, Northside, Dublin, 2013. [Google Scholar]
- Arezou, G.; Soleiman, M.; Fatemeh, T.; Farid, T. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. J. Intern Med. 2014, 5, 156–161. [Google Scholar]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Alonso. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef]
- Contri, R.V.; Fiel, L.A.; Alnasif, N.; Pohlmann, A.R.; Guterres, S.S.; Schäfer-Korting, M. Skin penetration and dermal tolerability of acrylic nanocapsules: Influence of the surface charge and a chitosan gel used as vehicle. Int. J. Pharm. 2016, 507, 12–20. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Rajan, M.; Raj, V. Encapsulation, characterisation and in-vitro release of anti-tuberculosis drug using chitosan poly ethylene glycol nanoparticles. Int. J. Pharm. Pharm. Sci. 2012, 4, 255–259. [Google Scholar]
- Manoj, K.S.; Sunil, K.P.; Alok, M.; Neha, R.; Singh, R. Chitosan: A novel excipient in pharmaceutical formulation: A review. Int. J. Pharma. Sci. Res. 2017, 2, 2266–2277. [Google Scholar]
- Chang, M.L.; Chang, C.M. Simultaneous HPLC determination of hydrophilic whitening agents in cosmetic products. J. Pharm. Biomed. Anal. 2003, 33, 617–626. [Google Scholar] [CrossRef]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv. Drug. Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Hussain, Z.; Katas, H.; Amin, M.C.; Kumolosasi, E.; Buang, F.; Sahudin, S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone/hydroxytyrosol: An ex vivo and in vivo study using an NC/Nga mouse model. Int. J. Pharm. 2013, 444, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Singka, G.S.; Samah, N.A.; Zulfakar, M.H.; Yurdasiper, A.; Heard, C.M. Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Eur. J. Pharm. Biopharm. 2010, 76, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Hoeller, S.; Sperge, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Cazares-Delgadillo, J.; Naik, A.; Kalia, Y.N.; Quintanar Guerrero, D.; Ganem-Quintanar, A. Skin permeation enhancement by sucrose esters: A pH-dependent phenomenon. Int. J. Pharm. 2005, 297, 204–212. [Google Scholar] [CrossRef]
- Prego, C.; Garcia, M.; Torres, D.; Alonso, M. Transdermal nano molecular chitosan drug delivery. J. Control. Release 2005, 101, 151. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Yu, W.; Xu, L.; Ge, C.; Liu, J.; Gu, N. Linear aggregation of gold nanoparticles in ethanol. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 177–183. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.N.; Sahudin, S.; Lazim, A.M.; Azfaralariff, A. Formulation and In-Vitro Characterisation of Cross-Linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021, 110, 3907–3918. [Google Scholar] [CrossRef]
- Jahagirdar, P.; Lokhande, A.S.; Dandekar, P.; Devarajan, P.V. Mannose receptor and targeting strategies. In Targeted Intracellular Drug Delivery by Receptor Mediated Endocytosis; Springer: Cham, Switzerland, 2019; pp. 433–456. [Google Scholar]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Mitra, S.; Biswas, S.; Banerjee, E.R. Mannose-rich guar gum nanoparticles as a novel therapeutic drug against inflammatory diseases. J. Mol. Biochem. 2018, 7, 14–27. [Google Scholar]

| β-Arbutin Concentration | Entrapment Efficiency (%) | Loading Capacity (%) |
|---|---|---|
| 0.1% | 43.3 | 56.2 |
| 0.2% | 45.0 | 45.2 |
| 0.3% | 50.3 | 47.0 |
| 0.4% | 68.0 | 73.8 |
| 0.5% | 54.3 | 58.1 |
| 0.6% | 56.3 | 61.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahudin, S.; Sahrum Ayumi, N.; Kaharudin, N. Enhancement of Skin Permeation and Penetration of β-Arbutin Fabricated in Chitosan Nanoparticles as the Delivery System. Cosmetics 2022, 9, 114. https://doi.org/10.3390/cosmetics9060114
Sahudin S, Sahrum Ayumi N, Kaharudin N. Enhancement of Skin Permeation and Penetration of β-Arbutin Fabricated in Chitosan Nanoparticles as the Delivery System. Cosmetics. 2022; 9(6):114. https://doi.org/10.3390/cosmetics9060114
Chicago/Turabian StyleSahudin, Shariza, Nursyafiqah Sahrum Ayumi, and Norsavina Kaharudin. 2022. "Enhancement of Skin Permeation and Penetration of β-Arbutin Fabricated in Chitosan Nanoparticles as the Delivery System" Cosmetics 9, no. 6: 114. https://doi.org/10.3390/cosmetics9060114




