Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions
Abstract
1. Introduction
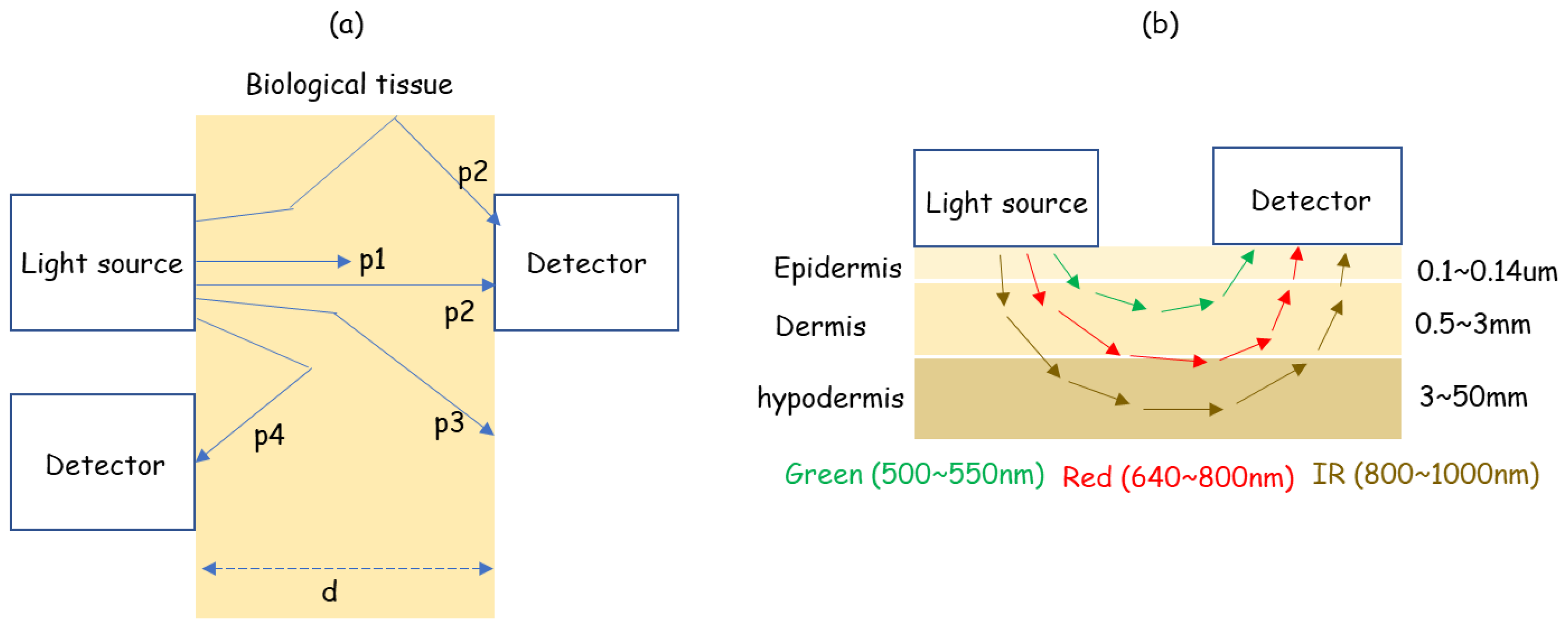
2. Working Principle
3. Technological Advances
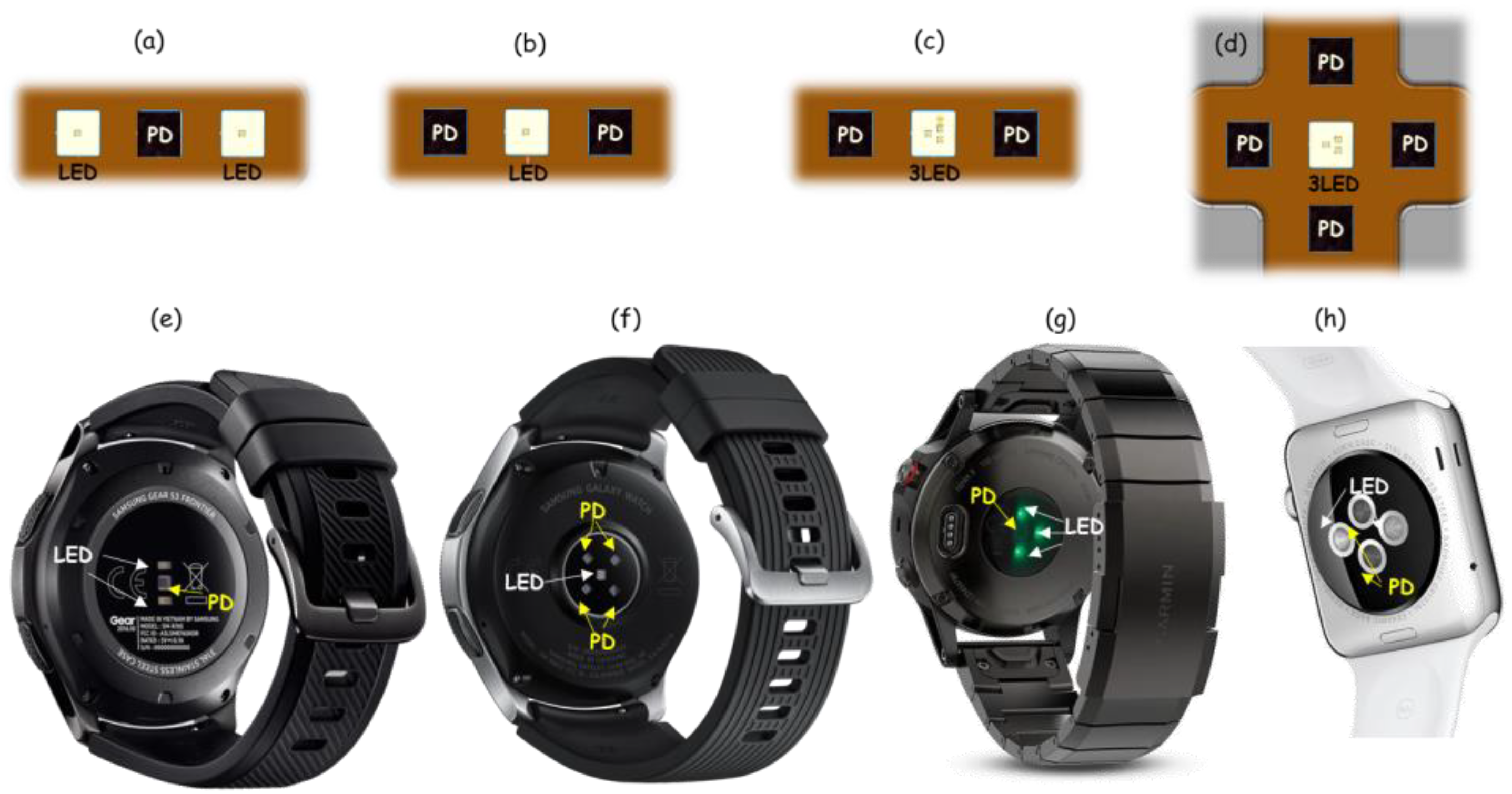
3.1. Sensor
3.2. Low-Power-Consumption System
3.3. PPG Signal Applications
4. Current Challenges
4.1. Sensor: LED Wavelength
4.2. Low-Power-Consumption System: Parabola Approximation
4.3. Multi-Wavelength PPG Signal Applications
5. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Lu, L.; Gao, F.; He, S.-J.; Zhao, H.-J.; Fang, Y.; Yang, J.-M.; An, Y.; Ye, Z.-W.; Dong, Z. Integration of artificial intelligence, blockchain, and wearable technology for chronic disease management: A new paradigm in smart healthcare. Curr. Med. Sci. 2021, 41, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.C.-P.; Mok, S.T.K.; Chow, I.S.W.; Chin, W.L. The opportunity to unlock the architecture of healthcare model: Chiropractic care-at-home. J. Contemp. Chiropr. 2022, 5, 44–49. [Google Scholar]
- Lim, Y.G.; Hong, K.H.; Kim, K.K.; Shin, J.H.; Lee, S.M.; Chung, G.S.; Baek, H.J.; Jeong, D.-U.; Park, K.S. Monitoring physiological signals using nonintrusive sensors installed in daily life equipment. Biomed. Eng. Lett. 2011, 1, 11–20. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, D. Healthcare wearable devices: An analysis of key factors for continuous use intention. Serv. Bus. 2020, 14, 503–531. [Google Scholar] [CrossRef]
- Ahmed, A.; Aziz, S.; Abd-alrazaq, A.; Farooq, F.; Sheikh, J. Overview of Artificial Intelligence–Driven Wearable Devices for Diabetes: Scoping Review. J. Med. Internet Res. 2022, 24, e36010. [Google Scholar] [CrossRef]
- Miller, D.J.; Sargent, C.; Roach, G.D. A Validation of Six Wearable Devices for Estimating Sleep, Heart Rate and Heart Rate Variability in Healthy Adults. Sensors 2022, 22, 6317. [Google Scholar] [CrossRef]
- Cho, J. Current status and prospects of health-related sensing technology in wearable devices. J. Healthc. Eng. 2019, 2019, 3924508. [Google Scholar] [CrossRef]
- Steven Eyobu, O.; Han, D.S. Feature Representation and Data Augmentation for Human Activity Classification Based on Wearable IMU Sensor Data Using a Deep LSTM Neural Network. Sensors 2018, 18, 2892. [Google Scholar] [CrossRef]
- Fotouhi-Ghazvini, F.; Abbaspour, S. Wearable Wireless Sensors for Measuring Calorie Consumption. J. Med. Signals Sens. 2020, 10, 19–34. [Google Scholar] [CrossRef]
- Pham, T.T.; Duong, H.T.; Suh, Y.S. Opportunistic Calibration Method for Walking Distance Estimation Using a Waist-Mounted Inertial Sensor. IEEE Trans. Instrum. Meas. 2020, 69, 7906–7913. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28, R1–R39. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar]
- Loh, H.W.; Xu, S.; Faust, O.; Ooi, C.P.; Barua, P.D.; Chakraborty, S.; Tan, R.-S.; Molinari, F.; Acharya, U.R. Application of photoplethysmography signals for healthcare systems: An in-depth review. Comput. Methods Programs Biomed. 2022, 216, 106677. [Google Scholar] [CrossRef]
- Biswas, D.; Simões-Capela, N.; Van Hoof, C.; Van Helleputte, N. Heart Rate Estimation from Wrist-Worn Photoplethysmography: A Review. IEEE Sens. J. 2019, 19, 6560–6570. [Google Scholar] [CrossRef]
- Maity, A.K.; Veeraraghavan, A.; Sabharwal, A. PPGMotion: Model-based detection of motion artifacts in photoplethysmography signals. Biomed. Signal Process. Control 2022, 75, 103632. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Schmitt, J.M. Simple photon diffusion analysis of the effects of multiple scattering on pulse oximetry. IEEE Trans. Biomed. Eng. 1991, 38, 1194–1203. [Google Scholar] [CrossRef]
- Rolfe, P. In Vivo Near-Infrared Spectroscopy. Annu. Rev. Biomed. Eng. 2000, 2, 715–754. [Google Scholar] [CrossRef]
- Kumar, G.; Schmitt, J.M. Optimal probe geometry for near-infrared spectroscopy of biological tissue. Appl. Opt. 1997, 36, 2286–2293. [Google Scholar] [CrossRef]
- McCully, K.; Hamaoka, T. Near-infrared spectroscopy: What can it tell us about oxygen saturation in skeletal muscle. Exerc. Sport Sci. Rev. 2000, 28, 123–127. [Google Scholar]
- Maikala, R.V. Modified Beer’s Law—Historical perspectives and relevance in near-infrared monitoring of optical properties of human tissue. Int. J. Ind. Ergon. 2010, 40, 125–134. [Google Scholar] [CrossRef]
- Wukitsch, M.W.; Petterson, M.T.; Tobler, D.R.; Pologe, J.A. Pulse oximetry: Analysis of theory, technology, and practice. J. Clin. Monit. Comput. 1998, 4, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.L.; Hill, A.V. The oxygen-dissociation curve of blood, and its thermodynamical basis. Proc. R. Soc. Lond. Ser. B 1923, 94, 297–334. [Google Scholar]
- Maxim Integrated. MAX30101 Data Sheet. 2020. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/MAX30101.pdf (accessed on 18 May 2023).
- Maxim Integrated. MAX86150 Data Sheet. 2018. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/MAX86150.pdf (accessed on 18 May 2023).
- Maxim Integrated. MAX86916 Data Sheet. 2019. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/MAX86916.pdf (accessed on 18 May 2023).
- Analog Devices. ADPD144RI Data Sheet. 2019. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/ADPD144RI.pdf (accessed on 18 May 2023).
- Analog Devices. ADPD188GG Data Sheet. 2018. Available online: https://www.analog.com/media/en/technical-documentation/data-sheets/adpd188gg.pdf (accessed on 18 May 2023).
- Osram Opto Semiconductors. SFH 7051 Data Sheet. 2016. Available online: https://look.ams-osram.com/m/1bc51f319852c566/original/SFH-7051.pdf (accessed on 18 May 2023).
- Osram Opto Semiconductors. SFH 7072 Data Sheet. 2022. Available online: https://look.ams-osram.com/m/682b32d8c8dd3713/original/SFH-7072.pdf (accessed on 18 May 2023).
- Osram Opto Semiconductors. SFH 7050 Data Sheet. 2016. Available online: https://look.ams-osram.com/m/470e18924403347a/original/SFH-7050.pdf (accessed on 18 May 2023).
- AMS AG. AS7024 Data Sheet. 2018. Available online: https://ams.com/documents/20143/36005/AS7024_DS000469_4-00.pdf (accessed on 18 May 2023).
- AMS AG. AS7-26GG Data Sheet. 2021. Available online: https://look.ams-osram.com/m/6615cb0b344731f0/original/AS7026GG-DS000622.pdf (accessed on 18 May 2023).
- Baek, H.J.; Shin, J.; Cho, J. The Effect of Optical Crosstalk on Accuracy of Reflectance-Type Pulse Oximeter for Mobile Healthcare. J. Healthc. Eng. 2018, 2018, 3521738. [Google Scholar] [CrossRef]
- NXP Semiconductors. Kinetis KL03 Data Sheet. 2022. Available online: https://www.nxp.com/docs/en/data-sheet/KL03P24M48SF0.pdf (accessed on 18 May 2017).
- Zhang, D.; Liu, Z.; Wu, Y.; Ji, S.; Yuan, Z.; Liu, J.; Zhu, M. In Situ Construction a Stable Protective Layer in Polymer Electrolyte for Ultralong Lifespan Solid-State Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104277. [Google Scholar] [CrossRef]
- Ownby, N.B.; Flynn, K.A.; Calhoun, B.H. Modeling Energy Aware Photoplethysmography for Personalized Healthcare Applications. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 570–579. [Google Scholar] [CrossRef]
- Lee, E.; Lee, C. PPG-Based Smart Wearable Device with Energy-Efficient Computing for Mobile Health-Care Applications. IEEE Sens. J. 2021, 21, 13564–13573. [Google Scholar] [CrossRef]
- Lin, B.; Ma, Z.; Atef, M.; Ying, L.; Wang, G. Low-Power High-Sensitivity Photoplethysmography Sensor for Wearable Health Monitoring System. IEEE Sens. J. 2021, 21, 16141–16151. [Google Scholar] [CrossRef]
- Ortegón-Aguilar, J.; Castillo-Atoche, A.; Becerra-Nuñez, G.; Estrada-López, J.J.; Osorio-de-la-Rosa, E.; Carrasco-Alvarez, R.; Datta, A.; Vázquez-Castillo, J. Multimodal Power Management Based on Decision Tree for Internet of Wearable Things Systems. Appl. Sci. 2023, 13, 4351. [Google Scholar] [CrossRef]
- Ghasemzadeh, H.; Amini, N.; Saeedi, R.; Sarrafzadeh, M. Power-Aware Computing in Wearable Sensor Networks: An Optimal Feature Selection. IEEE Trans. Mob. Comput. 2015, 14, 800–812. [Google Scholar] [CrossRef]
- Kalantarian, H.; Sideris, C.; Mortazavi, B.; Alshurafa, N.; Sarrafzadeh, M. Dynamic Computation Offloading for Low-Power Wearable Health Monitoring Systems. IEEE Trans. Biomed. Eng. 2017, 64, 621–628. [Google Scholar] [CrossRef]
- Liang, J.M.; Chen, J.J.; Cheng, H.H.; Tseng, Y.C. An energy-efficient sleep scheduling with QoS consideration in 3GPP LTE-advanced networks for Internet of things. IEEE J. Emerg. Sel. Topics Circuits Syst. 2013, 3, 13–22. [Google Scholar] [CrossRef]
- Wu, H.; Chen, C.; Weng, K. An Energy-Efficient Strategy for Microcontrollers. Appl. Sci. 2021, 11, 2581. [Google Scholar] [CrossRef]
- Rezaie, H.; Ghassemian, M. An Adaptive Algorithm to Improve Energy Efficiency in Wearable Activity Recognition Systems. IEEE Sens. J. 2017, 17, 5315–5323. [Google Scholar] [CrossRef]
- Covi, E.; Donati, E.; Liang, X.; Kappel, D.; Heidari, H.; Payvand, M.; Wang, W. Adaptive Extreme Edge Computing for Wearable Devices. Front. Neurosci. 2021, 15, 611300. [Google Scholar] [CrossRef]
- Choi, A.; Shin, H. Photoplethysmography sampling frequency: Pilot assessment of how low can we go to analyze pulse rate variability with reliability? Physiol. Meas. 2017, 38, 586. [Google Scholar] [CrossRef]
- Béres, S.; Hejjel, L. The minimal sampling frequency of the photoplethysmogram for accurate pulse rate variability parameters in healthy valunteers. Biomed. Signal Process. Control 2021, 68, 102589. [Google Scholar] [CrossRef]
- Peláez-Coca, M.D.; Hernando, A.; Lázaro, J.; Gil, E. Impact of the PPG Sampling Rate in the Pulse Rate Variability Indices Evaluating Several Fiducial Points in Different Pulse Waveforms. IEEE J. Biomed. Health Inform. 2022, 26, 539–549. [Google Scholar] [CrossRef]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Tobin, M.A.; Simango, B.; Buote, R.; Van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR mHealth uHealth 2020, 8, e18694. [Google Scholar] [CrossRef]
- Schäfer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies mhealth comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, R. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 2010, 31, 1271. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Yoon, C. Multilevel mental stress detection using ultra-short pulse rate variability series. Biomed. Signal Process. Control 2020, 57, 101736. [Google Scholar] [CrossRef]
- Pugliese, L.; Violante, M.; Groppo, S. A Novel Algorithm for Detecting the Drowsiness Onset in Real-Time. IEEE Access 2022, 10, 42601–42606. [Google Scholar] [CrossRef]
- Wulterkens, B.M.; Fonseca, P.; Hermans, L.W.; Ross, M.; Cerny, A.; Anderer, P.; Long, X.; van Dijk, J.P.; Vandenbussche, N.; Pillen, S.; et al. It is all in the wrist: Wearable sleep staging in a clinical population versus reference polysomnography. Nat. Sci. Sleep 2021, 13, 885–897. [Google Scholar] [CrossRef]
- Mukkamala, R.; Stergiou, G.S.; Avolio, A.P. Cuffless Blood Pressure Measurement. Annu. Rev. Biomed. Eng. 2022, 24, 203–230. [Google Scholar] [CrossRef]
- Islam, S.M.S.; Chow, C.K.; Daryabeygikhotbehsara, R.; Subedi, N.; Rawstorn, J.; Tegegne, T.; Karmakar, C.; Siddiqui, M.U.; Lambert, G.; Maddison, R. Wearable cuffless blood pressure monitoring devices: A systematic review and meta-analysis. Eur. Heart J. Digit. Health 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Yao, P.; Xue, N.; Yin, S.; You, C.; Guo, Y.; Shi, Y. Multi-Dimensional Feature Combination Method for Continuous Blood Pressure Measurement Based on Wrist PPG Sensor. IEEE J. Biomed. Health Inform. 2022, 26, 3708–3719. [Google Scholar] [CrossRef]
- Shi, W.; Zhou, C.; Zhang, Y.; Li, K.; Ren, X.; Liu, H.; Ye, X. Hybrid modeling on reconstitution of continuous arterial blood pressure using finger photoplethysmography. Biomed. Signal Process. Control 2023, 85, 104972. [Google Scholar] [CrossRef]
- Monte-Moreno, E. Non-invasive estimation of blood glucose and blood pressure from a photoplethysmography by means of machine learning techniques. Artif. Intell. Med. 2011, 53, 127–128. [Google Scholar] [CrossRef]
- Tsai, C.; Li, C.; Lam, R.; Li, C.; Ho, S. Diabetes Care in Motion: Blood Glucose Estimation Using Wearable Devices. IEEE Consum. Electron. Mag. 2020, 8, 30–34. [Google Scholar] [CrossRef]
- Islam, T.; Ahmed, M.; Hassanuzzaman, M.; Amir, S.B.; Rahman, T. Blood Glucose Level Regression for Smartphone PPG Signals Using Machine Learning. Appl. Sci. 2021, 11, 618. [Google Scholar] [CrossRef]
- Prabha, A.; Yadav, J.; Rani, A.; Singh, V. Design of intelligent diabetes mellitus detection system using hybrid feature selection based on XGBoost classifier. Comput. Biol. Med. 2021, 136, 104664. [Google Scholar] [CrossRef]
- Prabha, A.; Yadav, J.; Rani, A.; Singh, V. Intelligent estimation of blood glucose level using wristband PPG signal and physiological parameters. Biomed. Signal Process. Control 2022, 78, 103876. [Google Scholar] [CrossRef]
- Boukhechba, M.; Cai, L.; Wu, C.; Barnes, L.E. ActiPPG: Using deep neural networks for activity recognition from wrist-worn photoplethysmography (PPG) sensors. Smart Health 2019, 14, 100082. [Google Scholar] [CrossRef]
- Zonios, G.; Dimou, A.; Bassukas, I.; Galaris, D.; Tsolakidis, A.; Kaxiras, E. Melanin absorption spectroscopy: New method for noninvasive skin investigation and melanoma detection. J. Biomed. Opt. 2008, 13, 014017. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Kochubey, V.I.; Tuchin, V.V. Optical properties of human skin subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 2005, 38, 2543–2555. [Google Scholar] [CrossRef]
- Lemay, M.; Bertschi, M.; Sola, J.; Renevey, P.; Parak, J.; Korhonen, I. Application of optical heart rate monitoring. In Wearable Sensors: Fundamentals Implementation and Applications; Academic Press: Cambridge, MA, USA, 2014; pp. 105–129. [Google Scholar]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical properties of human skin. J. Biomed. Opt. 2012, 17, 909011. [Google Scholar] [CrossRef]
- Taroni, P.; Pifferi, A.; Torricelli, A.; Comelli, D.; Cubeddu, R. In vivo absorption and scattering spectroscopy of biological tissues. Photochem. Photobiol. Sci. 2003, 2, 124–129. [Google Scholar] [CrossRef]
- Mannheimer, P.D.; Cascini, J.R.; Fein, M.E.; Nierlich, S.L. Wavelength selection for low-saturation pulse oximetry. IEEE Trans. Biomed. Eng. 1997, 44, 148–158. [Google Scholar] [CrossRef]
- Sinex, J.E. Pulse oximetry: Principle and limitations. AM J. Emerg. Med. 1999, 17, 59–66. [Google Scholar] [CrossRef]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A smart health monitoring chair for nonintrusive measurement of biological signals. IEEE Trans. Inform. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Naschitz, J.E.; Bezobchuk, S.; Mussafia-Priselac, R.; Sundick, S.; Dreyfuss, D.; Khorshidi, I.; Karidis, A.; Manor, H.; Nagar, M.; Peck, E.R.; et al. Pulse Transit Time by R-Wave-Gated Infrared Photoplethysmography: Review of the Literature and Personal Experience. J. Clin. Monit. Comput. 2004, 18, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, R.; Karadaglic, D. A LED-LED-based photoplethysmography sensor. Physiol. Meas. 2007, 28, N19. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.F.; Zhang, Y.T. The effect of contacting force on photoplethysmographic signals. Physiol. Meas. 2004, 25, 1323. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T. The Advantages of Wearable Green Reflected Photoplethysmography. J. Med. Syst. 2011, 35, 829–834. [Google Scholar] [CrossRef]
- Mainster, M.A. Wavelength Selection in Macular Photocoagulation: Tissue Optics, Thermal Effects, and Laser Systems. Ophthalmology 1986, 93, 952–958. [Google Scholar] [CrossRef]
- Matsumura, K.; Rolfe, P.; Lee, J.; Yamakoshi, T. iPhone 4s photoplethysmography: Which light color yields the most accurate heart rate and normalized pulse volume using the iPhysioMeter application in the presence of motion artifact? PLoS ONE 2014, 9, e91205. [Google Scholar] [CrossRef]
- Shao, D.; Liu, C.; Tsow, F.; Yang, Y.; Du, Z.; Iriya, R.; Yu, H.; Tao, N. Noncontact Monitoring of Blood Oxygen Saturation Using Camera and Dual-Wavelength Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 1091–1098. [Google Scholar] [CrossRef]
- Wijshoff, R.W.C.G.R.; Veen, J.; Van der Lee, A.M.; Mulder, L.; Stijnen, M.; Van Tuijl, S.; Aarts, R.M. PPG motion artifact handling using a self-mixing interferometric sensor. Proc. SPIE 2011, 7894, 78940F. [Google Scholar]
- Lee, J.; Matsumura, K.; Yamakoshi, K.I.; Rolfe, P.; Tanaka, S.; Yamakoshi, T. Comparison between red, green and blue light reflection photoplethysmography for heart rate monitoring during motion. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2013, 1724–1727. [Google Scholar]
- Lee, B.; Han, J.; Baek, H.J.; Shin, J.H.; Park, K.S.; Yi, W.J. Improved elimination of motion artifacts from a photoplethysmographic signal using a Kalman smoother with simultaneous accelerometry. Physiol. Meas. 2010, 31, 1585. [Google Scholar] [CrossRef]
- Ram, M.R.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Reddy, K.A. A Novel Approach for Motion Artifact Reduction in PPG Signals Based on AS-LMS Adaptive Filter. IEEE Trans. Instrum. Meas. 2012, 61, 1445–1457. [Google Scholar] [CrossRef]
- Chowdhury, S.S.; Hyder, R.; Hafiz, M.S.B.; Haque, M.A. Real-Time Robust Heart Rate Estimation from Wrist-Type PPG Signals Using Multiple Reference Adaptive Noise Cancellation. IEEE J. Biomed. Health Inform. 2018, 22, 450–459. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S.; Vullings, R.; Biswas, D.; Simões-Capela, N.; van Helleputte, N.; van Hoof, C.; Groenendaal, W. Motion Artifact Reduction for Wrist-Worn Photoplethysmograph Sensors Based on Different Wavelengths. Sensors 2019, 19, 673. [Google Scholar] [CrossRef]
- Wang, C.Y.; Tang, K.T. Active noise cancellation of motion artifacts in pulse oximetry using isobestic wavelength light source. In Proceedings of the 2011 IEEE International Symposium of Circuits and Systems, Rio de Janeiro, Brazil, 15–18 May 2011; pp. 1029–1032. [Google Scholar]
- Ray, D.; Collins, T.; Wolley, S.I.; Ponnapalli, P.V.S. A Review of Wearable Multi-Wavelength Photoplethysmography. IEEE Rev. Biomed. Eng. 2023, 16, 136–158. [Google Scholar] [CrossRef]
- Liu, J.; Yan, B.P.; Dai, W.-X.; Ding, X.-R.; Zhang, Y.-T.; Zhao, N. Multi-wavelength photoplethysmography method for skin arterial pulse extraction. Biomed. Opt. Express 2016, 7, 4313–4326. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Park, H.-K.; Kim, I.Y. Motion Artifact Reduction in Wearable Photoplethysmography Based on Multi-Channel Sensors with Multiple Wavelengths. Sensors 2020, 20, 1493. [Google Scholar] [CrossRef]
- Baek, H.J.; Shin, J.; Jin, G.; Cho, J. Reliability of the Parabola Approximation Method in Heart Rate Variability Analysis Using Low-Sampling-Rate Photoplethysmography. J. Med. Syst. 2017, 41, 189. [Google Scholar] [CrossRef]
- Asare, L.; Kviesis-Kipge, E.; Grabovskis, A.; Rubins, U.; Erts, R.; Spigulis, J. Multi-spectral photoplethysmography biosensor. Proc. SPIE 2011, 8073, 374–379. [Google Scholar]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565–1588. [Google Scholar] [CrossRef]
- Yan, L.; Hu, S.; Alzahrani, A.; Alharbi, S.; Blanos, P. A multi-wavelength opto-electronic patch sensor to effectively detect physiological changes against human skin types. Biosensors 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.; Yoon, H.; Kang, H.; Yeom, H. Effects of skin surface temperature on photoplethysmography. J. Healthc. Eng. 2014, 5, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Spigulis, J.; Gailite, L.; Lihachev, A.; Erts, R. Simultaneous recording of skin blood pulsations at different vascular depths by multiwavelength photoplethysmography. Appl. Opt. 2007, 46, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, B.P.; Zhang, Y.T.; Ding, X.R.; Su, P.; Zhao, N. Multi-Wavelength Photoplethysmography Enabling Continuous Blood Pressure Measurement with Compact Wearable Electronics. IEEE Trans. Biomed. Eng. 2019, 66, 1514–1525. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, S.; Luo, N.; Lau, S.K.; Yu, H.; Kwok, T.; Zhang, Y.T.; Zhao, N. PCA-Based Multi-Wavelength Photoplethysmography Algorithm for Cuffless Blood Pressure Measurement on Elderly Subjects. IEEE J. Biomed. Health Inform. 2021, 25, 663–673. [Google Scholar] [CrossRef]
- Hossain, S.; Haque, C.A.; Kim, K.-D. Quantitative Analysis of Different Multi-Wavelength PPG Devices and Methods for Noninvasive In-Vivo Estimation of Glycated Hemoglobin. Appl. Sci. 2021, 11, 6867. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sens. Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Gorny, A.W.; Liew, S.J.; Tan, C.S.; Müller-Riemenschneider, F. Fitbit Charge HR Wireless Heart Rate Monitor: Validation Study Conducted Under Free-Living Conditions. JMIR mHealth uHealth 2017, 5, e157. [Google Scholar] [CrossRef]
- Nelson, B.W.; Allen, N.B. Accuracy of Consumer Wearable Heart Rate Measurement During an Ecologically Valid 24-Hour Period: Intraindividual Validation Study. JMIR mHealth uHealth 2019, 7, e10828. [Google Scholar] [CrossRef]
- Baek, H.J.; Cho, J. Novel heart rate variability index for wrist-worn wearable devices subject to motion artifacts that complicate measurement of the continuous pulse inverval. Physiol. Meas. 2019, 40, 105010. [Google Scholar]
- Daily Mail. Samsung Galaxy Watch User Claims Wearable Burned Their Wrist When Left on While They Were Sleeping. September 2022. Available online: https://www.dailymail.co.uk/sciencetech/article-11232191/Samsung-Galaxy-Watch-user-claims-wearable-BURNED-wrist-left-sleeping.html (accessed on 22 May 2023).
- 7 News Miami. Fort Lauderdale Woman Says Apple Watch Left Burn on Her Arm. April 2022. Available online: https://wsvn.com/news/local/fort-lauderdale-woman-says-apple-watch-left-burn-on-her-arm/ (accessed on 22 May 2023).
- ABC 7 News. Fitbit Smartwatch Recalled for Burn Risk 8 Years after Previous Tracker Recall. March 2022. Available online: https://abc7news.com/fitbit-ionic-recall-smartwatch-tracker-burn/11615270/ (accessed on 22 May 2023).




| AFE Module | Manufacturer | LED | PD | Size (mm) (L × W × H) | AFE | Unit Price (1) |
|---|---|---|---|---|---|---|
| MAX30101 [24] | Maxim Integrated | 1 Green, 1 Red, 1 IR | 1 PD | 5.6 × 3.3 × 1.55 | Pulse oximetry and heart rate monitor module | USD 11.21 |
| MAX86150 [25] | 1 Red, 1 IR | 1 PD | 5.6 × 3.3 × 1.3 | ECG, pulse oximetry, heart rate monitor module | USD 7.47 | |
| MAX86916 [26] | 1 Blue, 1 Green, 1 Red, 1 IR | 1 PD | 7.0 × 3.5 × 1.5 | Optical sensor module for PPG, proximity and color | USD 8.95 (2) | |
| ADPD144RI [27] | Analog Devices | 1 Red, 1 IR | 4 PD | 5.0 × 2.8 × 1.35 | Pulse oximetry and heart rate monitor module | USD 11.88 |
| ADPD188GG [28] | 2 Green | 2 PD | 5.0 × 3.8 × 0.9 | Heart rate monitor module | USD 11.8 | |
| SFH 7051 [29] | OSRAM | 3 Green | 1 PD | 4.7 × 2.5 × 0.9 | Heart rate monitor module | N.A (3) |
| SFH 7072 [30] | 2 Green, 1 Red, 1 IR | 2 PD | 7.5 × 3.9 × 0.9 | Pulse oximetry and heart rate monitor module | USD 3.47 | |
| SFH 7050 [31] | 1 Green, 1 Red, 1 IR | 1PD | 4.7 × 2.5 × 0.9 | Pulse oximetry and heart rate monitor module | N.A (3) | |
| AS7024 [32] | ams AG | 2 Green, 1 IR | 4 PD | 2.7 × 6.1 × N.A (4) | PPG and ECG | N.A (3) |
| AS7026GG [33] | 2 Green 1 IR | 4 PD | 2.75 × 6.2 × N.A (4) | PPG and ECG | USD 3.11 |
| Ref. | Application | Subjects | Data | Input | Algorithm | Results |
|---|---|---|---|---|---|---|
| [53] | Stress | 74 participants (72 M, 2 F) | PPG signal collected using pulse sensor amped on the index finger of the left-hand under mental arithmetic tasks and Stroop experimental design. | Selected features among time-domain, frequency-domain, nonlinear and point transition measure (PTM) parameters of PRV extracted from PPG. | Support vector machine (SVM) model | (5 levels of stress) Accuracy: 94.33% Sensitivity: 86.01% Specificity: 96.46% |
| [54] | Sleep (Drowsiness) | (At home) 17 participants (7 M, 10 F) Mean age: 44.3 (For driving simulator) 9 participants | (At home) EEG, EOG, EMG, ECG, respiration and PPG signals recorded continuously for 12 h, including nighttime sleep, using polysomnographic device. (For driving simulator) Polysomnographic device during pre-defined driving mission. | LF/HF ratio extracted PPG signal acquired with sampling rate of 50 Hz for the duration of one 2048 sample window. | Rule-based heuristic algorithm | (Drowsiness prediction) 6 m 39 s prior to the actual onset of sleep for at-home data, and 9 m 9 s prior to the actual onset of sleep for driving simulator data. |
| [55] | Sleep (Sleep Stage) | 292 participants (244 adults and 48 children) Mean age: 42.3 ± 19.7 | PPG and accelerometry signal measured from non-dominant wrist during sleep (SOMNIA dataset). Reference sleep measurement: clinical polysomnography. | PPG-derived PRV features combined with a measure of gross body movement for each 30-s epoch based on accelerometer signal. | Long short-term memory (LSTM) recurrent neural network | (4 stages of sleep) Accuracy: 76.4 ± 7.3 Kappa: 0.62 ± 0.12 |
| [58] | Blood Pressure | 33 participants (21 M, 12 F) Mean age: 41.1 ± 16.8 |
PPG signal collected using wrist-wearable device of their own design under the protocol in IEEE Standard for Wearable Cuffless BP. Reference blood pressure measurements: cuff electronic sphygmomanometer. | Feature sets derived by feature fusion with time-domain, morphological, statistical, and demographic features (gender, age, height, weight, and body mass index (BMI)). | A two-layer feed-forward artificial neural network | (SBP/DBP) ME = −0.07/0.00 mmHg MAE = 3.23/2.73 mmHg SDE = 4.47/3.61 mmHg |
| [63] | Blood Glucose Level |
217 participants (90 M, 127 F) Mean age: 49 ± 11 |
PPG signal collected using Empatica E4 wristband. Features extracted from 5-s segments. Reference blood glucose level measurements: laboratory test. | MFCC features of wristband PPG signal and physiological parameters (age, weight, and height) (total of 5 features) | Extreme gradient boost regression (XGBR) | R2 = 0.995 MAE = 1.76 mg/dL SEP = 5.53 mg/dL Accuracy = 98.24% |
| [65] | Activity | 12 participants (6 M, 6 F) | PPG signal collected using Huawei Watch 2 under 5 activities (standing, walking, jogging, jumping, sitting). Each activity lasted around 90 s. with around 1-min gap between two consecutive activities. | Cardiac, respiratory, and motion artifact signals extracted from PPG signal using bandpass filter. | Combination of convolutional and recurrent neural networks | (5 types of activity) F1 score = 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.B.; Baek, H.J. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics 2023, 12, 2923. https://doi.org/10.3390/electronics12132923
Kim KB, Baek HJ. Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics. 2023; 12(13):2923. https://doi.org/10.3390/electronics12132923
Chicago/Turabian StyleKim, Kwang Bok, and Hyun Jae Baek. 2023. "Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions" Electronics 12, no. 13: 2923. https://doi.org/10.3390/electronics12132923
APA StyleKim, K. B., & Baek, H. J. (2023). Photoplethysmography in Wearable Devices: A Comprehensive Review of Technological Advances, Current Challenges, and Future Directions. Electronics, 12(13), 2923. https://doi.org/10.3390/electronics12132923






