A Spiropyran-Doped Poly(methyl methacrylate) Matrix for Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
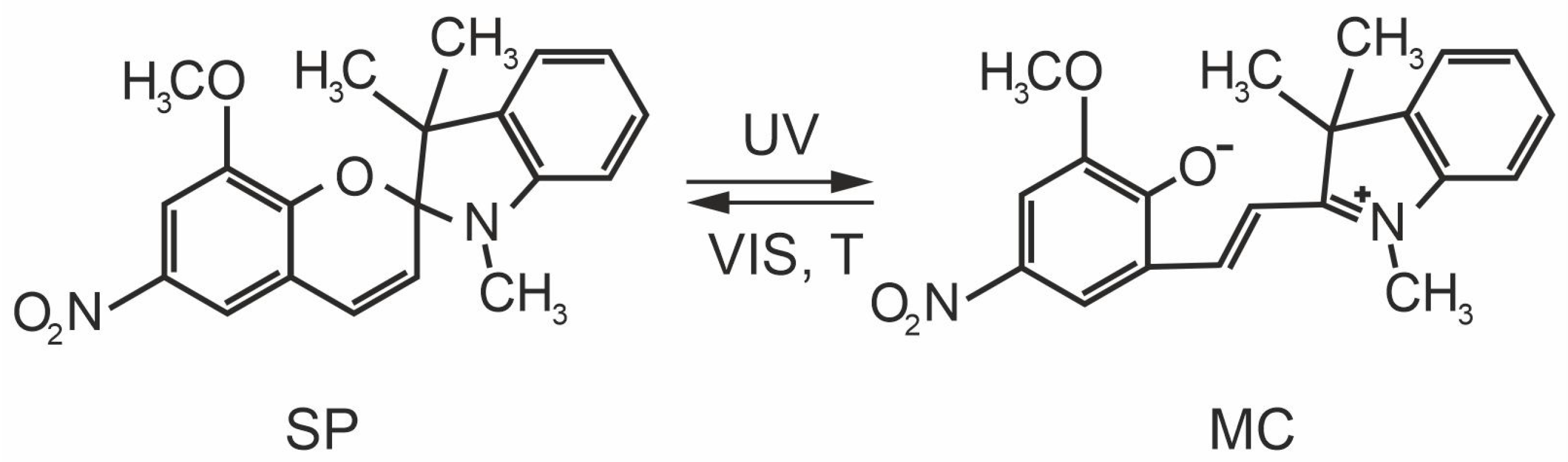
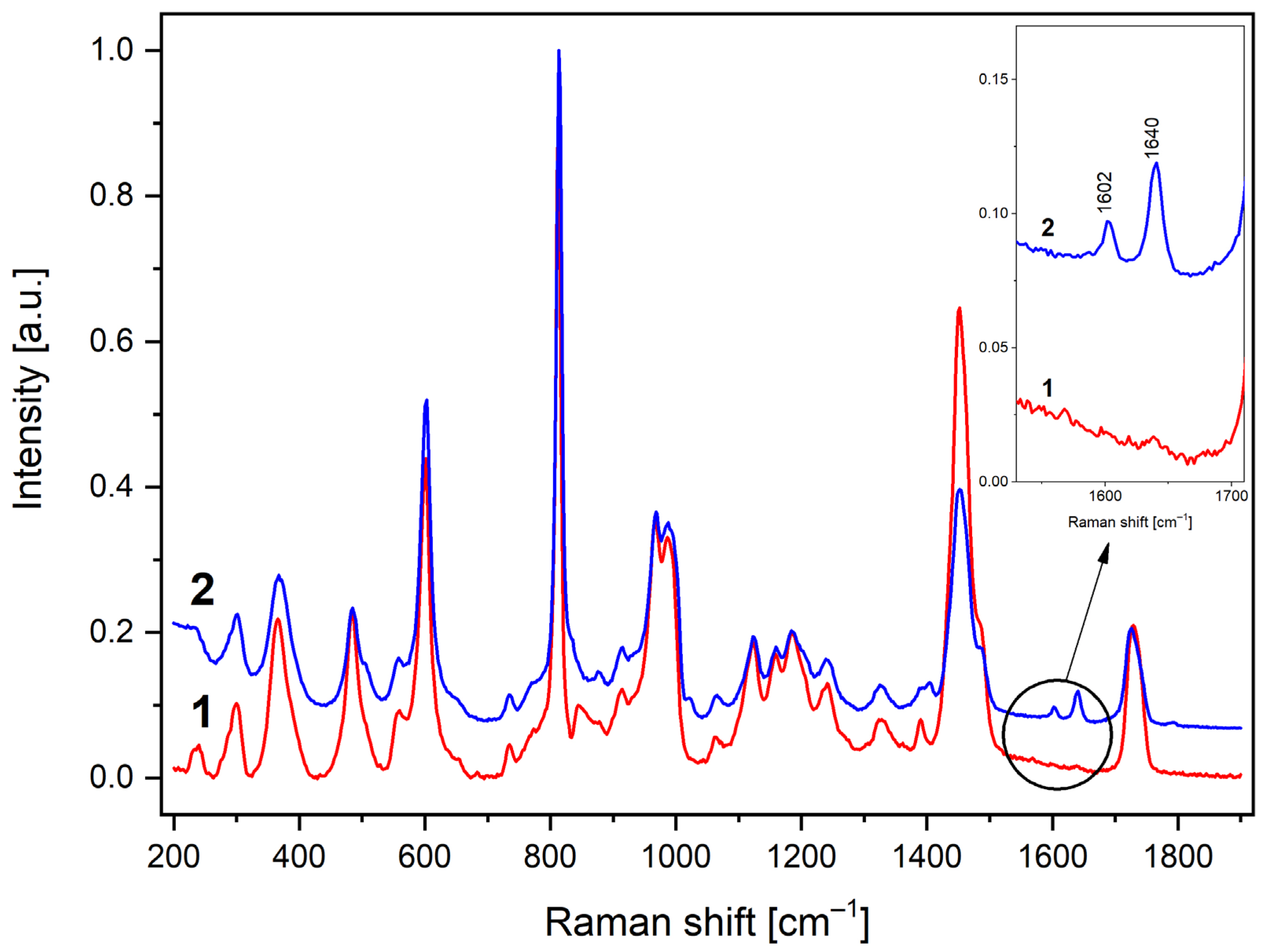
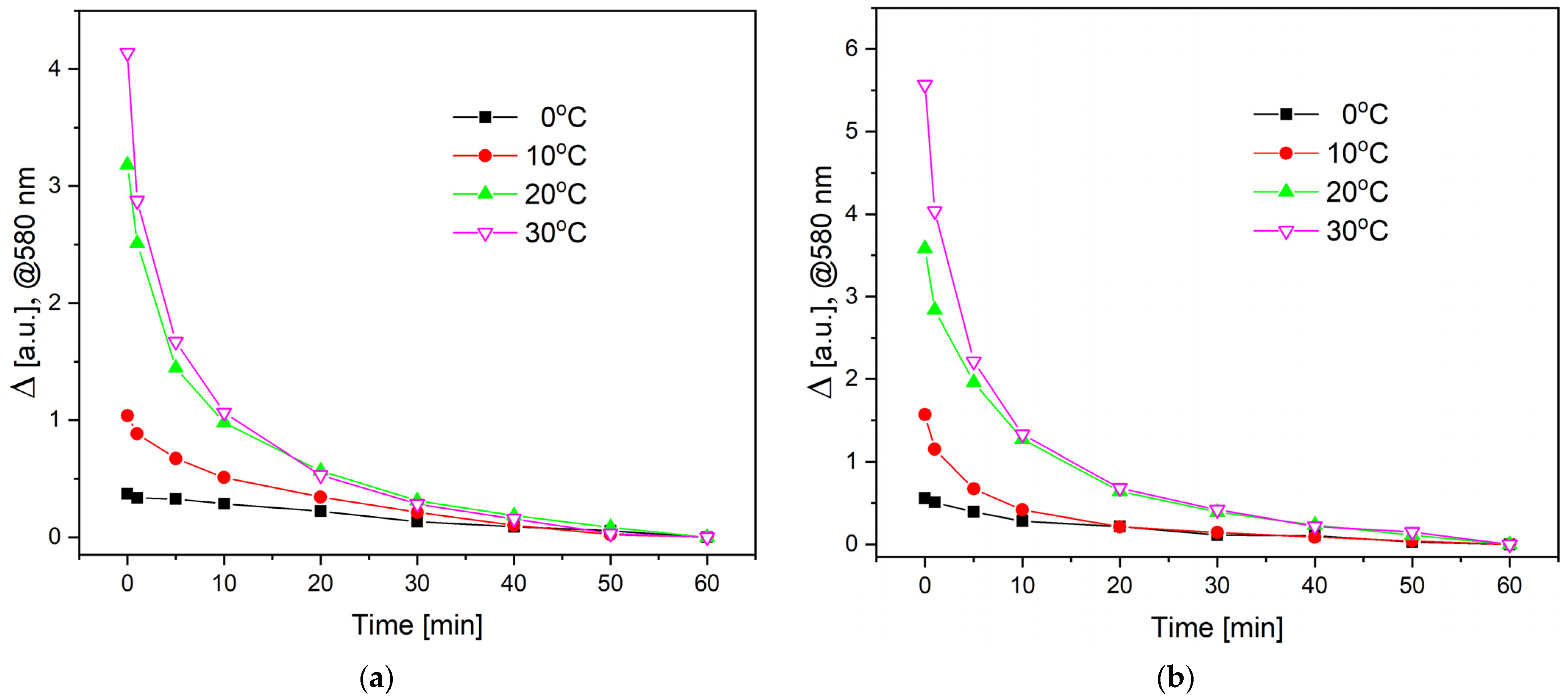
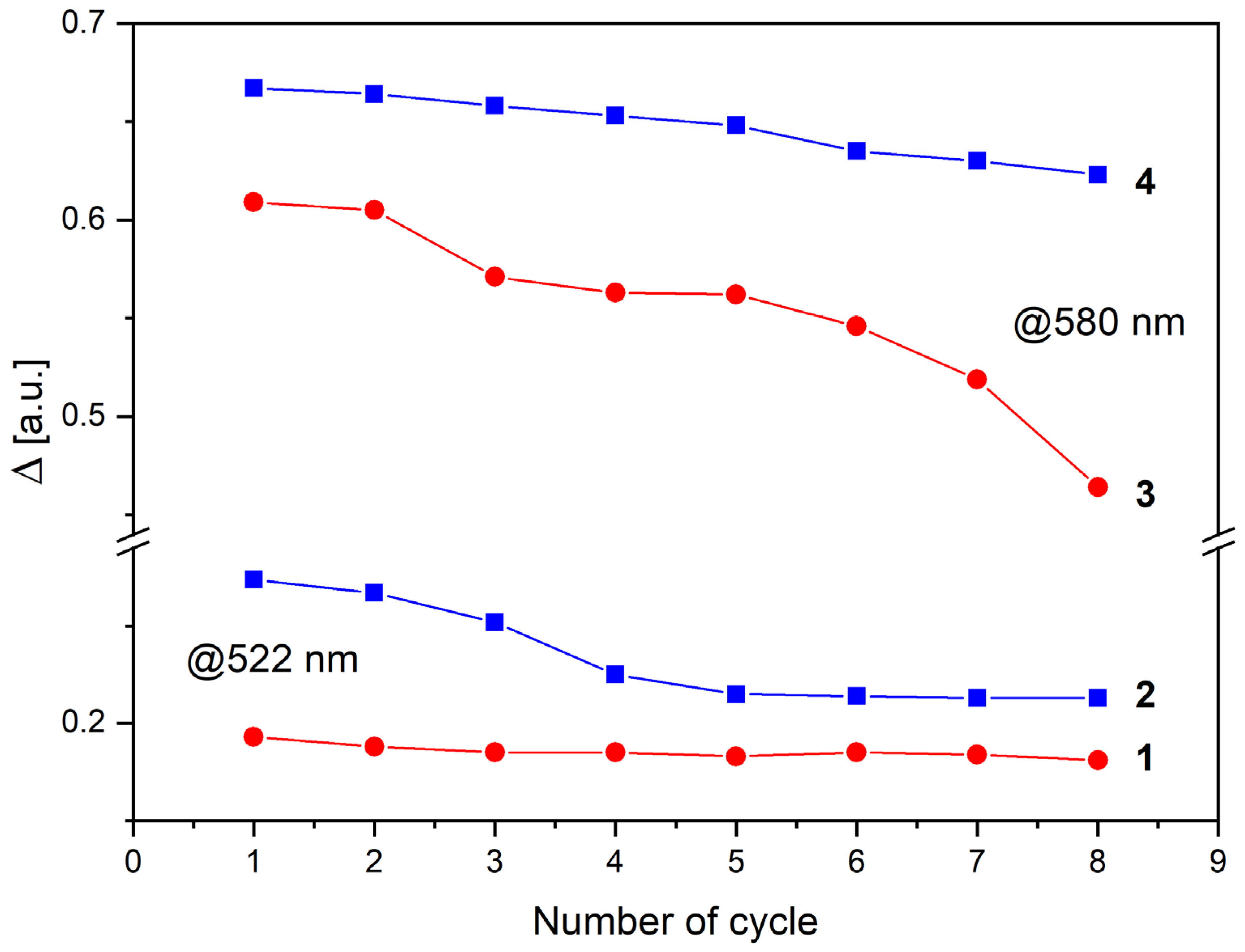
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching It up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-Based Advanced Photoswitchable Materials: A Fascinating Pathway to the Future Stimuli-Responsive Devices. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100487. [Google Scholar] [CrossRef]
- Sanjabi, S.; Rad, J.K.; Salehi-Mobarakeh, H.; Mahdavian, A.R. Preparation of Switchable Thermo- and Photo-Responsive Polyacrylic Nanocapsules Containing Leuco-Dye and Spiropyran: Multi-Level Data Encryption and Temperature Indicator. J. Ind. Eng. Chem. 2023, 119, 647–659. [Google Scholar] [CrossRef]
- Kim, D.W.; Jang, H.G.; Lee, H.S.; Kim, J. Force-Induced Fluorescence Spectrum Shift of Spiropyran-Based Polymer for Mechano-Response Sensing. Sens. Actuators Phys. 2023, 359, 114513. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Lee, C.S.; Pichika, M.R.; Cheng, S.F.; Hang Tan, R.Y. Light-Responsive Polyurethanes: Classification of Light-Responsive Moieties, Light-Responsive Reactions, and Their Applications. RSC Adv. 2022, 12, 15261–15283. [Google Scholar] [CrossRef]
- Arjmand, F.; Mohamadnia, Z. Fabrication of a Light-Responsive Polymer Nanocomposite Containing Spiropyran as a Sensor for Reversible Recognition of Metal Ions. Polym. Chem. 2022, 13, 937–945. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wan, Y.; Carvalho, W.; Hu, L.; Serpe, M.J. Stimuli-Responsive Polymers for Sensing and Reacting to Environmental Conditions. Prog. Polym. Sci. 2021, 116, 101386. [Google Scholar] [CrossRef]
- Yang, M.; Yuan, Z.; Liu, J.; Fang, Z.; Fang, L.; Yu, D.; Li, Q. Photoresponsive Actuators Built from Carbon-Based Soft Materials. Adv. Opt. Mater. 2019, 7, 1900069. [Google Scholar] [CrossRef]
- Pilz Da Cunha, M.; Foelen, Y.; Van Raak, R.J.H.; Murphy, J.N.; Engels, T.A.P.; Debije, M.G.; Schenning, A.P.H.J. An Untethered Magnetic- and Light-Responsive Rotary Gripper: Shedding Light on Photoresponsive Liquid Crystal Actuators. Adv. Opt. Mater. 2019, 7, 1801643. [Google Scholar] [CrossRef]
- Dürr, H.; Bouas-Laurent, H. Photochromism: Molecules and Systems, Rev. ed.; Dürr, H., Bouas-Laurent, H., Eds.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2003; p. XXVII–LIV. [Google Scholar]
- Lin, J.-S. Interaction between Dispersed Photochromic Compound and Polymer Matrix. Eur. Polym. J. 2003, 39, 1693–1700. [Google Scholar] [CrossRef]
- Balmond, E.I.; Tautges, B.K.; Faulkner, A.L.; Or, V.W.; Hodur, B.M.; Shaw, J.T.; Louie, A.Y. Comparative Evaluation of Substituent Effect on the Photochromic Properties of Spiropyrans and Spirooxazines. J. Org. Chem. 2016, 81, 8744–8758. [Google Scholar] [CrossRef]
- Kortekaas, L.; Chen, J.; Jacquemin, D.; Browne, W.R. Proton-Stabilized Photochemically Reversible E/Z Isomerization of Spiropyrans. J. Phys. Chem. B 2018, 122, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Julià-López, A.; Ruiz-Molina, D.; Hernando, J.; Roscini, C. Solid Materials with Tunable Reverse Photochromism. ACS Appl. Mater. Interfaces 2019, 11, 11884–11892. [Google Scholar] [CrossRef]
- Tork, A.; Boudreault, F.; Roberge, M.; Ritcey, A.M.; Lessard, R.A.; Galstian, T.V. Photochromic Behavior of Spiropyran in Polymer Matrices. Appl. Opt. 2001, 40, 1180. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, G.; Zheng, H.; Zhan, K.; Liu, B.; Zhao, L. Tracking of the Molecular Geometrical Changes in the Primary Event of Photoinduced Ring-Opening Reactions of a Spiropyran Model in Gas Phase. Mol. Phys. 2021, 119, e1814971. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, Y.; Coates, G.W.; Silberstein, M.N. Mechanoactivation of Spiropyran Covalently Linked PMMA: Effect of Temperature, Strain Rate, and Deformation Mode. Macromolecules 2015, 48, 1335–1342. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Mahdavian, A.R. Preparation of Fast Photoresponsive Cellulose and Kinetic Study of Photoisomerization. J. Phys. Chem. C 2016, 120, 9985–9991. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.S.A.; Saif, M.; Attia, M.S.; Abo-Aly, M.M.; Mobarez, S.N. Lanthanide Complexes of Spiropyran Photoswitch and Sensor: Spectroscopic Investigations and Computational Modelling. Photochem. Photobiol. Sci. 2018, 17, 221–230. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An Overview on Biodegradation of Polystyrene and Modified Polystyrene: The Microbial Approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Miluski, P.; Kochanowicz, M.; Zmojda, J.; Dorosz, D. Optical Properties of Spirooxazine-Doped PMMA Fiber for New Functional Applications. Opt. Eng. 2017, 56, 047105. [Google Scholar] [CrossRef]
- Miluski, P.; Kochanowicz, M.; Zmojda, J.; Silva, A.P.; Reis, P.N.B.; Ragin, T.; Dorosz, D. UV Sensing Optode for Composite Materials Environment Monitoring. Sci. Eng. Compos. Mater. 2019, 26, 240–243. [Google Scholar] [CrossRef]
- Alghunaim, N.S. Spectroscopic Analysis of PMMA/PVC Blends Containing CoCl2. Results Phys. 2015, 5, 331–336. [Google Scholar] [CrossRef]
- Malatesta, V. Organic Photochromic and Thermochromic Compounds; Crano, J.C., Guglielmetti, R.J., Eds.; Topics in Applied Chemistry; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 65–166. [Google Scholar] [CrossRef]
- Manouras, T.; Vamvakaki, M. Field Responsive Materials: Photo-, Electro-, Magnetic- and Ultrasound-Sensitive Polymers. Polym. Chem. 2017, 8, 74–96. [Google Scholar] [CrossRef]
- Keskin, İ.Ç.; Türemiş, M.; Katı, M.İ.; Kibar, R.; Çetin, A. Effects of CdS Quantum Dot in Polymer Nanocomposites: In Terms of Luminescence, Optic, and Thermal Results. Radiat. Phys. Chem. 2019, 156, 137–143. [Google Scholar] [CrossRef]
- Abozaid, R.M.; Lazarević, Z.Ž.; Radović, I.; Gilić, M.; Šević, D.; Rabasović, M.S.; Radojević, V. Optical Properties and Fluorescence of Quantum Dots CdSe/ZnS-PMMA Composite Films with Interface Modifications. Opt. Mater. 2019, 92, 405–410. [Google Scholar] [CrossRef]
- Pan, H.; Chu, H.; Li, Y.; Qi, N.; Zhao, S.; Li, G.; Li, D. Nonlinear Optical Properties of Colloidal CdSe/ZnS Quantum Dots in PMMA. Nanotechnology 2020, 31, 195703. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Llorent-Martínez, E.J.; Zougagh, M.; Ríos, A. Decoration of Multi-Walled Carbon Nanotubes with Metal Nanoparticles in Supercritical Carbon Dioxide Medium as a Novel Approach for the Modification of Screen-Printed Electrodes. Talanta 2016, 161, 775–779. [Google Scholar] [CrossRef]
- Moreno, V.; Zougagh, M.; Ríos, Á. Analytical Nanometrological Approach for Screening and Confirmation of Titanium Dioxide Nano/Micro-Particles in Sugary Samples Based on Raman Spectroscopy—Capillary Electrophoresis. Anal. Chim. Acta 2019, 1050, 169–175. [Google Scholar] [CrossRef]
- Hamdy, M.S.; AlFaifiy, S.; Al-Hajry, A.; Yahia, I.S. Disposable, Visual and Cost-Effective PMMA Sensor for UVC Radiation. Sens. Actuators B Chem. 2016, 229, 272–275. [Google Scholar] [CrossRef]
- Kawata, S.; Kawata, Y. Three-Dimensional Optical Data Storage Using Photochromic Materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and Spirooxazines for Memories and Switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Florea, L.; Diamond, D.; Benito-Lopez, F. Photo-Responsive Polymeric Structures Based on Spiropyran. Macromol. Mater. Eng. 2012, 297, 1148–1159. [Google Scholar] [CrossRef]
- Bertrand, O.; Gohy, J.-F. Photo-Responsive Polymers: Synthesis and Applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Li, X.; Lin, L.; Kanjwal, M.A.; Chronakis, I.S.; Liu, S.; Chen, Y. Preparing Photochromic Nanofibers and Animal Cells Using a Photochromic Compound of 1′,3′,3′-Trimethyl-6-Nitrospiro (2H-1-Benzopyran-2,2′-Indoline). Colloids Surf. B Biointerfaces 2012, 89, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Kumbasar, E.P.A.; Morsunbul, S.; Alır, S. Photochromic Nanofibers. In Novel Aspects of Nanofibers; Lin, T., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Chibisov, A.K.; Görner, H. Complexes of Spiropyran-Derived Merocyanines with Metal Ions: Relaxation Kinetics, Photochemistry and Solvent Effects. Chem. Phys. 1998, 237, 425–442. [Google Scholar] [CrossRef]
- Görner, H. Photochemical Ring Opening in Nitrospiropyrans: Triplet Pathway and the Role of Singlet Molecular Oxygen. Chem. Phys. Lett. 1998, 282, 381–390. [Google Scholar] [CrossRef]
- Minkin, V.I. Photo-, Thermo-, Solvato-, and Electrochromic Spiroheterocyclic Compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Bricks, J.L.; Slominskii, Y.L.; Panas, I.D.; Demchenko, A.P. Fluorescent J-Aggregates of Cyanine Dyes: Basic Research and Applications Review. Methods Appl. Fluoresc. 2017, 6, 012001. [Google Scholar] [CrossRef]
- Eilmes, A. Spiropyran to Merocyanine Conversion: Explicit versus Implicit Solvent Modeling. J. Phys. Chem. A 2013, 117, 2629–2635. [Google Scholar] [CrossRef]
- Atassi, Y.; Chauvin, J.; Delaire, J.A.; Delouis, J.-F.; Fanton-Maltey, I.; Nakatani, K. Photoinduced Manipulations of Photochromes in Polymers: Anisotropy, Modulation of the NLO Properties and Creation of Surface Gratings. Pure Appl. Chem. 1998, 70, 2157–2166. [Google Scholar] [CrossRef]
- Ali, A.A.; Kharbash, R.; Kim, Y. Chemo- and Biosensing Applications of Spiropyran and Its Derivatives—A Review. Anal. Chim. Acta 2020, 1110, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Takagi, S.; Yomo, K.; Hirai, T. Spontaneous Isomerization of a Hydroxynaphthalene-Containing Spiropyran in Polar Solvents Enhanced by Hydrogen Bonding Interactions. ACS Omega 2021, 6, 35619–35628. [Google Scholar] [CrossRef] [PubMed]
- Irie, M. Photoresponsive Polymers. 7.l Reversible Solubility Change of Polystyrene Having Pendant Spirobenzopyran Groups and Its Application to Photoresists. Macromolecules 1985, 18, 2418–2422. [Google Scholar] [CrossRef]
- Aiken, S.; Edgar, R.J.L.; Gabbutt, C.D.; Heron, B.M.; Hobson, P.A. Negatively Photochromic Organic Compounds: Exploring the Dark Side. Dye. Pigment. 2018, 149, 92–121. [Google Scholar] [CrossRef]
- Gardlund, Z.G. Effect of a Polymer Matrix on Photochromism of Some Benzospirans. J. Polym. Sci. B 1968, 6, 57–61. [Google Scholar] [CrossRef]
- Raman Life. Polymethyl Methacrylate Raman Spectrum. Available online: https://ramanlife.com/library/pmma/ (accessed on 14 November 2023).
- Willis, H.A.; Zichy, V.J.I.; Hendra, P.J. The Laser-Raman and Infra-Red Spectra of Poly(Methyl Methacrylate). Polymer 1969, 10, 737–746. [Google Scholar] [CrossRef]
- Xingsheng, X.; Hai, M.; Qijing, Z.; Yunsheng, Z. Properties of Raman Spectra and Laser-Induced Birefringence in Polymethyl Methacrylate Optical Fibres. J. Opt. Pure Appl. Opt. 2002, 4, 237–242. [Google Scholar] [CrossRef]
- Chaurasia, S.; Rao, U.; Mishra, A.K.; Sijoy, C.D.; Mishra, V. Raman Spectroscopy of Poly (Methyl Methacrylate) under Laser Shock and Static Compression. J. Raman Spectrosc. 2020, 51, 860–870. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kaneko, S.; Tamaki, T.; Kiguchi, M.; Tsukagoshi, K.; Terao, J.; Nishino, T. Principal Component Analysis of Surface-Enhanced Raman Scattering Spectra Revealing Isomer-Dependent Electron Transport in Spiropyran Molecular Junctions: Implications for Nanoscale Molecular Electronics. ACS Omega 2022, 7, 5578–5583. [Google Scholar] [CrossRef] [PubMed]
- Vasilyuk, G.T.; Maskevich, S.A.; Podtynchenko, S.G.; Stepuro, V.I.; Luk’yanov, B.S.; Alekseenko, Y.S. Structural Thermo- and Phototransformations of Oxaindane Spiropyrans Adsorbed on Silver Films. J. Appl. Spectrosc. 2002, 69, 344–350. [Google Scholar] [CrossRef]
- Bonefacino, J.; Tse, M.-L.V.; Pun, C.-F.J.; Cheng, X.; Chan, W.K.E.; Boersma, A.; Tam, H.-Y. Characterization of Spirooxazine and Spiropyran Hosted in Poly(Methyl Methacrylate) for Germicidal UV Source Indicator Application. Opt. Photonics J. 2013, 3, 11–16. [Google Scholar] [CrossRef]
- Perkowski, J.; Kos, L.; Żyłła, R.; Ledakowicz, S. A Kinetic Model of Decoloration of Water Solution of Anthraquinone Dye Initiated by Generality Hydroxyl Radicals. FIBRES Text. East. Eur. 2005, 13, 59–64. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askirka, V.; Miluski, P.; Kochanowicz, M. A Spiropyran-Doped Poly(methyl methacrylate) Matrix for Sensor Applications. Electronics 2023, 12, 4997. https://doi.org/10.3390/electronics12244997
Askirka V, Miluski P, Kochanowicz M. A Spiropyran-Doped Poly(methyl methacrylate) Matrix for Sensor Applications. Electronics. 2023; 12(24):4997. https://doi.org/10.3390/electronics12244997
Chicago/Turabian StyleAskirka, Valiantsin, Piotr Miluski, and Marcin Kochanowicz. 2023. "A Spiropyran-Doped Poly(methyl methacrylate) Matrix for Sensor Applications" Electronics 12, no. 24: 4997. https://doi.org/10.3390/electronics12244997





