New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review
Abstract
:1. Introduction
- To update and simplify the work of medical staff by automating or semi-automating certain procedures—such as sleep staging or sleep disorders diagnosis—through new instrumentation.
- To verify medical treatment efficacy and, eventually, to optimize it, through sleep monitoring.
- To ensure frequent or continuous follow-up by providing instrumentation and protocols to be used in non-hospital settings.
1.1. Background of Sleep Monitoring in Neurodegenerative Diseases
- Insomnia.
- Excessive daytime sleepiness (EDS).
- Rapid eye movement (REM) sleep behavior disorder (RBD).
- Periodic leg movements in sleep (PLMS).
- Restless legs syndrome (RLS).
- Central or obstructive sleep apnea (CSA, OSA).
- Sleep disordered Breathing (SDS).
- Nocturnal stridor.
- Circadian rhythm disorders.
1.2. Overview of Technologies for Neurodegenerative Diseases
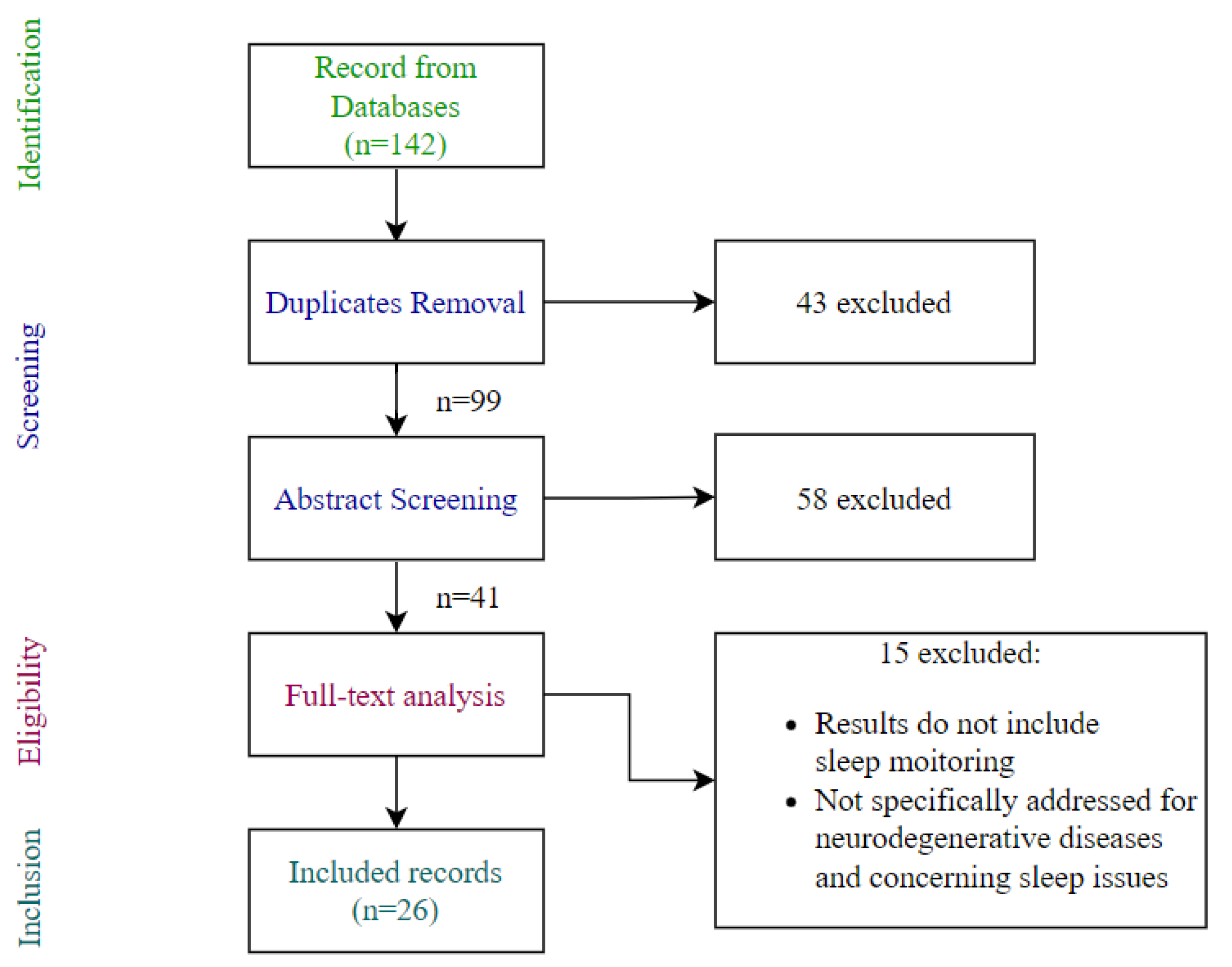
2. Materials and Methods
- Customized queries using keywords and Boolean operators in the form “(Neurodegenerative Disorder OR Parkinson OR Alzheimer OR Huntington OR Lewy Body OR amyotrophic lateral sclerosis OR Ataxia OR Dementia OR Tremor) AND (sleep monitoring) AND (sensor OR IoT OR smart sensor OR environmental sensor OR inertial sensor OR wearable sensor OR optical sensor OR camera OR bed sensor)”.
- Year range restriction to 2010–2022.
- Exclusion of pharmacology, veterinary and construction engineering categories.
- Writing language limitation to English.
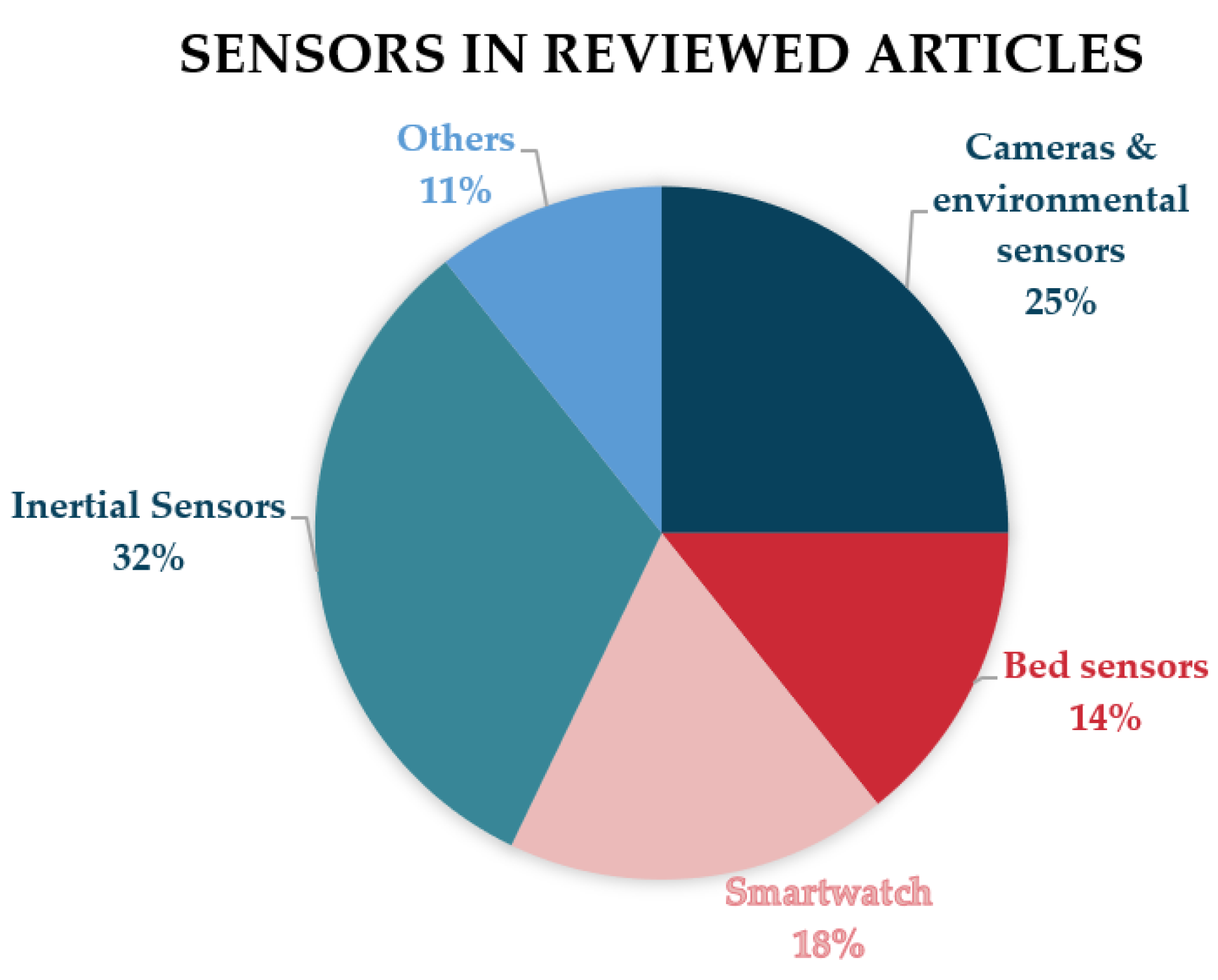
3. Results
3.1. Automatic Sleep-Staging Techniques
3.2. At-Home Sleep Monitoring
3.3. Sleep Quality and Movement Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADL | activity of daily living |
| ALS | amyotrophic lateral sclerosis |
| BK | bradykinesia |
| CAP | cyclic alternating pattern |
| DK | dyskinesia |
| CNN | convolutional neural network |
| CNS | central nervous system |
| DL | deep learning |
| DLB | dementia with Lewy body |
| ECG | electrocardiography |
| EDS | excessive daytime sleepiness |
| EEG | electroencephalography |
| EMG | electromyography |
| EOG | electrooculography |
| ESS | Epworth Sleepiness Scale |
| FDA | American Food and Drug Administration |
| GPS | global positioning system |
| HY | Hoehn andYahr |
| IoT | Internet of Things |
| k-NN | k-nearest neighbour |
| LSTM | long short-term memory |
| MCI | mild cognitive impairment |
| MSA | multiple system atrophy |
| ND | neurodegenerative diseases |
| NREM | non-REM |
| ORCATECH | Oregon Center for Aging and Technology |
| OSA | obstructive sleep apnea |
| PD | Parkinson’s disease |
| PLMS | periodic leg movements in sleep |
| PSG | polysomnography |
| PSQI | Pittsburg Sleep Quality Index |
| PSS | Parkinson’s Disease Sleep Scale |
| RBD | REM sleep behavior disorder |
| REM | rapid eye movements |
| RFID | radio frequency identification |
| RLS | restless legs syndrome |
| SARA | Scale for the Assessment and Rating of Ataxia |
| SD | sleep disorders incidence |
| SDB | sleep-disordered breathing |
| SVM | support vector machine |
| TST | total sleep time |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| WASO | wake after sleep onset |
References
- Killgore, W.D.S. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar]
- Spiegel, K. Effect of sleep deprivation on response to immunizaton. JAMA 2002, 288, 1471–1472. [Google Scholar] [CrossRef]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halász, P. Hierarchy of micro-arousals and the microstructure of sleep. Neurophysiol. Clin. Neurophysiol. 1998, 28, 461–475. [Google Scholar] [CrossRef]
- Parrino, L.; Ferrillo, F.; Smerieri, A.; Spaggiari, M.C.; Palomba, V.; Rossi, M.; Terzano, M.G. Is insomnia a neurophysiological disorder? The role of sleep EEG microstructure. Brain Res. Bull. 2004, 63, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.; Ancoli-Israel, S.; Britz, P.; Walsh, J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America Survey. J. Psychosom. Res. 2004, 56, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Santamaria, J. Sleep in Neurodegenerative Diseases. In Sleep Medicine; Springer: New York, NY, USA, 2015; pp. 271–283. [Google Scholar]
- Iranzo, A. Sleep in dementia and other neurodegenerative diseases. In Sleep Disorders in Neurology; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 229–240. [Google Scholar]
- Abbott, S.M.; Videnovic, A. Chronic sleep disturbance and neural injury: Links to neurodegenerative disease. Nat. Sci. Sleep 2016, 8, 55–61. [Google Scholar]
- Priano, L.; Bigoni, M.; Albani, G.; Sellitti, L.; Giacomotti, E.; Picconi, R.; Cremascoli, R.; Zibetti, M.; Lopiano, L.; Mauro, A. Sleep microstructure in Parkinson’s disease: Cycling alternating pattern (CAP) as a sensitive marker of early NREM sleep instability. Sleep Med. 2019, 61, 57–62. [Google Scholar] [CrossRef]
- Iranzo, A. Sleep in neurodegenerative diseases. Sleep Med. Clin. 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Mendonca, F.; Mostafa, S.S.; Ravelo-Garcia, A.G.; Morgado-Dias, F.; Penzel, T. A review of obstructive sleep apnea detection approaches. IEEE J. Biomed. Health Inform. 2019, 23, 825–837. [Google Scholar] [CrossRef]
- Younes, M.; Raneri, J.; Hanly, P. Staging sleep in polysomnograms: Analysis of inter-scorer variability. J. Clin. Sleep Med. 2016, 12, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, Đ.; Muheim, C.; Bauer, S.; Spinnler, A.; Noain, D.; Bandarabadi, M.; Gallusser, B.; Krummenacher, G.; Baumann, C.; Adamantidis, A.; et al. SPINDLE: End-to-end learning from EEG/EMG to extrapolate animal sleep scoring across experimental settings, labs and species. PLoS Comput. Biol. 2019, 15, e1006968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggi, A.; Ferri, R. Sleep disorders in neurodegenerative diseases: Sleep in neurodegenerative diseases. Eur. J. Neurol. 2010, 17, 1326–1338. [Google Scholar] [CrossRef]
- Fifel, K.; Videnovic, A. Circadian and sleep dysfunctions in neurodegenerative disorders-an update. Front. Neurosci. 2020, 14, 627330. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Yang, L.; Zhang, H.; Shi, Y.; Okhravi, H.R.; Vitiello, M.V.; Sanford, L.D.; Tang, X. Sleep in Alzheimer’s disease: A systematic review and meta-analysis of polysomnographic findings. Transl. Psychiatry 2022, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Lyons, B.E.; Austin, D.; Seelye, A.; Petersen, J.; Yeargers, J.; Riley, T.; Sharma, N.; Mattek, N.; Dodge, H.; Wild, K.; et al. Corrigendum: Pervasive computing technologies to continuously assess Alzheimer’s disease progression and intervention efficacy. Front. Aging Neurosci. 2015, 7, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voysey, Z.J.; Barker, R.A.; Lazar, A.S. The treatment of sleep dysfunction in neurodegenerative disorders. Neurotherapeutics 2021, 18, 202–216. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders—Third Edition (ICSD-3); American Academy of Sleep Medicine (AASM): Darien, IL, USA, 2014. [Google Scholar]
- Wood, E.A.; McCall, W.V. Assessment methodologies in sleep medicine clinical trials. Clin. Investig. 2013, 3, 791–800. [Google Scholar] [CrossRef]
- Shrivastava, D.; Jung, S.; Saadat, M.; Sirohi, R.; Crewson, K. How to interpret the results of a sleep study. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 24983. [Google Scholar] [CrossRef] [Green Version]
- Zampogna, A.; Manoni, A.; Asci, F.; Liguori, C.; Irrera, F.; Suppa, A. Shedding light on nocturnal movements in Parkinson’s disease: Evidence from wearable technologies. Sensors 2020, 20, 5171. [Google Scholar] [CrossRef]
- Urrestarazu, E.; Iriarte, J. Clinical management of sleep disturbances in Alzheimer’s disease: Current and emerging strategies. Nat. Sci. Sleep 2016, 8, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claassen, D.; Josephs, K.; Ahlskog, J.; Silber, M.; Tippmann-Peikert, M.; Boeve, B. Rem sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology 2011, 77, 1155. [Google Scholar] [CrossRef] [Green Version]
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis. 2016, 2016, 9832839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lajoie, A.C.; Lafontaine, A.L.; Kaminska, M. The spectrum of sleep disorders in Parkinson disease: A review. Chest 2021, 159, 818–827. [Google Scholar] [CrossRef]
- Lin, J.Y.; Zhang, L.Y.; Cao, B.; Wei, Q.Q.; Ou, R.W.; Hou, Y.B.; Liu, K.C.; Xu, X.R.; Jiang, Z.; Gu, X.J.; et al. Sleep-related symptoms in multiple system atrophy: Determinants and impact on disease severity. Chin. Med. J. 2020, 134, 690–698. [Google Scholar] [CrossRef]
- Ferman, T.J.; Smith, G.E.; Dickson, D.W.; Graff-Radford, N.R.; Lin, S.C.; Wszolek, Z.; Van Gerpen, J.A.; Uitti, R.; Knopman, D.S.; Petersen, R.C.; et al. Abnormal daytime sleepiness in dementia with Lewy bodies compared to Alzheimer’s disease using the Multiple Sleep Latency Test. Alzheimer Res. Ther. 2014, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Duncan, M.J.; Veasey, S.C.; Zee, P. Editorial: Roles of sleep disruption and circadian rhythm alterations on neurodegeneration and Alzheimer’s disease. Front. Neurosci. 2021, 15, 737895. [Google Scholar] [CrossRef]
- Herzog-Krzywoszanska, R.; Krzywoszanski, L. Sleep disorders in Huntington’s disease. Front. Psychiatry 2019, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Boentert, M. Sleep disturbances in patients with amyotrophic lateral sclerosis: Current perspectives. Nat. Sci. Sleep 2019, 11, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Corben, L.A.; Ho, M.; Copland, J.; Tai, G.; Delatycki, M.B. Increased prevalence of sleep-disordered breathing in Friedreich ataxia. Neurology 2013, 81, 46–51. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef]
- Kohnen, R.; Allen, R.P.; Benes, H.; Garcia-Borreguero, D.; Hening, W.A.; Stiasny-Kolster, K.; Zucconi, M. Assessment of restless legs syndrome—Methodological approaches for use in practice and clinical trials. Mov. Disord. 2007, 22, S485–S494. [Google Scholar] [CrossRef] [PubMed]
- Skorvanek, M.; Feketeova, E.; Kurtis, M.M.; Rusz, J.; Sonka, K. Accuracy of rating scales and clinical measures for screening of rapid eye movement sleep behavior disorder and for predicting conversion to Parkinson’s disease and other synucleinopathies. Front. Neurol. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- McWhirter, D.; Bae, C.; Budur, K. The assessment, diagnosis, and treatment of excessive sleepiness: Practical considerations for the psychiatrist. Psychiatry 2007, 4, 26–35. [Google Scholar]
- Riek, L.D. Healthcare robotics. Commun. ACM 2017, 60, 68–78. [Google Scholar] [CrossRef]
- Sivaparthipan, C.B.; Muthu, B.A.; Manogaran, G.; Maram, B.; Sundarasekar, R.; Krishnamoorthy, S.; Hsu, C.H.; Chandran, K. Innovative and efficient method of robotics for helping the Parkinson’s disease patient using IoT in big data analytics. Trans. Emerg. Telecommun. Technol. 2020, 31, e3838. [Google Scholar] [CrossRef]
- Moro, C.; Štromberga, Z.; Raikos, A.; Stirling, A. The effectiveness of virtual and augmented reality in health sciences and medical anatomy: VR and AR in Health Sciences and Medical Anatomy. Anat. Sci. Educ. 2017, 10, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Walper, S.A.; Lasarte Aragonés, G.; Sapsford, K.E.; Brown, C.W., 3rd; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting biothreat agents: From current diagnostics to developing sensor technologies. ACS Sens. 2018, 3, 1894–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lai, F.; Vespa, P. Enabling technologies facilitate new healthcare delivery models for acute stroke. Stroke 2010, 41, 1076–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillodo, E.; Lemey, C.; Simonnet, M.; Walter, M.; Baca-García, E.; Masetti, V.; Moga, S.; Larsen, M.; HUGOPSY Network; Ropars, J.; et al. Clinical applications of mobile health wearable-based sleep monitoring: Systematic review. JMIR mHealth uHealth 2020, 8, e10733. [Google Scholar] [CrossRef] [PubMed]
- Rune Labs Secures FDA Clearance for Parkinson’s Disease Monitoring through StrivePD Ecosystem on Apple Watch. 2022. Available online: https://www.prnewswire.com/news-releases/rune-labs-secures-fda-clearance-for-parkinsons-disease-monitoring-through-strivepd-ecosystem-on-apple-watch-301566472.html (accessed on 16 December 2022).
- Ben-Sadoun, G.; Manera, V.; Alvarez, J.; Sacco, G.; Robert, P. Recommendations for the design of serious games in neurodegenerative diseases. Front. Aging Neurosci. 2018, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.L.M.; Mesquita, B.S.; Morais, W.S.; Leal, J.C.; Satler, C.E.; Dos Santos Mendes, F.A. Nintendo Wii™ versus Xbox Kinect™ for assisting people with Parkinson’s Disease. Percept. Mot. Skills 2018, 125, 31512518769204. [Google Scholar] [CrossRef] [Green Version]
- Amprimo, G.; Masi, G.; Priano, L.; Azzaro, C.; Galli, F.; Pettiti, G.; Mauro, A.; Ferraris, C. Assessment tasks and virtual exergames for remote monitoring of Parkinson’s disease: An integrated approach based on Azure Kinect. Sensors 2022, 22, 8173. [Google Scholar] [CrossRef]
- Fino, E.; Mazzetti, M. Monitoring healthy and disturbed sleep through smartphone applications: A review of experimental evidence. Sleep Breath. 2019, 23, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qiu, L.; Wu, Y.; Tang, Y.; Cao, G. SleepMonitor: Monitoring respiratory rate and body position during sleep using smartwatch. In Proceedings of the ACM on Interactive Mobile, Wearable and Ubiquitous Technologies Association for Computing Machinery (ACM), New York, NY, USA, 11–15 September 2017; Volume 1, pp. 1–22. [Google Scholar]
- Giannakopoulou, K.M.; Roussaki, I.; Demestichas, K. Internet of Things technologies and machine learning methods for Parkinson’s disease diagnosis, monitoring and management: A systematic review. Sensors 2022, 22, 1799. [Google Scholar] [CrossRef]
- Sheikhtaheri, A.; Sabermahani, F. Applications and outcomes of Internet of Things for patients with Alzheimer’s disease/dementia: A scoping review. Biomed. Res. Int. 2022, 2022, 6274185. [Google Scholar] [CrossRef] [PubMed]
- Simonet, C.; Noyce, A.J. Domotics, smart homes, and Parkinson’s disease. J. Parkinsons. Dis. 2021, 11, S55–S63. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, A.; Weakley, A.; Schmitter-Edgecombe, M.; Cook, D.J.; Aztiria, A.; Basarab, A.; Barrenechea, M. Smart home-based prediction of multidomain symptoms related to Alzheimer’s Disease. IEEE J. Biomed. Health Inform. 2018, 22, 1720–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ROSETTA. 2012. Available online: http://www.aal-europe.eu/projects/rosetta/ (accessed on 16 December 2022).
- Home. 2014. Available online: https://www.neurodegenerationresearch.eu/ (accessed on 16 December 2022).
- Cooray, N.; Andreotti, F.; Lo, C.; Symmonds, M.; Hu, M.T.M.; De Vos, M. Proof of concept: Screening for REM sleep behaviour disorder with a minimal set of sensors. Clin. Neurophysiol. 2021, 132, 904–913. [Google Scholar] [CrossRef]
- Rechichi, I.; Zibetti, M.; Borzì, L.; Olmo, G.; Lopiano, L. Single-channel EEG classification of sleep stages based on REM microstructure. Healthc. Technol. Lett. 2021, 8, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Choi, S.H. Smart technologies toward sleep monitoring at home. Biomed. Eng. Lett. 2019, 9, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Nochino, T.; Ohno, Y.; Kato, T.; Taniike, M.; Okada, S. Sleep stage estimation method using a camera for home use. Biomed. Eng. Lett. 2019, 9, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lin, M.; Chen, F.; Lane, N.; Cardone, G.; Wang, R.; Li, T.; Chen, Y.; Choudhury, T.; Cambell, A. Unobtrusive Sleep Monitoring using Smartphones. In Proceedings of the Proceedings of the ICTs for improving Patients Rehabilitation Research Techniques, Venice, Italy, 5–8 May 2013. [Google Scholar]
- Ya-Ti, P.; Ching-Yung, L.; Ming-Ting, S.; Landis, C.A. Multimodality sensor system for long-term sleep quality monitoring. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 217–227. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent advances in wearable sensors and portable electronics for sleep monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef]
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler radar-based non-contact health monitoring for obstructive sleep apnea diagnosis: A comprehensive review. Big Data Cogn. Comput. 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, S.Y.; Cen, S.S.; Li, Y.; Zhang, H.; Han, C.; Gu, Z.Q.; Mao, W.; Ma, J.H.; Zhou, Y.T.; et al. Detection of motor dysfunction with wearable sensors in patients with idiopathic rapid eye movement disorder. Front. Bioeng. Biotechnol. 2021, 9, 627481. [Google Scholar] [CrossRef]
- Febriana, N.; Rizal, A.; Susanto, E. Sleep monitoring system based on body posture movement using Microsoft Kinect sensor. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2019. [Google Scholar]
- Lee, J.; Hong, M.; Ryu, S. Sleep monitoring system using Kinect sensor. Int. J. Distrib. Sens. Netw. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Jakkaew, P.; Onoye, T. Non-contact respiration monitoring and body movements detection for sleep using thermal imaging. Sensors 2020, 20, 6307. [Google Scholar] [CrossRef]
- Veauthier, C.; Ryczewski, J.; Mansow-Model, S.; Otte, K.; Kayser, B.; Glos, M.; Schöbel, C.; Paul, F.; Brandt, A.U.; Penzel, T. Contactless recording of sleep apnea and periodic leg movements by nocturnal 3-D-video and subsequent visual perceptive computing. Sci. Rep. 2019, 9, 16812. [Google Scholar] [CrossRef] [Green Version]
- Ancona, S.; Faraci, F.D.; Khatab, E.; Fiorillo, L.; Gnarra, O.; Nef, T.; Bassetti, C.L.A.; Bargiotas, P. Wearables in the home-based assessment of abnormal movements in Parkinson’s disease: A systematic review of the literature. J. Neurol. 2022, 269, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Breasail, M.Ó.; Biswas, B.; Smith, M.D.; Mazhar, M.K.A.; Tenison, E.; Cullen, A.; Lithander, F.E.; Roudaut, A.; Henderson, E.J. Wearable GPS and accelerometer technologies for monitoring mobility and physical activity in neurodegenerative disorders: A systematic review. Sensors 2021, 21, 8261. [Google Scholar] [CrossRef] [PubMed]
- Channa, A.; Popescu, N.; Ciobanu, V. Wearable solutions for patients with Parkinson’s disease and neurocognitive disorder: A systematic review. Sensors 2020, 20, 2713. [Google Scholar] [CrossRef]
- Mughal, H.; Javed, A.R.; Rizwan, M.; Almadhor, A.S.; Kryvinska, N. Parkinson’s disease management via wearable sensors: A systematic review. IEEE Access 2022, 10, 35219–35237. [Google Scholar] [CrossRef]
- Woodberry, E.; Browne, G.; Hodges, S.; Watson, P.; Kapur, N.; Woodberry, K. The use of a wearable camera improves autobiographical memory in patients with Alzheimer’s disease. Memory 2015, 23, 340–349. [Google Scholar] [CrossRef]
- Lussier, M.; Lavoie, M.; Giroux, S.; Consel, C.; Guay, M.; Macoir, J.; Hudon, C.; Lorrain, D.; Talbot, L.; Langlois, F.; et al. Early detection of mild cognitive impairment with in-home monitoring sensor technologies using functional measures: A systematic review. IEEE J. Biomed. Health Inform. 2019, 23, 838–847. [Google Scholar] [CrossRef]
- Saner, H.; Schütz, N.; Botros, A.; Urwyler, P.; Buluschek, P.; du Pasquier, G.; Nef, T. Potential of ambient sensor systems for early detection of health problems in older adults. Front. Cardiovasc. Med. 2020, 7, 110. [Google Scholar] [CrossRef]
- Varatharajan, R.; Manogaran, G.; Priyan, M.K.; Sundarasekar, R. Wearable sensor devices for early detection of Alzheimer disease using dynamic time warping algorithm. Cluster Comput. 2018, 21, 681–690. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Del Din, S.; Galna, B.; Thomas, A.; Rochester, L. Differentiating dementia disease subtypes with gait analysis: Feasibility of wearable sensors? Gait Posture 2020, 76, 372–376. [Google Scholar] [CrossRef]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/ wearable devices opportunity. NPJ Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Sigcha, L.; Domínguez, B.; Borzì, L.; Costa, N.; Costa, S.; Arezes, P.; López, J.M.; De Arcas, G.; Pavón, I. Bradykinesia detection in Parkinson’s disease using smartwatches’ inertial sensors and deep learning methods. Electronics 2022, 11, 3879. [Google Scholar] [CrossRef]
- Casciola, A.A.; Carlucci, S.K.; Kent, B.A.; Punch, A.M.; Muszynski, M.A.; Zhou, D.; Kazemi, A.; Mirian, M.S.; Valerio, J.; McKeown, M.J.; et al. A deep learning strategy for automatic sleep staging based on two-channel EEG headband data. Sensors 2021, 21, 3316. [Google Scholar] [CrossRef] [PubMed]
- Shustak, S.; Inzelberg, L.; Steinberg, S.; Rand, D.; David Pur, M.; Hillel, I.; Katzav, S.; Fahoum, F.; De Vos, M.; Mirelman, A.; et al. Home monitoring of sleep with a temporary-tattoo EEG, EOG and EMG electrode array: A feasibility study. J. Neural Eng. 2019, 16, 026024. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Enayati, M.; Keller, J.M.; Popescu, M.; Skubic, M. Non-Invasive In-Home Sleep Stage Classification Using a Ballistocardiography Bed Sensor. In Proceedings of the IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Chicago, IL, USA, 19–22 May 2019. [Google Scholar]
- Ko, Y.F.; Kuo, P.H.; Wang, C.F.; Chen, Y.J.; Chuang, P.C.; Li, S.Z.; Chen, B.W.; Yang, F.C.; Lo, Y.C.; Yang, Y.; et al. Quantification analysis of sleep based on smartwatch sensors for Parkinson’s disease. Biosensors 2022, 12, 74. [Google Scholar] [CrossRef]
- Mahoney, E.L.; Mahoney, D.F. Acceptance of wearable technology by people with Alzheimer’s disease: Issues and accommodations. Am. J. Alzheimer Dis. Other Demen. 2010, 25, 527–531. [Google Scholar] [CrossRef]
- Bate, G.; Richardson, S.; Taylor, J.P.; Burn, D.; Allan, L.; Yarnall, A.; Guan, Y.; Del-Din, S.; Lawson, R. Feasibility of using wearable sensors to monitor activity and sleep patterns in inpatients with delirium and Parkinson’s disease. Mov. Disord. 2022, 37, S365–S366. [Google Scholar]
- Lazarou, I.; Karakostas, A.; Stavropoulos, T.G.; Tsompanidis, T.; Meditskos, G.; Kompatsiaris, I.; Tsolaki, M. A novel and intelligent home monitoring system for care support of elders with cognitive impairment. J. Alzheimer Dis. 2016, 54, 1561–1591. [Google Scholar] [CrossRef]
- Stavropoulos, T.G.; Meditskos, G.; Tsompanidis, T.; Andreadis, S.; Kompatsiaris, I. Dem@Home: Ambient Monitoring and Clinical Support for People Living with Dementia. In Proceedings of the 13th European Semantic Web Conference (ESWC), Crete, Greece, 29 May–2 June 2016; Volume 9989, pp. 26–29. [Google Scholar]
- Andreadis, S.; Stavropoulos, T.G.; Meditskos, G.; Kompatsiaris, I. Dem@Home: Ambient Intelligence for Clinical Support of People Living with Dementia. In Proceedings of the 13th European Semantic Web Conference (ESWC), Crete, Greece, 29 May–2 June 2016. [Google Scholar]
- Lazarou, I.; Stavropoulos, T.G.; Meditskos, G.; Andreadis, S.; Kompatsiaris, I.Y.; Tsolaki, M. Long-term impact of intelligent monitoring technology on people with cognitive impairment: An observational study. J. Alzheimer Dis. 2019, 70, 757–792. [Google Scholar] [CrossRef]
- Thomas, N.W.D.; Beattie, Z.; Marcoe, J.; Wright, K.; Sharma, N.; Mattek, N.; Dodge, H.; Wild, K.; Kaye, J. An Ecologically Valid, longitudinal, and Unbiased Assessment of Treatment Efficacy in Alzheimer disease (the EVALUATE-AD trial): Proof-of-concept study. JMIR Res. Protoc. 2020, 9, e17603. [Google Scholar] [CrossRef]
- Kikhia, B.; Stavropoulos, T.G.; Meditskos, G.; Kompatsiaris, I.; Hallberg, J.; Sävenstedt, S.; Melander, C. Utilizing ambient and wearable sensors to monitor sleep and stress for people with BPSD in nursing homes. J. Ambient Intell. Humaniz. Comput. 2018, 9, 261–273. [Google Scholar] [CrossRef]
- Rose, K.M.; Lach, J.; Perkhounkova, Y.; Gong, J.; Dandu, S.R.; Dickerson, R.; Emi, I.A.; Fan, D.; Specht, J.; Stankovic, J. Use of body sensors to examine nocturnal agitation, sleep, and urinary incontinence in individuals with Alzheimer’s disease. J. Gerontol. Nurs. 2018, 44, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.L.; Riley, T.; Mattek, N.; Pavel, M.; Kaye, J.A. Sleep habits in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2014, 28, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au-Yeung, W.T.M.; Miller, L.; Beattie, Z.; May, R.; Cray, H.V.; Kabelac, Z.; Katabi, D.; Kaye, J.; Vahia, I.V. Monitoring behaviors of patients with late-stage dementia using passive environmental sensing approaches: A case series. Am. J. Geriatr. Psychiatry 2022, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rawtaer, I.; Mahendran, R.; Kua, E.H.; Tan, H.P.; Tan, H.X.; Lee, T.S.; Ng, T.P. Early detection of mild cognitive impairment with in-home sensors to monitor behavior patterns in community-dwelling senior citizens in Singapore: Cross-sectional feasibility study. J. Med. Internet Res. 2020, 22, e16854. [Google Scholar] [CrossRef]
- Abbate, S.; Avvenuti, M.; Light, J. MIMS: A minimally invasive monitoring sensor platform. IEEE Sens. J. 2012, 12, 677–684. [Google Scholar] [CrossRef]
- Branco, D.; Bouça, R.; Ferreira, J.; Guerreiro, T. Designing free-living reports for Parkinson’s disease. In Proceedings of the Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems—CHI EA ’19 Glasgow, Scotland, UK, 4–9 May 2019; ACM Press: New York, NY, USA, 2019. [Google Scholar]
- Silva de Lima, A.L.; Hahn, T.; de Vries, N.M.; Cohen, E.; Bataille, L.; Little, M.A.; Baldus, H.; Bloem, B.R.; Faber, M.J. Large-scale wearable sensor deployment in Parkinson’s patients: The Parkinson@home study protocol. JMIR Res. Protoc. 2016, 5, e172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wamelen, D.J.; Sringean, J.; Trivedi, D.; Carroll, C.B.; Schrag, A.E.; Odin, P.; Antonini, A.; Bloem, B.R.; Bhidayasiri, R.; Chaudhuri, K.R.; et al. Digital health technology for non-motor symptoms in people with Parkinson’s disease: Futile or future? Parkinsonism Relat. Disord. 2021, 89, 186–194. [Google Scholar] [CrossRef]
- Boroojerdi, B.; Ghaffari, R.; Mahadevan, N.; Markowitz, M.; Melton, K.; Morey, B.; Otoul, C.; Patel, S.; Phillips, J.; Sen-Gupta, E.; et al. Clinical feasibility of a wearable, conformable sensor patch to monitor motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 61, 70–76. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Rizos, A.; Sauerbier, A.; McGregor, S.; Martinez-Martin, P.; Reichmann, H.; Horne, M.; Chaudhuri, K.R. Night-time sleep in Parkinson’s disease - the potential use of Parkinson’s KinetiGraph: A prospective comparative study. Eur. J. Neurol. 2016, 23, 1275–1288. [Google Scholar] [CrossRef]
- Xue, F.; Wang, F.Y.; Mao, C.J.; Guo, S.P.; Chen, J.; Li, J.; Wang, Q.J.; Bei, H.Z.; Yu, Q.; Liu, C.F. Analysis of nocturnal hypokinesia and sleep quality in Parkinson’s disease. J. Clin. Neurosci. 2018, 54, 96–101. [Google Scholar] [CrossRef]
- Bhidayasiri, R.; Sringean, J.; Taechalertpaisarn, P.; Thanawattano, C. Capturing nighttime symptoms in Parkinson disease: Technical development and experimental verification of inertial sensors for nocturnal hypokinesia. J. Rehabil. Res. Dev. 2016, 53, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Hillel, I.; Rochester, L.; Del Din, S.; Bloem, B.R.; Avanzino, L.; Nieuwboer, A.; Maidan, I.; Herman, T.; Thaler, A.; et al. Tossing and turning in bed: Nocturnal movements in Parkinson’s disease: Nocturnal movement in pd. Mov. Disord. 2020, 35, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, C.; Thomik, A.A.C.; Lourencco, P.R.; Nageshwaran, S.; Athanasopoulos, S.; Sylaidi, A.; Festenstein, R.; Faisal, A.A. Kinematic body sensor networks and behaviourmetrics for objective efficacy measurements in neurodegenerative disease drug trials. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015. [Google Scholar]
- Gavriel, C.; Thomik, A.A.C.; Lourenco, P.R.; Nageshwaran, S.; Athanasopoulos, S.; Sylaidi, A.; Festenstein, R.; Faisal, A.A. Towards neurobehavioral biomarkers for longitudinal monitoring of neurodegeneration with wearable body sensor networks. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015. [Google Scholar]
- Wei, J.; Boger, J. Sleep detection for younger adults, healthy older adults, and older adults living with dementia using wrist temperature and actigraphy: Prototype testing and case study analysis. JMIR mHealth uHealth 2021, 9, e26462. [Google Scholar] [CrossRef] [PubMed]
- Cesari, M.; Kohn, B.; Holzknecht, E.; Ibrahim, A.; Heidbreder, A.; Bergmann, M.; Brandauer, E.; Hogl, B.; Garn, H.; Stefani, A. Automatic 3D video analysis of upper and lower body movements to identify isolated REM sleep behavior disorder: A pilot study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 7050–7053. [Google Scholar] [PubMed]
- Waser, M.; Stefani, A.; Holzknecht, E.; Kohn, B.; Hackner, H.; Brandauer, E.; Bergmann, M.; Taupe, P.; Gall, M.; Garn, H.; et al. Automated 3D video analysis of lower limb movements during REM sleep: A new diagnostic tool for isolated REM sleep behavior disorder. Sleep 2020, 43, zsaa100. [Google Scholar] [CrossRef]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM sleep behaviour disorder and neurodegeneration—An update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef]
- Filardi, M.; Stefani, A.; Holzknecht, E.; Pizza, F.; Plazzi, G.; Högl, B. Objective rest-activity cycle analysis by actigraphy identifies isolated rapid eye movement sleep behavior disorder. Eur. J. Neurol. 2020, 27, 1848–1855. [Google Scholar] [CrossRef]
- Hjelm, N.M. Benefits and drawbacks of telemedicine. J. Telemed. Telecare 2005, 11, 60–70. [Google Scholar] [CrossRef]
- Dhanvijay, M.M.; Patil, S.C. Internet of Things: A survey of enabling technologies in healthcare and its applications. Comput. Netw. 2019, 153, 113–131. [Google Scholar] [CrossRef]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med.. 2018, 14, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Conti, M.; Orcioni, S.; Madrid, N.M.; Gaiduk, M.; Seepold, R. A review of health monitoring systems using sensors on bed or cushion. In Bioinformatics and Biomedical Engineering; Springer International Publishing: Cham, Switzerland, 2018; pp. 347–358. [Google Scholar]

| ND | Symptoms | Sleep Symptoms | SD |
|---|---|---|---|
| Parkinson’s disease [27,28] | Motor: tremors, postural issues, bradykinesia, ON/OFF states, dystonia, rigidity, dyskinesias. Non-motor: orthostatic hypotension, depression, gastrointestinal symptoms, speech and writing change | RBD (also prodromal), sleep-disordered breathing, EDS, Insomnia, RLS, periodic leg movements in sleep (PLMS) | 60–90% |
| Multiple system atrophy [29] | Parkinsonism, breathing problems | RBD, fragmented sleep, insomnia, stridor, EDS | 80–100% |
| Dementia with Lewy body [30] | Dementia, parkinsonism, fluctuations, and visual hallucinations | Insomnia, circadian rhythm disorder, RBD 1 (also prodromal), confusional awakenings, EDS | 80% |
| Alzheimer’s Disease [31] | Cognitive impairment, dementia. Altered behavior, confusion, aggressiveness | Frequent daytime napping, difficulty in falling asleep and early wakeups, sleep fragmentation, reduced deep and REM sleep amounts, OSA, circadian rhythm alterations, slowdown of sleep EEG rhythms. | 45% |
| Huntington Disease [32] (genetic) | Dementia, psychiatric disturbances | Sleep quality loss, insomnia, sleep fragmentation, EDS, circadian rhythm sleep disorders, reduced NREM and REM sleep. | 87% |
| Amyotrophic lateral sclerosis [33] | Weakness, muscle atrophy, spasticity, respiratory dysfunction | Sleep-disordered breathing, nocturnal hypoventilation, nocturia, cramps, insomnia, EDS | 17–76% |
| Friedreich ataxia [34] (genetic) | Impaired gait, balance, coordination, and speech | RBD, RLS, OSA | 50% |
| Sleep Investigation | Clinical Assessing Methods |
|---|---|
| Sleep quality [35] | Anamnesis, diaries such as Consensus Sleep Diary (CSD), clinical scales such as Pittsburgh Sleep Quality Index (PSQI) for sleep disturbances, sleep duration, sleep latency, sleep efficiency, use of sleep medication, daytime dysfunction, and sleep-quality subjective evaluation in the past months. |
| SD: restless leg syndrome (RLS) [36] | Anamnesis, PSG for detecting associated PLMS, International Restless Legs Scale (IRLS). |
| SD: REM behavior disorder (RBD) [37] | Anamnesis; PSG 1 with sleep staging and REM sleep without atonia scorings; Video-PSG; screening questionnaires; rating scales: RBD Screening Questionnaire (RBDSQ), RBD Single-Question Screen (RBD1Q). |
| Sleep-related problems severity in PD | Rating scales: Parkinson’s disease sleep scale (PDSS), ESS, SCOPA-SLEEP; PSG. |
| Nocturnal movements in PD [24] | Anamnesis; PSG; Video-PSG, Actigraphy; rating scales:
|
| Sleep disturbances in AD [25] | Anamnesis (manifestations of the sleep disorders can be atypical, cognitive impairment can make it difficult); RLS and breathing-disorders assessment; PSG; Actigraphy. |
| EDS [38] | Anamnesis, PSG, Multiple sleep latency test (MSLT), Maintenance of wakefulness test (MWT), Epworth sleepiness scale (ESS) |
| Article | Subjects | Instrumentation | Methods | Results |
|---|---|---|---|---|
| Casciola et al. [81] | 12 healthy subjects (12 nights) | (W 1) two-channel EEG headband (HB) | DL approach to overcome low-quality signals from EEG HB in sleep staging. Manual and automatic corrupted-epoch recognition and discard. Data augmentation. DL training in CNN plus LSTM configuration. | Accuracy: 74 ± 10 % with EEG HB signals, 77 ± 10 % with PSG signals. |
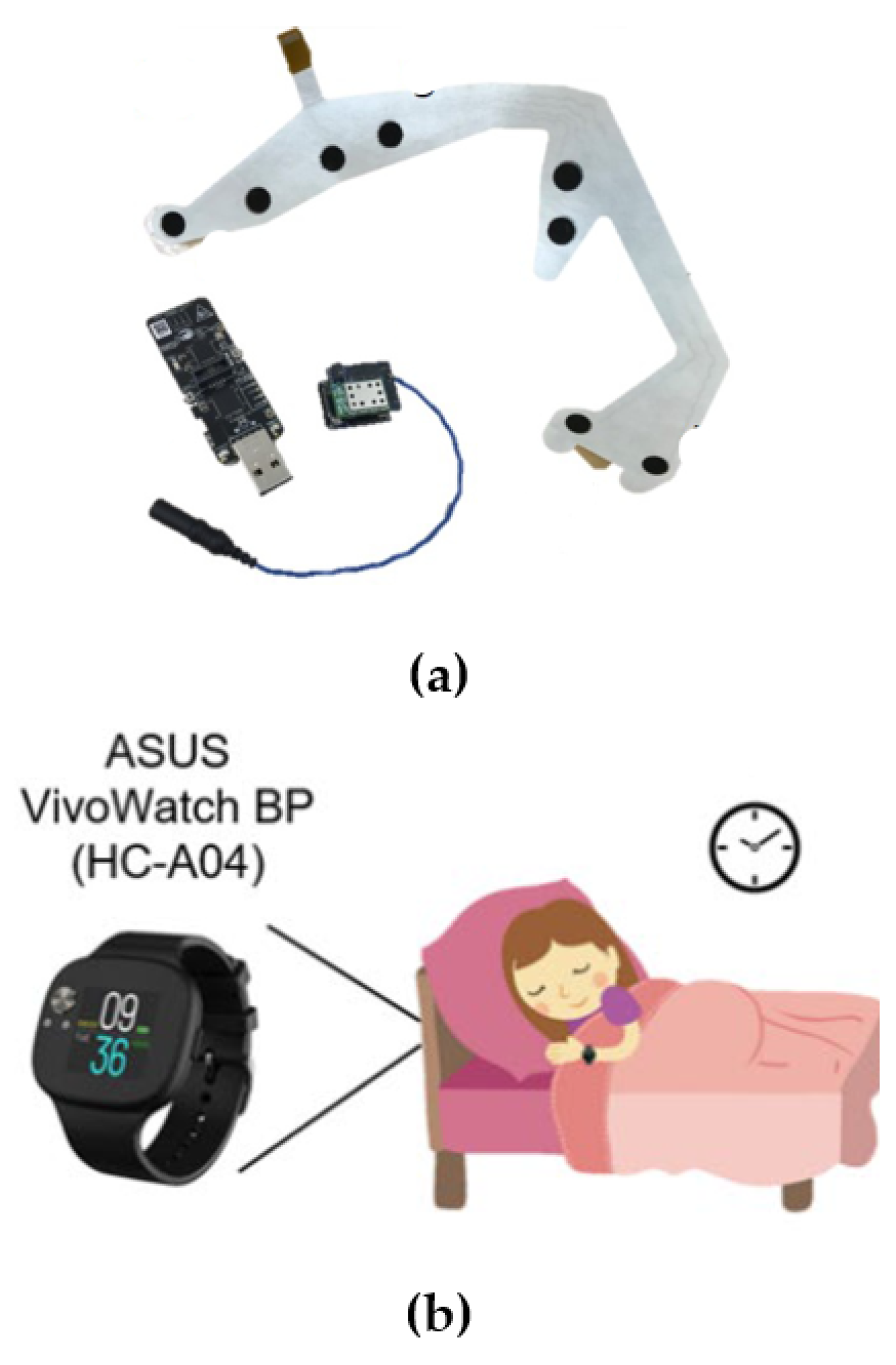
| Shustak et al. [82] | 9 healthy subjects (5 nights) | (W) temporary tattooed dry electrode array: two submental EMG, two EOG and four forehead EEG electrodes. The signals were acquired through a customized wireless recording system and Bluetooth connection. See Figure 4a. | Assessment of sensing performance in three ways: by observing signal behavior in typical facial expression; in comparison with standard video-PSG, through qualitative and correlation measures; and in-home settings for feasibility and electrode-stability evaluation. In addition, the opinions of sleep technicians were collected. | Signals recorded with the temporary tattoo and the 10–20 system were visually similar (e.g., eye blinking, k-complexes, sleep spindles), making them easily interpretable for sleep technicians. Amplitude signal parameters and noise were evaluated in the presence of artifacts such rolling in bed or blinking. |
| Yi et al. [83] | 5 healthy subjects (1 night) | (NW) hydraulic bed sensor. | 74 features extraction from cardiac and respiratory signals. Classification into awake, REM, and non-REM stages by SVM and k-NN. Accuracy referred to manual PSG scoring. | Accuracy 85% with 0.74 kappa, in the detection of awake, REM, and non-REM stages. |
| Ko et al. [84] | 30 healthy subjects, 27 PD patients divided into two subgroups: 15 PD patients taking clonezepam (PDcC), 12 PD patients without clonezepam (PDnC) | (W) Smartwatch (PPG). See Figure 4b. | Quantification analysis of light sleep, deep sleep, REM, and abnormal REM sleep. Classification into sleep/awake, light/deep sleep and REM sleep using Cole–Kripke algorithm and k-means clustering. Definition of abnormal REM epochs. Comparison between control group and PD group was conducted in the quantitative analysis of sleep stages. | Statistically significant differences between PD and controls were measured in the percentage of deep sleep and abnormal REM. Abnormal REM sleep was also able to distinguish between PDcC and PDnC. |
| Article | Stage | Instrumentation | Subjects | Results |
|---|---|---|---|---|
| Dem@Care FP71 project [87,90] | Platform tested on patients | (NW 1) Commercial under-mattress sensor providing sleep duration and stages | 4 in [87]; 22 MCI + 4AD in [90]; | Adaptation of treatment based on clinicians’ observation of the platform output resulted in the improvement of the sleep quality, also comparing the results with subjects who received a standard intervention. |
| Thomas et al. [91] | System feasibility | (W) Smartwatch and automatic measures. See Figure 5. | 30 AD + 30 spouses | Evaluation of feasibility, compliance in wearing watch, and total sleep-time extraction. |
| Kikhia et al. [92] | System feasibility and preliminary results | (NW) Smart clock with a smartphone (movement and respiration detection) able to provide sleep staging (awake, light sleep and deep sleep) and a sleep score. | 4 subjects with Dementia | Good acceptability of the system by clinical staff, who were able to assess patients based on the output of the system. |
| Rose et al. in [93] | Platform tested on patients | (NW) Matress sensor, TEMPO nodes on wrists and a microphone, from which data are transmitted to an online platform where automatic event detection is performed and available for users’ consultation. | 12 AD subjects | Monitoring and correlation of symptoms, such as nighttime agitation and incontinence in AD, were performed. The correlation inference process showed a pattern for the time occurrence of symptoms. |
| Hayes et al. [94] | Platform tested on patients | (NW) Passive infrared sensors with custom automatic algorithm extracting sleep features (ORCATECH platform) | 45 seniors, including 16 MCI (amnestic, aMCI, and non-amnestic MCI, naMCI) over 6 months | The comparisons of self-reported and platform measures in the three groups (healthy seniors, aMCI, naMCI) showed that movement in bed during the night, wake after sleep onset, and times up during the night were significantly different. |
| Au-Yeung et al. [95] | Case study with existing platforms | (NW) Aging & Technology (ORCATECH) platform + Emerald device | 2AD, 1 frontotemporal dementia, and a major neurocognitive disorder affected subjects. | Sleep-score comparison in the presence/absence of drug administration. Night-time agitation and PLM assessment. |
| Rawtaer et al. [96] | Feasibility study | (NW) Bed-occupancy sensor based on fiberoptic technology, providing sleep duration and quality metrics (sleep duration, number of sleep interruptions) | 28 MCI and 21 healthy controls (>65 years) subjects (HC) | Comparison of sleep duration and interruptions between MCI and HC subjects. |
| Abbate et al. [97] | Feasibility study | (W+NW) Bed sensor + EEG HB. | - | General discussion on the feasibility of sleep studies based on Enobio EEG HB and inference of risk of fall. |
| Branco et al. [98] | Feasibility study | (W) Inertial sensor included in the Datapark platform | 22 PD subjects in rehabilitation center, for 2 months | Report of changes in sleep position and wakeups were provided to clinicians and patients along with other measures of general activity. Good acceptability of the system. |
| Silva de Lima [99] | Study presentation and beginning of recruiting | (W) Smartwatch + app | To be: 1000 PD subjects | The system aims to provide sleep-movement analyses. |
| Article | Subjects | Instrumentation | Methods | Results |
|---|---|---|---|---|
| Boroojerdi et al. in [101] | 21 PD subjects | (W 1) NIMBLE patch contains an accelerometer and an EMG | Tremor, postural instability, and sleep-quality-measures computation with different patch locations. Comparison with standard clinical scales. Feasibility evaluation. | No correlation between sleep measures and sleep diaries. General good usability and acceptability of the system. |
| Klingelhoefer in [102] | 30 PD subjects with EDS and 33 PD subjects without EDS | (W) PKG (Parkinson’s Kineti-Graph) | Bradykinesia and dyskinesia scores to determine disturbed nights. Comparison of the two groups by PKG and sleep-diary data (immobility, sleep duration, sleep interruptions). | In the PD-EDS group, correlation between subjective sleep reports and PKG parameters for quantity and quality of sleep. No correlation in the other group. |
| Xue in [103] | 29 PD subjects, 17 with IBM | (W) multisite inertial sensors | Sleep-quality measure with traditional measures (total sleep time and sleep efficiency) and inertial sensors (acceleration, angular velocity, wakeups, turning in bad, limbs movements). Comparison between the two groups. | Negative correlation between turning-over events and disease duration. Positive correlation between TST and sleep-efficiency parameters and the number of turns in bed. Significant correlation between the number of turns and TST. |
| Bhidayasiri et al. in [104] | 6 PD subjects and 6 spouses | (W) Inertial sensors | Night-time movement analysis, hypokinesia, rolling over description (degrees, duration, velocity, and acceleration) and wakeups | Impairment in turning in PD subjects (less frequent, slower, smaller). |
| Mirelman et al. in [105] | 305 PD + 205 HC subjects | (W) Accelerometer | Nocturnal symptom assessment through lying, turning, and upright time. | Advanced PD subjects showed more upright periods, and a reduction in the number and velocity of their turns. Correlation between the reduction in nocturnal movements and increased PD motor severity, worse dysautonomia and cognition, and dopaminergic medication. |
| Gavriel et al. in [106,107] | 9 F.Ataxia subjects | (W) 1 or 4 of wireless BSN nodes (inertial). | Extraction of biomarkers of Ataxia and Ataxia progression from segmentation of acceleration. They are based on movements and stillness intervals and were correlated to SARA (traditional Ataxia assessment method). | Correlation between the proposed biomarker and SARA assessment. |
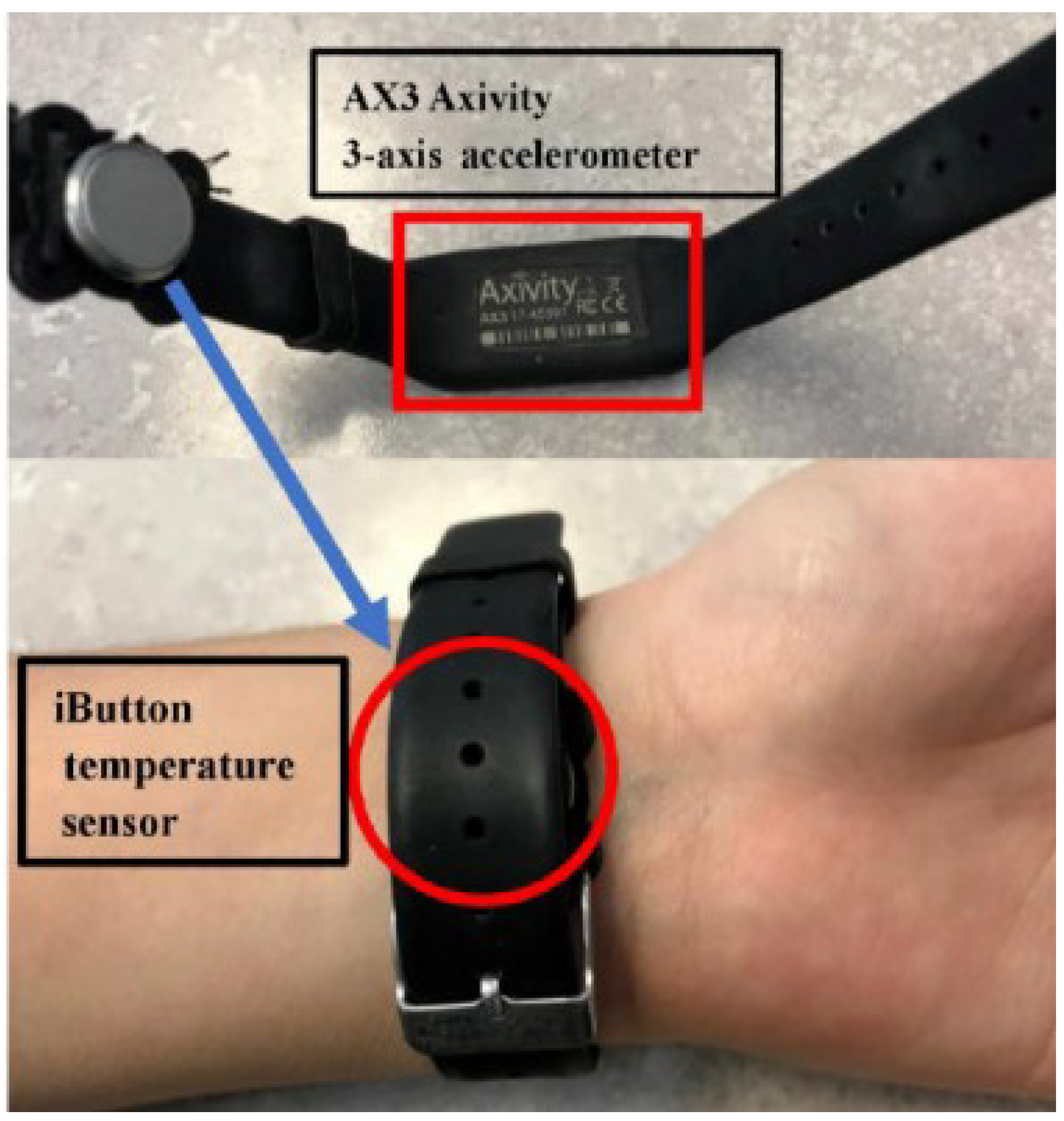
| Wei et al. in [108] | 10 healthy young subjects, 10 healthy elders, 8 subjects affected by Dementia | (W) Smartwatch (accelerometer) + actigraph and temperature sensors. See Figure 6. | Confront sleep diaries and accelerometer data. Sleep onset, sleep offset, and sleep duration and nighttime wakeups were calculated. Interday stability and intraday variability were calculated from temperature. | More movement during sleep, measured by actigraphy, in older adults than in the young, with an increasing trend in those with dementia. In addition, less temperature variation between night and day was measured in the elderly. |
| Article | Subjects | Instrumentation | Methods | Results |
|---|---|---|---|---|
| Waser et al. in [110] | 122 (40 iRBD, 18 prodromal RBD, 64 participants with mimic symptoms). | (NW1) 3D cameras | Custom algorithm for lower limb movement identification in REM. Feature extraction (movements rate, duration, extent, and intensity) and comparison with video-polysomnographic findings. | Significant increase in features analyzed among subjects with iRBD and prodromal RBD and mimic groups. In addition, leg movements with a duration <2 seconds discriminated iRBD with the highest accuracy (90.4%) from other motor activity during sleep. |
| Cesari et al. in [109] | 20 RBD, 24 SDB subjects | (NW) 3D cameras | Custom algorithm for lower and upper limb movement identification in REM with a max. duration of 5s. Exclusion of breathing movements. Feature extraction (3D rate: the number of movements in REM sleep per hour of REM sleep, and 3D ratio: the total movement-duration time in seconds in REM sleep divided by the total REM-sleep time in seconds) and patient classification were performed (receiver operating characteristic curve to distinguish iRBD, positive class from SDB, negative class). | RBD vs. SDB classification provided an accuracy of 0.91 and F1-score of 0.90 |
| Filardi et al. [112] | 19 with iRBD, 19 RLS and 20 with untreated SAS and 16 healthy controls | (W) Micro Motionlogger® Actigraphy Watch (Ambulatory Monitoring, Inc.; NY) + light sensor. | Comparison of video-PSG and RBD-screening-questionnaires findings with the analysis of rest–activity cycles as derived from actigraphy. Features of rest–activity rhythm such as bedtime, wake-up time, midpoint of sleep, estimated wake after sleep onset (eWASO), estimated sleep efficiency (eSE) and activity bouts were extracted. | Lower sleep efficiency, augmented eWASO and increased frequency of prolonged activity bouts for subjects with iRBD compared with those with RLS and controls; no difference compared with SAS patients. In addition, features computed on 24h recording allowed to distinguish iRBD subjects better than screening questionnaires. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, G.; Amprimo, G.; Priano, L.; Ferraris, C. New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review. Electronics 2023, 12, 1098. https://doi.org/10.3390/electronics12051098
Masi G, Amprimo G, Priano L, Ferraris C. New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review. Electronics. 2023; 12(5):1098. https://doi.org/10.3390/electronics12051098
Chicago/Turabian StyleMasi, Giulia, Gianluca Amprimo, Lorenzo Priano, and Claudia Ferraris. 2023. "New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review" Electronics 12, no. 5: 1098. https://doi.org/10.3390/electronics12051098
APA StyleMasi, G., Amprimo, G., Priano, L., & Ferraris, C. (2023). New Perspectives in Nonintrusive Sleep Monitoring for Neurodegenerative Diseases—A Narrative Review. Electronics, 12(5), 1098. https://doi.org/10.3390/electronics12051098






