Random Body Movement Removal Using Adaptive Motion Artifact Filtering in mmWave Radar-Based Neonatal Heartbeat Sensing
Abstract
:1. Introduction
- (1)
- A novel method is proposed to enhance the quality of heartbeat measurements using MIMO mmWave radar in the presence of RBM;
- (2)
- The non-continuous nature of RBM is leveraged to mitigate its impact on the calculation of respiration and heart rates;
- (3)
- By analyzing the time–frequency information on the chest surface, the spectra of RBM and heartbeat are separated in the temporal domain, the continuously changing heartbeat spectra are extracted, and the influence of RBM is reduced.
2. Methodology
2.1. MIMO Millimeter Wave Radar Induction Technology
2.2. The Impact of RBM
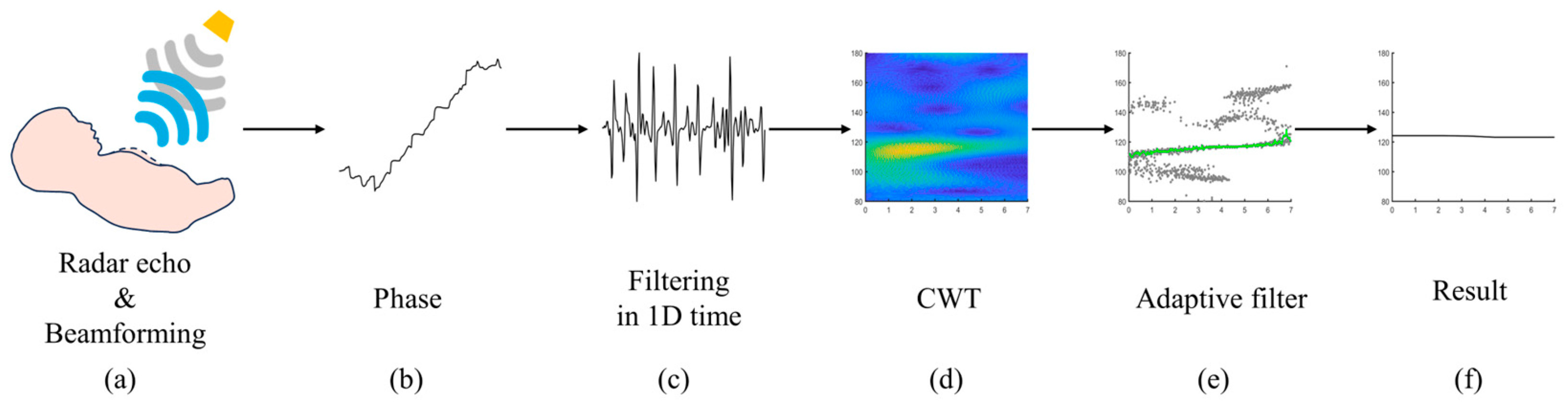
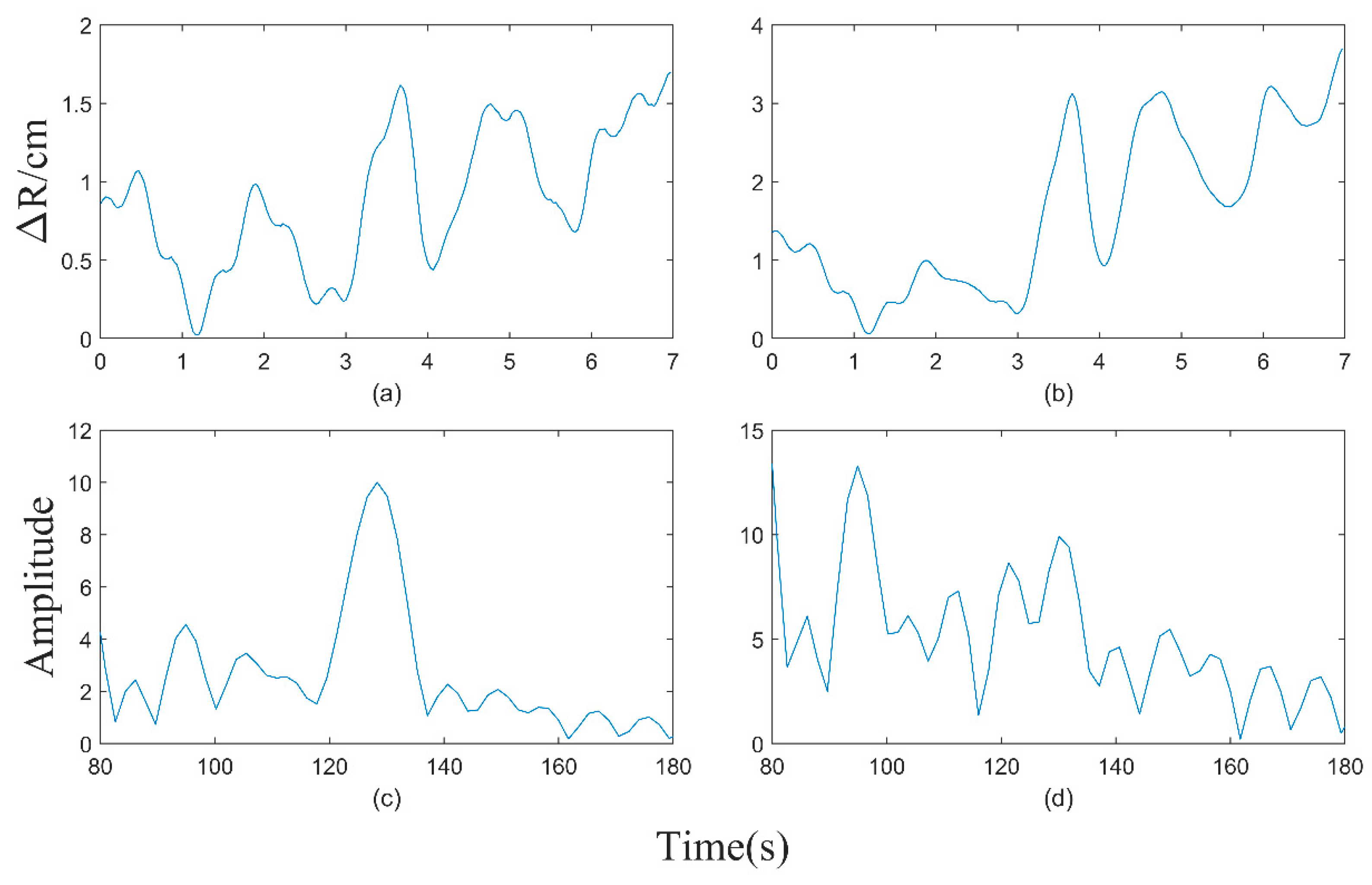
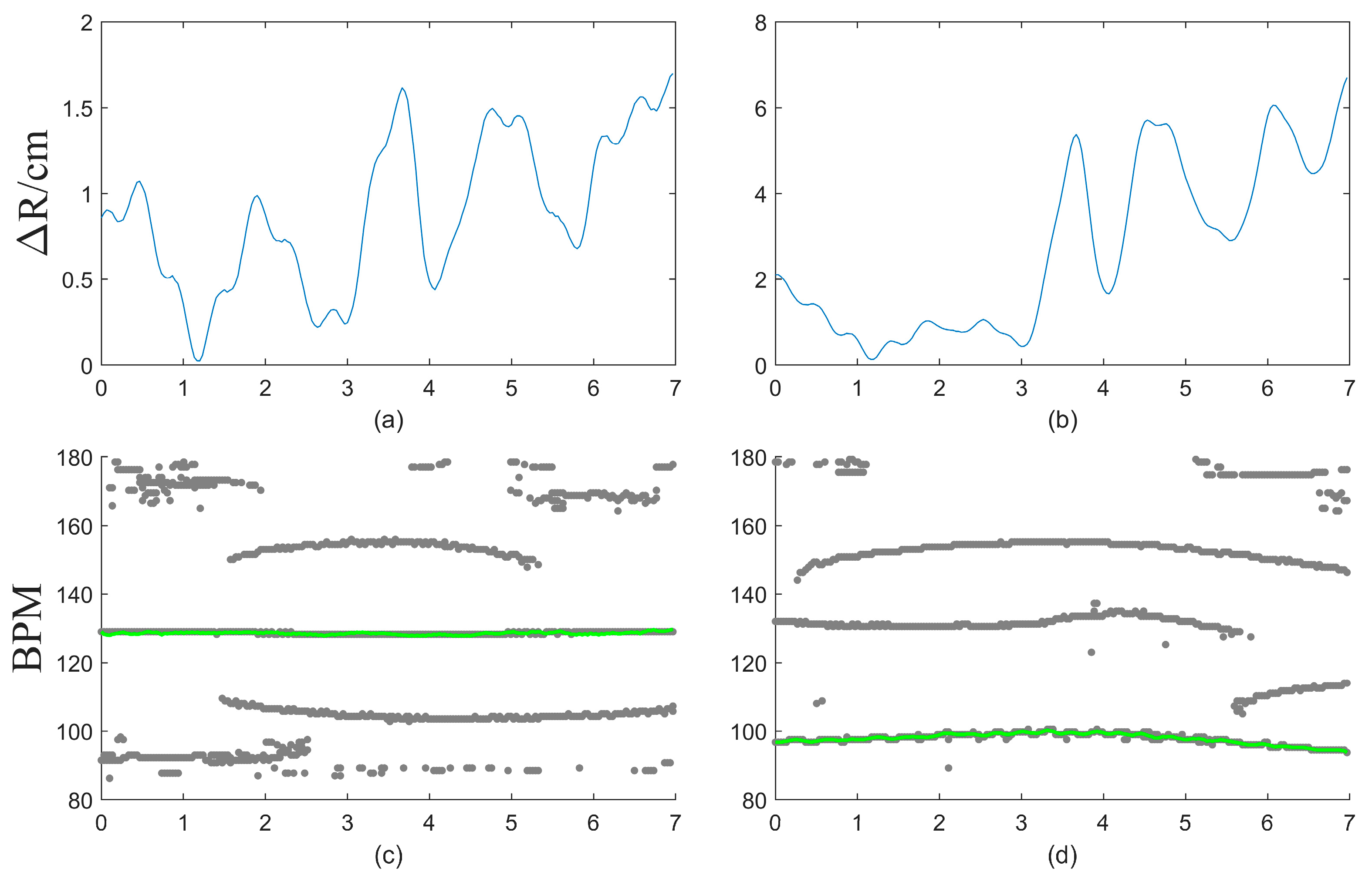
2.3. Adaptive Motion Artifact Filtering
- (a)
- Determine the position of each spectral peak at every time point;
- (b)
- The spectral peaks within the time at t are selected as the starting points for component fitting;
- (c)
- Along the temporal axis, the frequency of the spectral peak corresponding to tk and the differences in spectral peak frequencies between [tk+1, tk+2, tk+3] are calculated, respectively. The spectral peak with the smallest difference is se-lected for fitting;
- (d)
- The process described in (c) is repeated until all time points have been traversed;
- (e)
- The peak-to-peak value of the fitted curve is calculated, and the signal component with the smallest peak-to-peak value is selected as the heart rate curve.
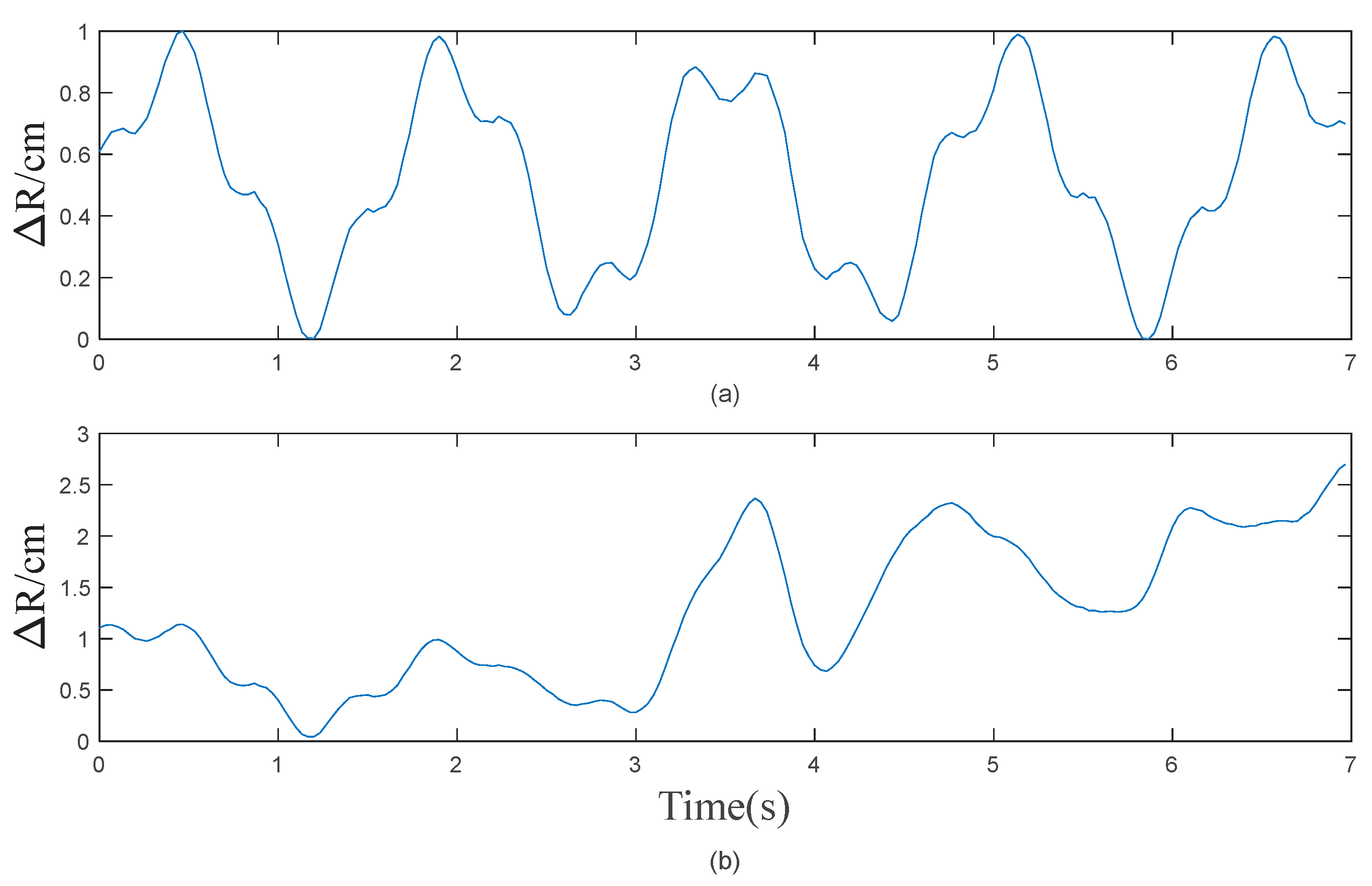
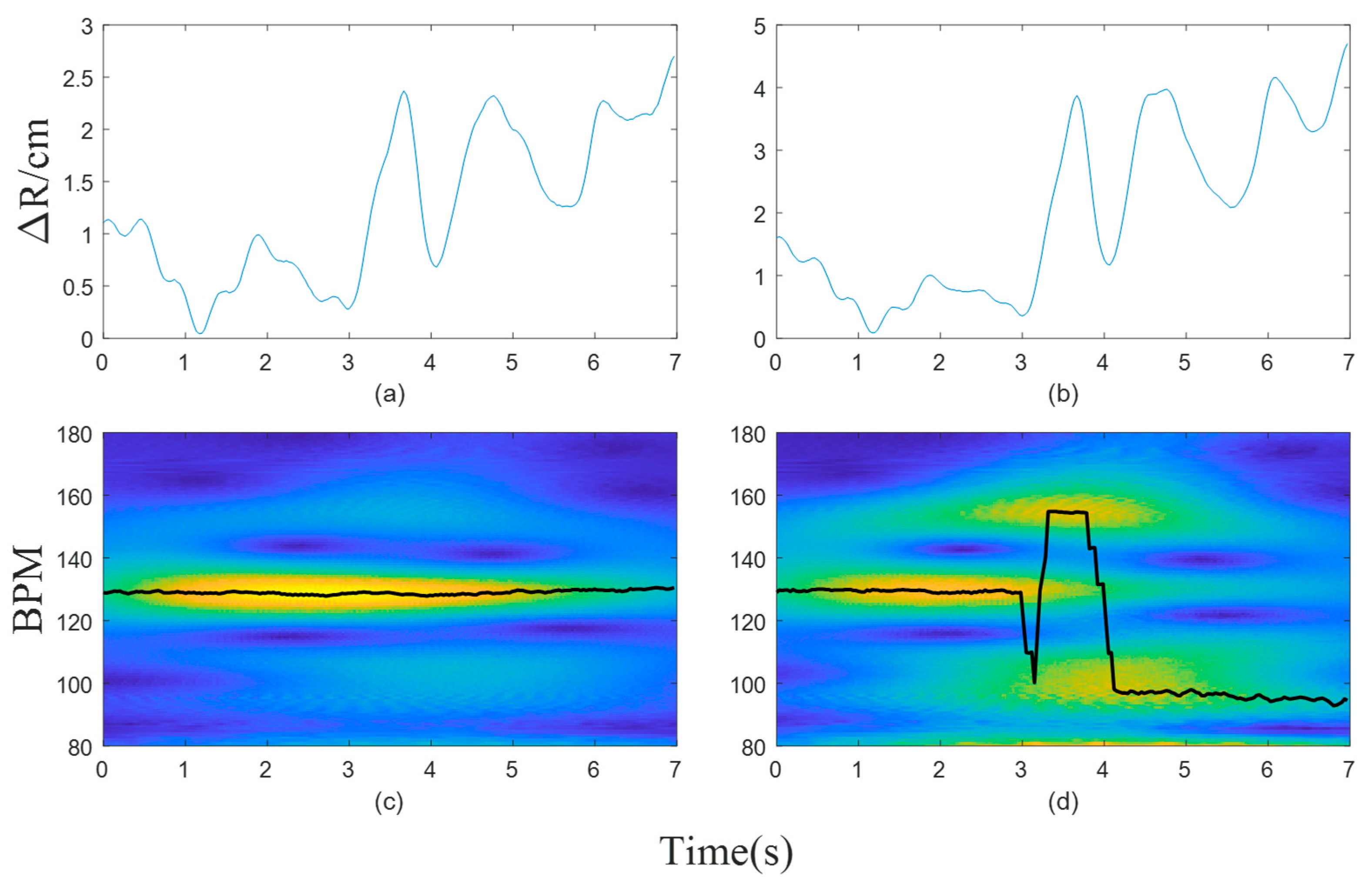
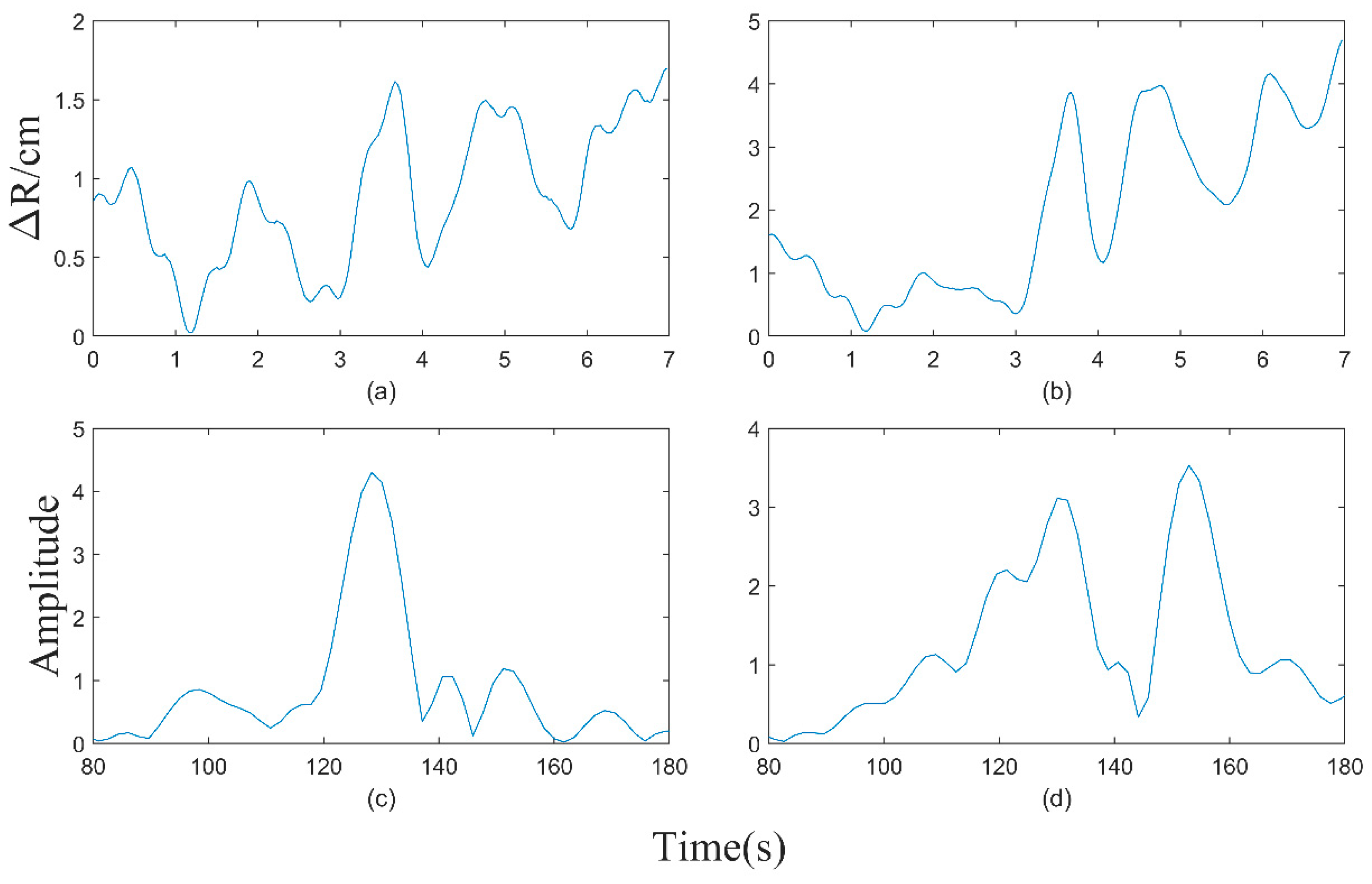
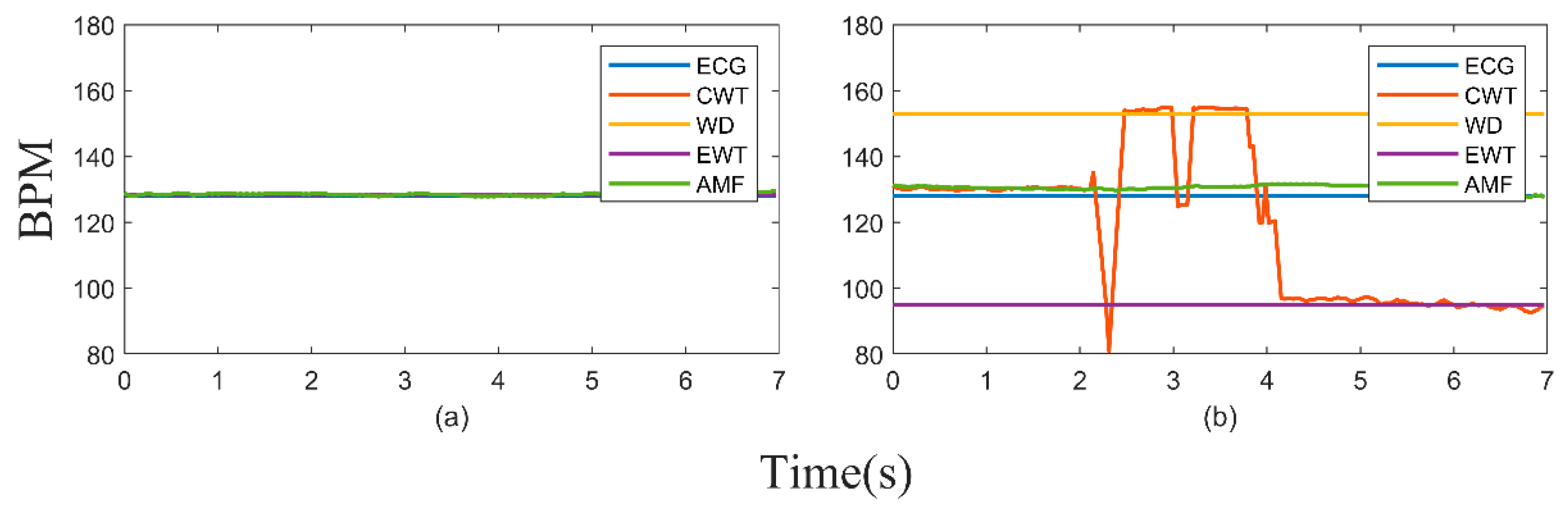
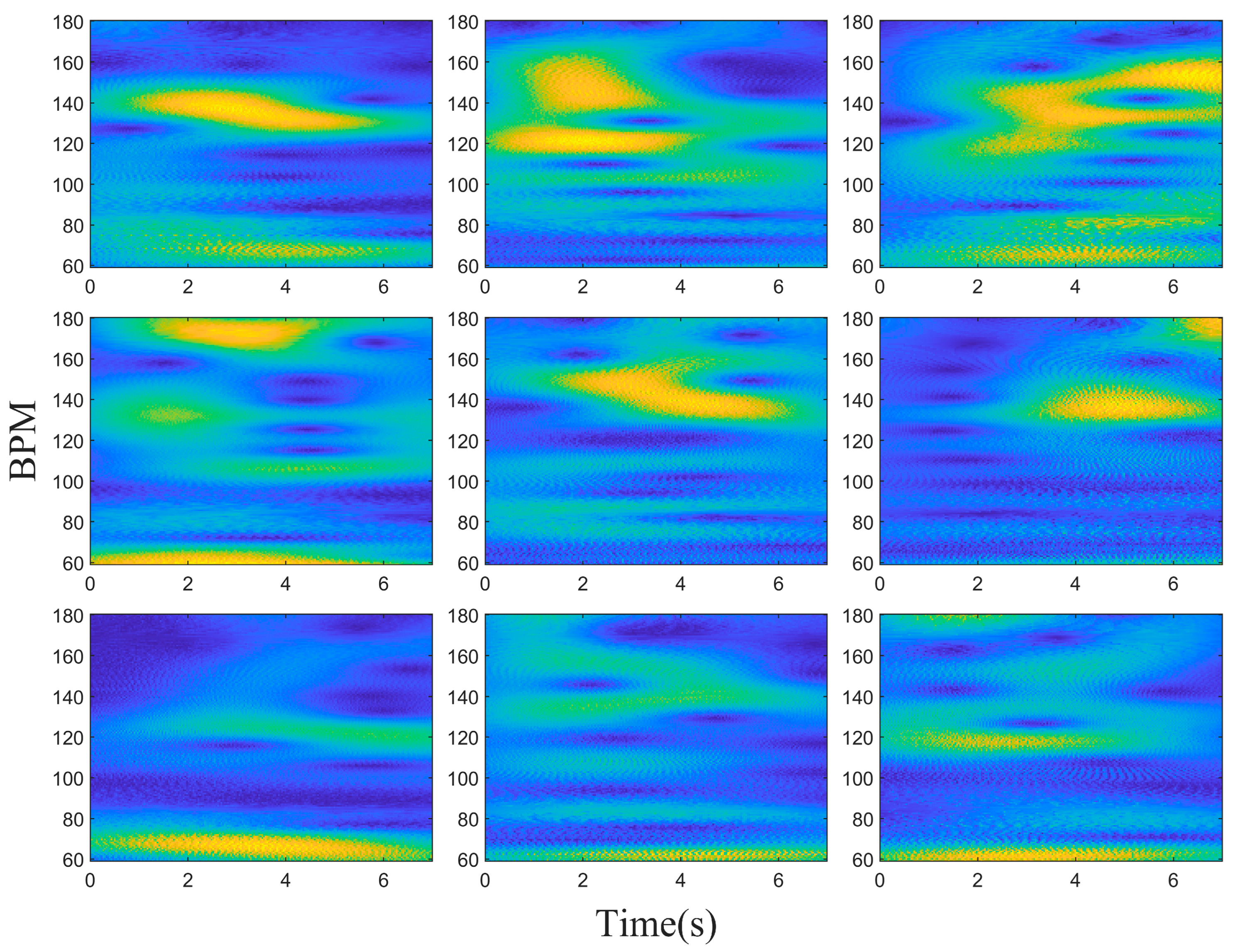
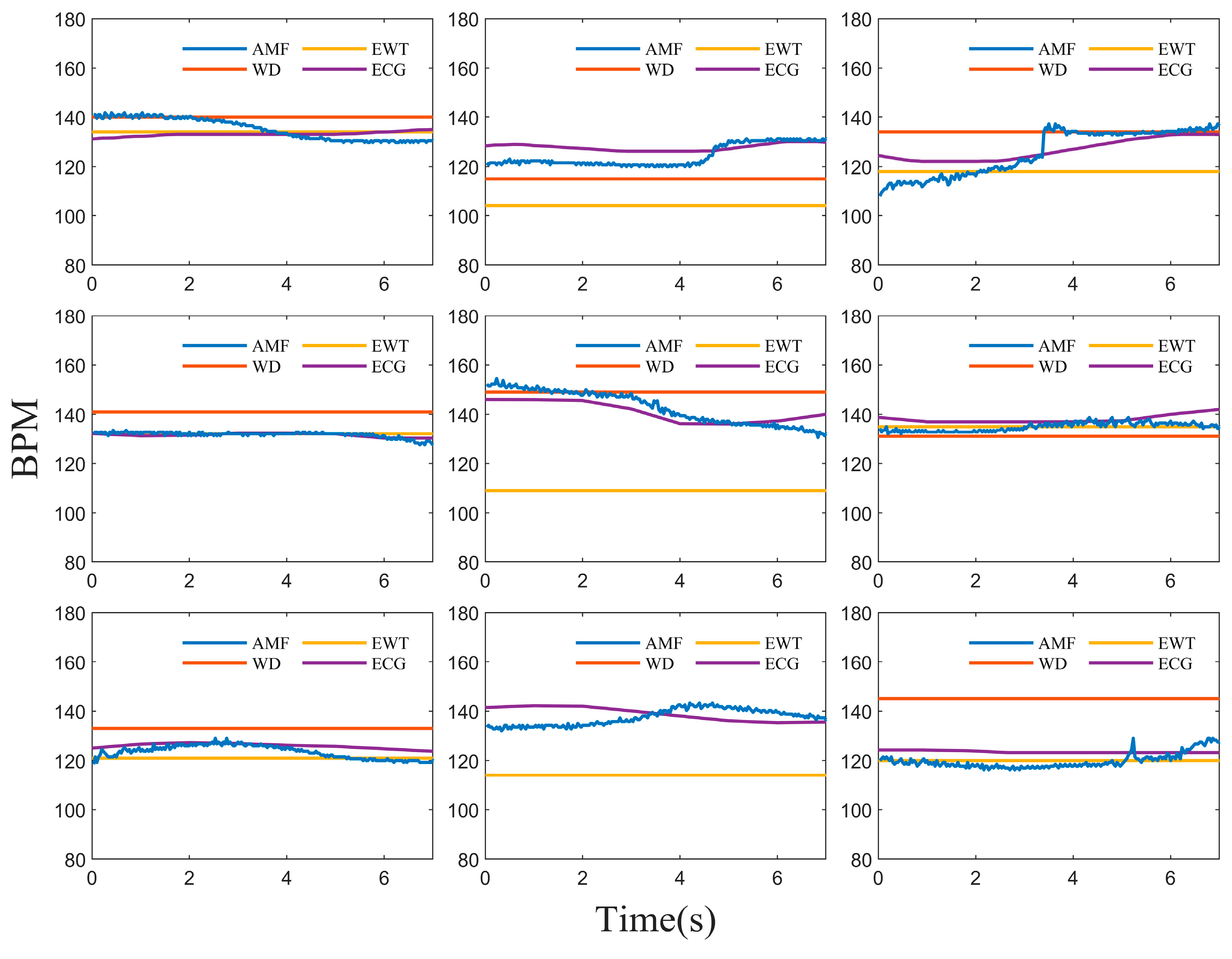
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E. Neonatal resuscitation. Respir. Care 2003, 48, 288–294; discussion 294–295. [Google Scholar] [PubMed]
- Garvey, A.A.; Dempsey, E.M. Simulation in Neonatal Resuscitation. Front. Pediatr. 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.L.; Umoren, R.A. Telesimulation for Neonatal Resuscitation Training. Semin. Perinatol. 2023, 47, 151827. [Google Scholar] [CrossRef] [PubMed]
- Flower, A.A.; Moorman, J.R.; Lake, D.E.; Delos, J.B. Periodic heart rate decelerations in premature infants. Exp. Biol. Med. 2010, 235, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.A.; Schmölzer, G.M. Heart rate assessment during neonatal resuscitation. Healthcare 2020, 8, 43. [Google Scholar] [CrossRef]
- Topfer, F.; Oberhammer, J. Millimeter-wave tissue diagnosis: The most promising fields for medical applications. IEEE Microw. Mag. 2015, 16, 97–113. [Google Scholar] [CrossRef]
- Anton, O.; Fernandez, R.; Rendon-Morales, E.; Aviles-Espinosa, R.; Jordan, H.; Rabe, H. Heart rate monitoring in newborn babies: A systematic review. Neonatology 2019, 116, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust non-contact vital signs monitoring using a camera. Biomed. Opt. Express 2015, 6, 1565–1588. [Google Scholar] [CrossRef]
- Ravichandran, R.; Saba, E.; Chen, K.-Y.; Goel, M.; Gupta, S.; Patel, S.N. WiBreathe: Estimating respiration rate using wireless signals in natural settings in the home. In Proceedings of the 2015 IEEE International Conference on Pervasive Computing and Communications (PerCom), St. Louis, MO, USA, 23–27 March 2015; pp. 131–139. [Google Scholar]
- Patwari, N.; Brewer, L.; Tate, Q.; Kaltiokallio, O.; Bocca, M. Breathfinding: A wireless network that monitors and locates breathing in a home. IEEE J. Sel. Top. Signal Process. 2013, 8, 30–42. [Google Scholar] [CrossRef]
- Liu, X.; Cao, J.; Tang, S.; Wen, J. Wi-sleep: Contactless sleep monitoring via wifi signals. In Proceedings of the 2014 IEEE Real-Time Systems Symposium, Rome, Italy, 2–5 December 2014; pp. 346–355. [Google Scholar]
- Lin, J.C. Noninvasive microwave measurement of respiration. Proc. IEEE 1975, 63, 1530. [Google Scholar] [CrossRef]
- Li, C.; Ling, J.; Li, J.; Lin, J. Accurate Doppler radar noncontact vital sign detection using the RELAX algorithm. IEEE Trans. Instrum. Meas. 2009, 59, 687–695. [Google Scholar]
- Xiong, Y.; Chen, S.; Dong, X.; Peng, Z.; Zhang, W. Accurate measurement in Doppler radar vital sign detection based on parameterized demodulation. IEEE Trans. Microw. Theory Tech. 2017, 65, 4483–4492. [Google Scholar] [CrossRef]
- Saluja, J.; Casanova, J.; Lin, J. A supervised machine learning algorithm for heart-rate detection using Doppler motion-sensing radar. IEEE J. Electromagn. RF Microw. Med. Biol. 2019, 4, 45–51. [Google Scholar] [CrossRef]
- Feng, C.; Jiang, X.; Jeong, M.-G.; Hong, H.; Fu, C.-H.; Yang, X.; Wang, E.; Zhu, X.; Liu, X. Multitarget vital signs measurement with chest motion imaging based on MIMO radar. IEEE Trans. Microw. Theory Tech. 2021, 69, 4735–4747. [Google Scholar] [CrossRef]
- Cardillo, E.; Caddemi, A. A review on biomedical MIMO radars for vital sign detection and human localization. Electronics 2020, 9, 1497. [Google Scholar] [CrossRef]
- Upadhyay, B.R.; Baral, A.B.; Torlak, M. Vital sign detection via angular and range measurements with mmWave MIMO radars: Algorithms and trials. IEEE Access 2022, 10, 106017–106032. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, W.H.; Lee, Y.; Lee, H.J.; Cha, T.; Kim, S.H.; Song, K.-M.; Lim, Y.-H.; Cho, S.H.; Cho, S.H. Non-contact respiration monitoring using impulse radio ultrawideband radar in neonates. R. Soc. Open Sci. 2019, 6, 190149. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, Y.; Na, J.Y.; Kim, S.H.; Lee, H.J.; Lim, Y.-H.; Cho, S.H.; Cho, S.H.; Park, H.-K. Feasibility of non-contact cardiorespiratory monitoring using impulse-radio ultra-wideband radar in the neonatal intensive care unit. PLoS ONE 2020, 15, e0243939. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.; Heo, R.; Park, H.-K.; Cho, S.-H.; Cho, S.H.; Lim, Y.-H. Preclinical evaluation of noncontact vital signs monitoring using real-time IR-UWB radar and factors affecting its accuracy. Sci. Rep. 2021, 11, 23602. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, G.; Inoue, T.; Li, C. Doppler radar vital sign detection with random body movement cancellation based on adaptive phase compensation. In Proceedings of the 2013 IEEE MTT-S International Microwave Symposium Digest (MTT), Seattle, WC, USA, 2–7 June 2013; pp. 1–3. [Google Scholar]
- Li, C.; Lin, J. Random Body Movement Cancellation in Doppler Radar Vital Sign Detection. IEEE Trans. Microw. Theory Tech. 2008, 56, 3143–3152. [Google Scholar] [CrossRef]
- Wang, F.K.; Horng, T.S.; Peng, K.C.; Jau, J.K.; Li, J.Y.; Chen, C.C. Single-Antenna Doppler Radars Using Self and Mutual Injection Locking for Vital Sign Detection With Random Body Movement Cancellation. IEEE Trans. Microw. Theory Tech. 2011, 59, 3577–3587. [Google Scholar] [CrossRef]
- Yu, X.; Li, C.; Lin, J. Two-dimensional noncontact vital sign detection using Doppler radar array approach. In Proceedings of the 2011 IEEE MTT-S International Microwave Symposium, Baltimore, MD, USA, 5–10 June 2011; pp. 1–4. [Google Scholar]
- Zhang, X.; Yang, X.; Ding, Y.; Wang, Y.; Zhou, J.; Zhang, L. Contactless simultaneous breathing and heart rate detections in physical activity using ir-uwb radars. Sensors 2021, 21, 5503. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Chen, L.; An, K.; Wang, J.; Li, H.; Ye, D.; Huangfu, J.; Li, C.; Ran, L. Doppler Vital Signs Detection in the Presence of Large-Scale Random Body Movements. IEEE Trans. Microw. Theory Tech. 2018, 66, 4261–4270. [Google Scholar] [CrossRef]
- Tariq, A.; Shiraz, H. Doppler radar vital signs monitoring using wavelet transform. In Proceedings of the 2010 Loughborough Antennas & Propagation Conference, Loughborough, UK, 8–9 November 2010; pp. 293–296. [Google Scholar]
- Mercuri, M.; Lorato, I.R.; Liu, Y.-H.; Wieringa, F.; Hoof, C.V.; Torfs, T. Vital-sign monitoring and spatial tracking of multiple people using a contactless radar-based sensor. Nat. Electron. 2019, 2, 252–262. [Google Scholar] [CrossRef]
- He, M.; Nian, Y.; Xu, L.; Qiao, L.; Wang, W. Adaptive separation of respiratory and heartbeat signals among multiple people based on empirical wavelet transform using UWB radar. Sensors 2020, 20, 4913. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Kong, L.; Foroughian, F.; Wang, H.; Theilmann, P.; Fathy, A.E. Comparison Study of Noncontact Vital Signs Detection Using a Doppler Stepped-Frequency Continuous-Wave Radar and Camera-Based Imaging Photoplethysmography. IEEE Trans. Microw. Theory Tech. 2017, 65, 3519–3529. [Google Scholar] [CrossRef]
- Harter, M.; Mahler, T.; Schipper, T.; Ziroff, A.; Zwick, T. 2-D antenna array geometries for MIMO radar imaging by Digital Beamforming. In Proceedings of the 2013 European Microwave Conference, Nuremberg, Germany, 6–10 October 2013; pp. 1695–1698. [Google Scholar]
- Wang, G.; Muñoz-Ferreras, J.M.; Gu, C.; Li, C.; Gómez-García, R. Application of Linear-Frequency-Modulated Continuous-Wave (LFMCW) Radars for Tracking of Vital Signs. IEEE Trans. Microw. Theory Tech. 2014, 62, 1387–1399. [Google Scholar] [CrossRef]
- Zhang, X.-D. Modern Signal Processing; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022. [Google Scholar]
- Rana, M.J.; Alam, M.S.; Islam, M.S. Continuous wavelet transform based analysis of low frequency oscillation in power system. In Proceedings of the 2015 International Conference on Advances in Electrical Engineering (ICAEE), Dhaka, Bangladesh, 17–19 December 2015; pp. 320–323. [Google Scholar]
- Hu, X.; Jin, T. Short-range vital signs sensing based on EEMD and CWT using IR-UWB radar. Sensors 2016, 16, 2025. [Google Scholar] [CrossRef]
- Addison, P.S.; Watson, J.N.; Feng, T. Low-oscillation complex wavelets. J. Sound Vib. 2002, 254, 733–762. [Google Scholar] [CrossRef]
- Gilles, J. Empirical wavelet transform. IEEE Trans. Signal Process. 2013, 61, 3999–4010. [Google Scholar] [CrossRef]
- Jähne, B. Digital Image Processing; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Xiong, Y.; Peng, Z.; Gu, C.; Li, S.; Wang, D.; Zhang, W. Differential Enhancement Method for Robust and Accurate Heart Rate Monitoring via Microwave Vital Sign Sensing. IEEE Trans. Instrum. Meas. 2020, 69, 7108–7118. [Google Scholar] [CrossRef]
- Li, Z.; Jin, T.; Dai, Y.; Song, Y. Motion-Robust Contactless Heartbeat Sensing Using 4-D Imaging Radar. IEEE Trans. Instrum. Meas. 2023, 72, 4011110. [Google Scholar] [CrossRef]

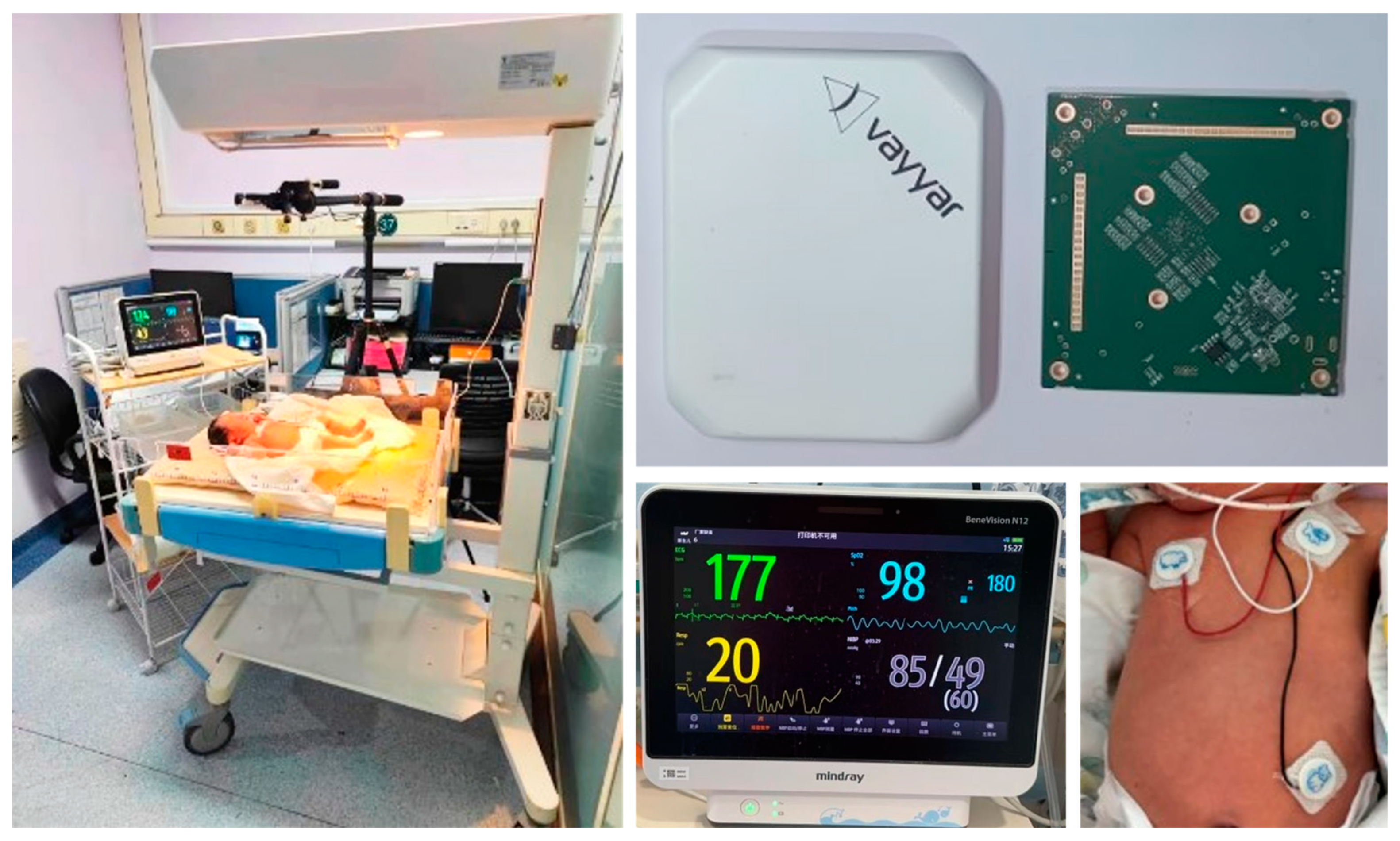
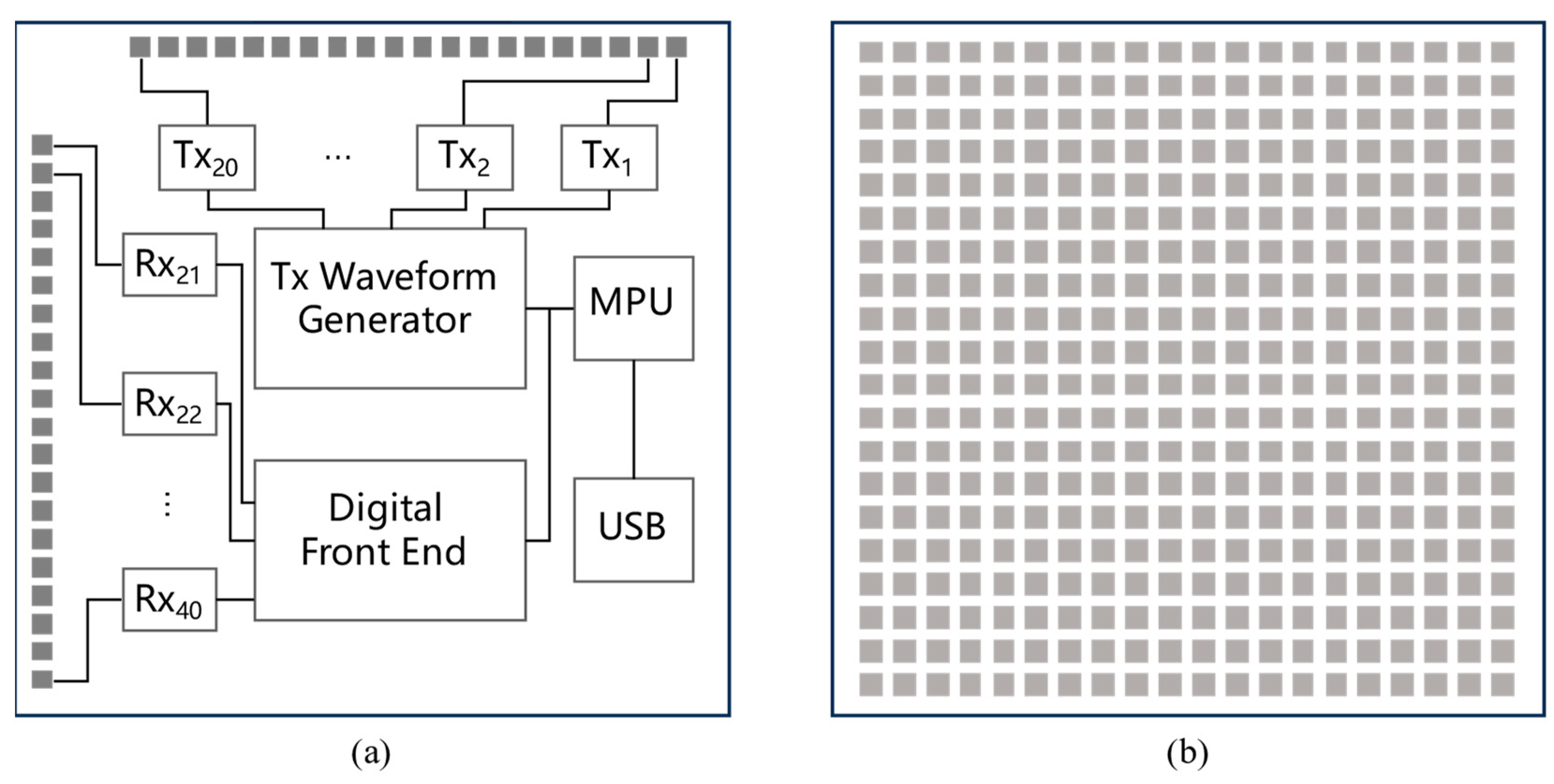


| Study Participants | Gestational Age | Weight | Age | Symptoms |
|---|---|---|---|---|
| Baby1 | 39 weeks and 5 days | 3180 g | 4 days | Newborn wet lung |
| Baby2 | 33 weeks and 5 days | 1840 g | 14 days | Premature birth |
| Baby3 | 39 weeks and 2 days | 3710 g | 4 days | Amniotic fluid inhalation, mild suffocation |
| Parameters | Value |
|---|---|
| Frequency Band | 62–69 GHz |
| ADC Samples | 151 |
| Stop–Start Min Step | 150 MHz |
| EIRP (Effective Isotropic Radiated Power) | −5 dBm |
| Max Range Resolution | 2.14 cm |
| Max Angular Resolution | 6.7° |
| Study Participants | ACC (%) | MAE (bpm) | RMSE (bpm) | |||
|---|---|---|---|---|---|---|
| Static | Movement | Static | Movement | Static | Movement | |
| Baby1 | 96.4 | 96.3 | 4.6 | 4.7 | 5.4 | 5.8 |
| Baby2 | 99.4 | 96.4 | 0.7 | 3.1 | 1.0 | 3.6 |
| Baby3 | 97.6 | 96.2 | 3.0 | 4.6 | 3.6 | 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liang, X.; Dang, X.; Jiang, N.; Cao, J.; Zeng, Z.; Li, Y. Random Body Movement Removal Using Adaptive Motion Artifact Filtering in mmWave Radar-Based Neonatal Heartbeat Sensing. Electronics 2024, 13, 1471. https://doi.org/10.3390/electronics13081471
Yang S, Liang X, Dang X, Jiang N, Cao J, Zeng Z, Li Y. Random Body Movement Removal Using Adaptive Motion Artifact Filtering in mmWave Radar-Based Neonatal Heartbeat Sensing. Electronics. 2024; 13(8):1471. https://doi.org/10.3390/electronics13081471
Chicago/Turabian StyleYang, Shiguang, Xuerui Liang, Xiangwei Dang, Nanyi Jiang, Jiasheng Cao, Zhiyuan Zeng, and Yanlei Li. 2024. "Random Body Movement Removal Using Adaptive Motion Artifact Filtering in mmWave Radar-Based Neonatal Heartbeat Sensing" Electronics 13, no. 8: 1471. https://doi.org/10.3390/electronics13081471
APA StyleYang, S., Liang, X., Dang, X., Jiang, N., Cao, J., Zeng, Z., & Li, Y. (2024). Random Body Movement Removal Using Adaptive Motion Artifact Filtering in mmWave Radar-Based Neonatal Heartbeat Sensing. Electronics, 13(8), 1471. https://doi.org/10.3390/electronics13081471







