Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota
Abstract
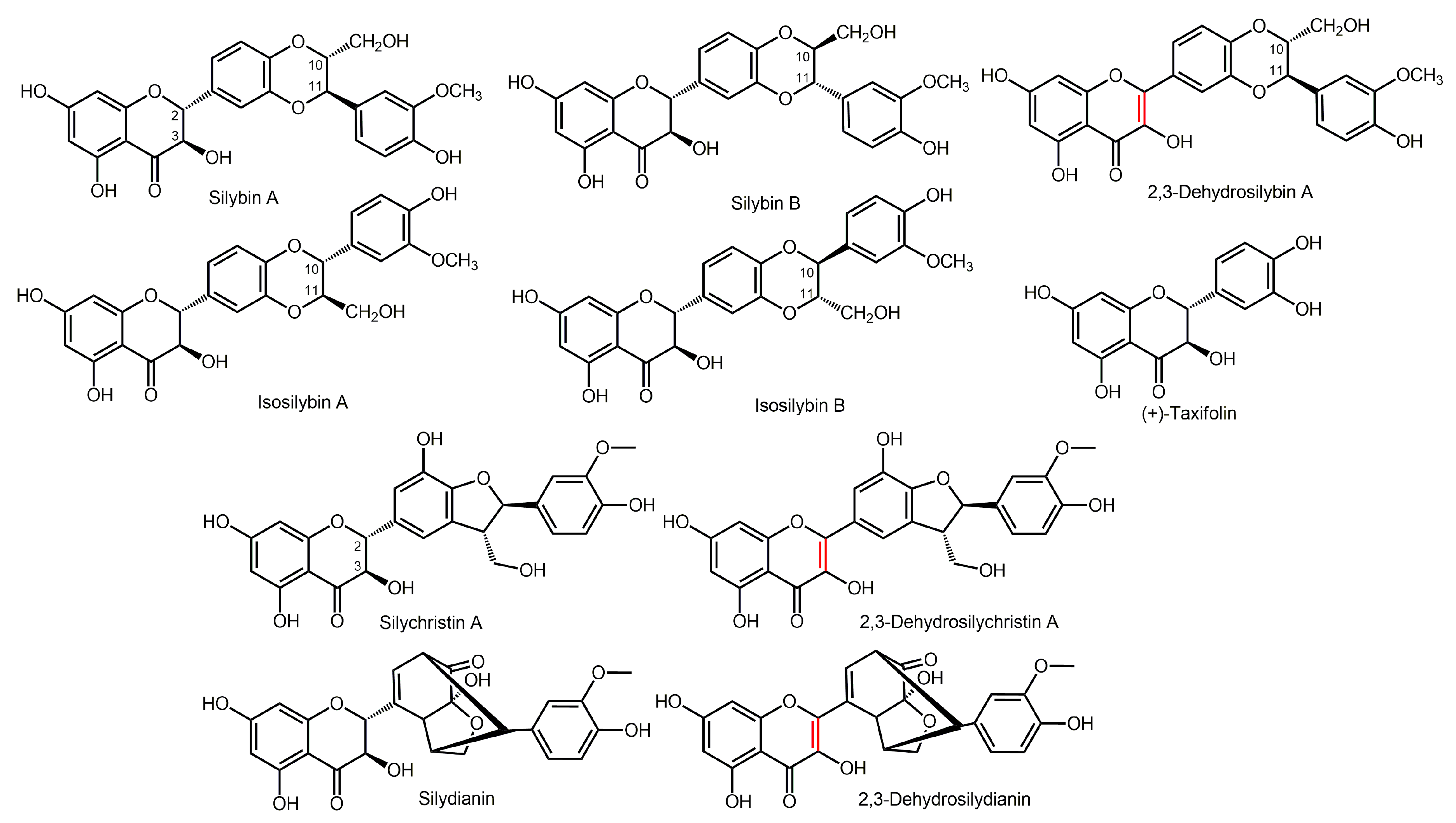
:1. Introduction
2. Results and Discussion
2.1. Pilot Study
2.2. Fecal Fermentation of Silymarin Flavonolignans Ex Vivo
2.3. Inter-Individual Variability
3. Materials and Methods
3.1. Flavonolignans and Other Chemicals
3.2. Fecal Samples and Ethics Statement
3.3. Pilot Study
3.4. Fecal Fermentation of Silymarin Flavonolignans Ex Vivo
3.5. Sample Preparation
3.6. Analysis of the Metabolites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


| Compound | Elemental Composition | m/z | tR (min) | Δppm | MSE |
|---|---|---|---|---|---|
| Taxifolin | |||||
| Taxifolin | C15H12O7 | 303.0490 | 2.5 | −4.9 | 175.0391; 125.0224 |
| Taxifolin isomer | C15H12O7 | 303.0486 | 2.8 | −6.3 | 125.0221 |
| C15H10O4 | C15H10O4 | 253.0488 | 10.3 | −5.1 | xxx |
| Sulfate conjugation | C15H12O10S | 383.0034 | 1.5 | −7.3 | 285.0424 |
| Loss of O | C15H12O6 | 287.0540 | 3.6 | −5.6 | 177.0523; 125.0225 |
| Loss of O | C15H12O6 | 287.0538 | 5.2 | −6.3 | xxx |
| Loss of O | C15H12O6 | 287.0541 | 1.6 | −5.2 | xxx |
| Methylation | C16H14O7 | 317.0650 | 4.2 | −3.5 | 125.0263 |
| 2x Loss of O | C15H12O5 | 271.0609 | 6.8 | 1.1 | xxx |
| Silybin | |||||
| Silybin A | C25H22O10 | 481.1118 | 7.7 | −3.5 | 301.0304; 125.0217 |
| Silybin B | C25H22O10 | 481.1123 | 7.8 | −2.5 | 301.0349; 125.0210 |
| Isosilybin | C25H22O10 | 481.1165 | 8.2 | 6.2 | xxx |
| Demethylation | C24H20O10 | 467.0946 | 6.1 | −6.9 | 301.0317; 125.0212 |
| Demethylation | C24H20O10 | 467.0964 | 6.2 | −3.0 | 301.0322; 125.0217 |
| Isosilybin | |||||
| Isosilybin | C25H22O10 | 481.1133 | 8.3 | −0.4 | 453.1169; 125.0224 |
| Demethylation | C24H20O10 | 467.0954 | 6.4 | −5.1 | 125.0221 |
| Demethylation | C24H20O10 | 467.0969 | 6.5 | −1.9 | 285.0389; 125.0227 |
| Decarbonylation | C24H21O9 | 453.1164 | 6.3 | −4.9 | 181.0141; 125.0203 |
| Silychristin | |||||
| Silychristin A | C25H22O10 | 481.1129 | 5.1 | −1.2 | 325.0701; 125.0232 |
| Silychristin B | C25H22O10 | 481.1124 | 5.3 | −2.3 | 325.0687 |
| Reduction | C25H24O10 | 483.1268 | 5.3 | −4.8 | 357.0912; 125.0242 |
| Reduction | C25H24O10 | 483.1346 | 4.8 | 11.4 | xxx |
| Desaturation + demethylation + sulfate conjugation | C24H18O13S | 545.0369 | 4.8 | −3.9 | xxx |
| Desaturation + demethylation | C24H18O10 | 465.0819 | 6.5 | −0.6 | xxx |
| + C3H6O | C28H28O11 | 539.1550 | 5.9 | −0.6 | 183.0648; 237.0384 |
| + C3H6O + desaturation | C28H26O11 | 537.1390 | 7.0 | −1.3 | 235.0247 |
| + C3H6O isomer + desaturation | C28H26O11 | 537.1394 | 7.3 | −0.6 | 183.0655; 235.0224 |
| + C5H8O2 | C30H30O12 | 581.1659 | 6.2 | −0.2 | 553.1713; 279.0490 |
| C4H8O6 | C4H8O6 | 151.0239 | 0.7 | −2.6 | xxx |
| C4H8O7 | C4H8O7 | 167.0200 | 0.6 | 4.8 | xxx |
| Silydianin | |||||
| Silydianin | C25H22O10 | 481.1135 | 6.6 | 0.0 | 178.9972; 151.0023 |
| Reduction | C25H24O10 | 483.1280 | 4.7 | −2.3 | 125.0236 |
| Cysteine conjugation | C28H27NO11S | 584.1212 | 6.1 | −2.6 | 326.0632; 178.9949 |
| Cysteine conjugation | C28H27NO11S | 584.1229 | 7.7 | 0.3 | 125.0215; 326.0641 |
| Cysteine conjugation | C28H27NO11S | 584.1216 | 6.3 | −1.9 | 326.0647; 125.0211 |
| Cysteine conjugation | C28H27NO11S | 584.1207 | 6.0 | −3.4 | 326.0670 |
| Decarbonylation | C24H22O9 | 453.1169 | 6.3 | −3.8 | 125.0215; 182.0191 |
| Desaturation | C25H20O10 | 479.0982 | 7.2 | 0.8 | 299.0183 |
| +C3H6O | C28H28O11 | 539.1563 | 6.9 | −0.6 | 209.0441; 237.0386 |
| +C3H6O + desaturation | C28H26O11 | 537.1393 | 7.7 | −0.7 | 235.0241 |
| +C3H6O isomer + desaturation | C28H26O11 | 537.1396 | 8.2 | −0.2 | 235.0220; 207.0274 |
| + C5H8O2 | C30H30O12 | 581.1656 | 7.2 | −0.5 | xxx |
| C4H8O6 | C4H8O6 | 151.0252 | 0.8 | 6.0 | 135.0308 |
| C4H8O7 | C4H8O7 | 167.0205 | 0.7 | 7.8 | xxx |
| 2,3-Dehydrosilybin | |||||
| 2,3-Dehydrosilybin | C25H20O10 | 479.0985 | 10.2 | 1.5 | 299.0185; 271.0237 |
| 2,3-Dehydrosilybin isomer | C25H20O10 | 479.0971 | 10.0 | −1.5 | 299.0186; 271.0241 |
| Demethylation | C24H18O10 | 465.0825 | 8.7 | 0.6 | 299.0188; 271.0238 |
| Silybin A | C25H22O10 | 481.1115 | 8.0 | −4.2 | 301.0331; 125.0248 |
| Silybin B | C25H22O10 | 481.1134 | 8.1 | −0.2 | 301.0341 |
| Reduction + demethylation | C24H20O10 | 467.0970 | 6.1 | −1.7 | 125.0215; 301.0320 |
| Reduction + demethylation | C24H20O11 | 467.0963 | 6.2 | −3.2 | 125.0213; 301.0315 |
| 2,3-Dehydrosilychristin | |||||
| 2,3-Dehydrosilychristin | C25H20O10 | 479.0964 | 7.4 | −2.9 | 449.0852; 151.0018 |
| Dehydrosilychristin isomer | C25H20O10 | 479.0958 | 7.7 | −4.2 | 449.0944; 461.0851 |
| 2,3-Dehydrosilybin | C25H20O10 | 479.0941 | 10.0 | −7.7 | 299.0178 |
| Silychristin A | C25H22O10 | 481.1119 | 5.1 | −3.3 | 125.0229; 325.0711 |
| Silychristin B | C25H22O10 | 481.1115 | 5.3 | −4.2 | 125.0253 |
| Demethylation | C24H18O10 | 465.0816 | 5.9 | −1.3 | xxx |
| Reduction + demethylation | C24H20O10 | 467.0950 | 6.4 | −6.0 | xxx |
| Reduction + demethylation | C24H20O10 | 467.0934 | 6.1 | −9.4 | xxx |
| Reduction + demethylation | C24H20O10 | 467.0948 | 6.2 | −6.4 | xxx |
| 2x reduction | C25H24O10 | 483.1307 | 5.3 | 3.3 | 125.0245 |
| 2,3-Dehydrosilydianin | |||||
| 2,3-Dehydrosilydianin | C25H20O10 | 479.0955 | 7.0 | −4.8 | 299.0168; 127.0477 |
| Silydianin | C25H22O10 | 481.1135 | 6.3 | −0.2 | 151.0013; 178.9931 |
| Cysteine conjugation | C28H25NO11S | 582.1053 | 8.2 | −3 | 324.0479 |
| Cysteine conjugation | C28H25NO11S | 582.1066 | 9.1 | −0.7 | 324.0489 |
| Decarbonylation | C24H20O9 | 451.1004 | 7.1 | −5.5 | 315.1245; 300.1013 |
| Decarbonylation | C24H20O9 | 451.1029 | 9.2 | 0.0 | 301.0341 |
| Demethylation | C24H18O10 | 465.0805 | 7.1 | −3.7 | xxx |
| Reduction + demethylation | C24H20O10 | 467.0966 | 6.2 | −2.6 | 125.0244; 151.0105 |
| Reduction + cysteine conjg. | C28H27NO11S | 584.1221 | 7.7 | −1 | 326.0655; 125.0227 |

References
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Zholobenko, A.; Modrianský, M. Silymarin and its constituents in cardiac preconditioning. Fitoterapia 2014, 97, 122–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Škottová, N.; Krečman, V. Silymarin as a potential hypocholesterolaemic drug. Physiol Res. 1998, 47, 1–7. [Google Scholar] [PubMed]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Ferreri, K.; Todorov, I.; Kuroda, Y.; Smith, C.V.; Kandeel, F.; Mullen, Y. Silymarin protects pancreatic β-cells against cytokine-mediated toxicity: Implication of c-jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology 2005, 146, 175–185. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Gažák, R.; Walterová, D.; Křen, V. Silybin and silymarin-new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Kvasnička, F.; Biba, B.; Ševčík, R.; Voldřich, M.; Kratká, J. Analysis of the active components of silymarin. J. Chromatogr. A 2003, 990, 239–245. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Romanucci, V.; Zarrelli, A.; Monaco, I.; Lolicato, F.; Spinella, N.; Galati, C.; Grasso, G.; D’Urso, L.; Romeo, M.; et al. Inhibition of Aβ amyloid growth and toxicity by silybins: The crucial role of stereochemistry. ACS Chem. Neurosci. 2017, 8, 1767–1778. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7-1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Rajamanickam, S.; Singh, R.P.; Deep, G.; Chittezhath, M.; Agarwal, R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008, 68, 6822–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [Green Version]
- Křen, V.; Marhol, P.; Purchartová, K.; Gabrielová, E.; Modrianský, M. Biotransformation of silybin and its congeners. Curr. Drug Metab. 2013, 14, 1009–1021. [Google Scholar] [CrossRef]
- Miranda, S.R.; Lee, J.K.; Brouwer, K.L.; Wen, Z.; Smith, P.C.; Hawke, R.L. Hepatic metabolism and biliary excretion of silymarin flavonolignans in isolated perfused rat livers: Role of multidrug resistance-associated protein 2 (Abcc2). Drug Metab. Dispos. 2008, 36, 2219–2226. [Google Scholar] [CrossRef] [Green Version]
- Vrba, J.; Papoušková, B.; Roubalová, L.; Zatloukalová, M.; Biedermann, D.; Křen, V.; Valentová, K.; Ulrichová, J.; Vacek, J. Metabolism of flavonolignans in human hepatocytes. J. Pharm. Biomed. Anal. 2018, 152, 94–101. [Google Scholar] [CrossRef]
- Vrba, J.; Papoušková, B.; Lněničková, K.; Kosina, P.; Křen, V.; Ulrichová, J. Identification of UDP-glucuronosyltransferases involved in the metabolism of silymarin flavonolignans. J. Pharm. Biomed. Anal. 2019, 178, 112972. [Google Scholar] [CrossRef]
- Jančová, P.; Anzenbacherová, E.; Papoušková, B.; Lemr, K.; Lužná, P.; Veinlichová, A.; Anzenbacher, P.; Šimánek, V. Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab. Dispos. 2007, 35, 2035–2039. [Google Scholar] [CrossRef] [Green Version]
- Jančová, P.; Šiller, M.; Anzenbacherová, E.; Křen, V.; Anzenbacher, P.; Šimánek, V. Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica 2011, 41, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, transport and drug–drug interactions of silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yang, D.-H.; Zhang, Y.-T.; Chen, X.-M.; Li, L.-L.; Cai, S.-Q. Biotransformation on the flavonolignan constituents of Silybi Fructus by an intestinal bacterial strain Eubacterium limosum ZL-II. Fitoterapia 2014, 92, 61–71. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Li, X.-Y.; Ji, H.-F. Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl. Microbiol. Biotechnol. 2019, 103, 7141–7149. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food - an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef]
- Aura, A.M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimia, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef]
- de Oliveira, D.R.; Tintino, S.R.; Braga, M.F.; Boligon, A.A.; Athayde, M.L.; Coutinho, H.D.; de Menezes, I.R.; Fachinetto, R. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. Biomed. Res. Int. 2015, 2015, 292797. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Hong, D.Y.; Kim, E.S.; Lee, H.G. Improving the water solubility and antimicrobial activity of silymarin by nanoencapsulation. Colloids Surf. B Biointerfaces 2017, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Evren, E.; Yurtcu, E. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia Microbiol. 2015, 60, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharm. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Koren, E.; Kohen, R.; Ovadia, H.; Ginsburg, I. Bacteria coated by polyphenols acquire potent oxidant-scavenging capacities. Exp. Biol. Med. 2009, 234, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vidlář, A.; Zatloukalová, M.; Stuchlík, M.; Vacek, J.; Šimánek, V.; Ulrichová, J. Biosafety and antioxidant effects of a beverage containing silymarin and arginine. A pilot, human intervention cross-over trial. Food Chem. Toxicol. 2013, 56, 178–183. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. bioavailability of quercetin in humans with a focus on interindividual variation. Comp. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, J.; Valentová, K.; Vacek, J.; Paliková, I.; Zatloukalová, M.; Kosina, P.; Ulrichová, J.; Vrbková, J.; Šimánek, V. Metabolic profiling of phenolic acids and oxidative stress markers after consumption of Lonicera caerulea L. fruit. J. Agric. Food Chem. 2013, 61, 4526–4532. [Google Scholar] [CrossRef]
- Havlík, J.; Edwards, C. Non-extractable polyphenols and the gut microbiome. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 241–262. [Google Scholar]
- Jarošová, V.; Veselý, O.; Maršík, P.; Jaimes, J.D.; Smejkal, K.; Klouček, P.; Havlík, J. Metabolism of stilbenoids by human faecal microbiota. Molecules 2019, 24, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gažák, R.; Fuksová, K.; Marhol, P.; Kuzma, M.; Agarwal, R.; Křen, V. Preparative method for isosilybin isolation based on enzymatic kinetic resolution of silymarin mixture. Process Biochem. 2013, 48, 184–189. [Google Scholar] [CrossRef]
- Petrásková, L.; Káňová, K.; Valentová, K.; Kuzma, M.; Biedermann, D.; Křen, V. A simple and rapid HPLC separation and quantification of flavonoid, flavonolignans and 2,3-dehydroflavonolignans in silymarin. Foods 2020. submitted. [Google Scholar]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barrio, R.; Edwards, C.A.; Crozier, A. Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: In vivo and in vitro studies. Drug Metab. Dispos. 2011, 39, 1680–1688. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Borges, G.; Ky, I.; Ribas, A.; Calani, L.; Del Rio, D.; Clifford, M.N.; Roberts, S.A.; Crozier, A. In vitro colonic catabolism of orange juice (poly)phenols. Mol. Nutr. Food Res. 2015, 59, 465–475. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Manuel Moreno-Rojas, J.; Brindani, N.; Del Rio, D.; Lean, M.E.J.; Hara, Y.; Crozier, A. Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agric. Food Chem. 2017, 65, 5365–5374. [Google Scholar] [CrossRef]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef]


| Compound | 0 | 6 | 12 | 24 | 48 h |
|---|---|---|---|---|---|
| Silybin A | 7.0 ± 1.2 | 7.6 ± 0.8 | 7.8 ± 1.2 | 10.4 ± 0.5 | 8.5 ± 1.8 |
| Silybin B | 5.8 ± 1.1 | 6.7 ± 0.7 | 6.5 ± 1.1 | 9.5 ± 0.8 | 6.7 ± 2.0 |
| Silychristin A | 29.0 ± 0.5 | 29.6 ± 0.4 | 33.5 ± 1.3 | 5.1 ± 1.2 | 3.5 ± 0.2 |
| Silychristin B | 4.8 ± 0.1 | 3.8 ± 0.1 | 1.2 ± 0.1 | 0.4 ± 0.0 | 0.5 ± 0.1 |
| Silydianin | 69.9 ± 0.9 | 70.1 ± 8.0 | 55.8 ± 5.4 | 19.7 ± 3.6 | 13.1 ± 1.0 |
| 2,3-Dehydrosilybin (A + B) | 57.4 ± 3.8 | 47.8 ± 2.3 | 54.2 ± 1.0 | 60.6 ± 5.4 | 57.5 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentová, K.; Havlík, J.; Kosina, P.; Papoušková, B.; Jaimes, J.D.; Káňová, K.; Petrásková, L.; Ulrichová, J.; Křen, V. Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota. Metabolites 2020, 10, 29. https://doi.org/10.3390/metabo10010029
Valentová K, Havlík J, Kosina P, Papoušková B, Jaimes JD, Káňová K, Petrásková L, Ulrichová J, Křen V. Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota. Metabolites. 2020; 10(1):29. https://doi.org/10.3390/metabo10010029
Chicago/Turabian StyleValentová, Kateřina, Jaroslav Havlík, Pavel Kosina, Barbora Papoušková, José Diógenes Jaimes, Kristýna Káňová, Lucie Petrásková, Jitka Ulrichová, and Vladimír Křen. 2020. "Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota" Metabolites 10, no. 1: 29. https://doi.org/10.3390/metabo10010029
APA StyleValentová, K., Havlík, J., Kosina, P., Papoušková, B., Jaimes, J. D., Káňová, K., Petrásková, L., Ulrichová, J., & Křen, V. (2020). Biotransformation of Silymarin Flavonolignans by Human Fecal Microbiota. Metabolites, 10(1), 29. https://doi.org/10.3390/metabo10010029







