Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae)
Abstract
:1. Introduction
2. Results and Discussion
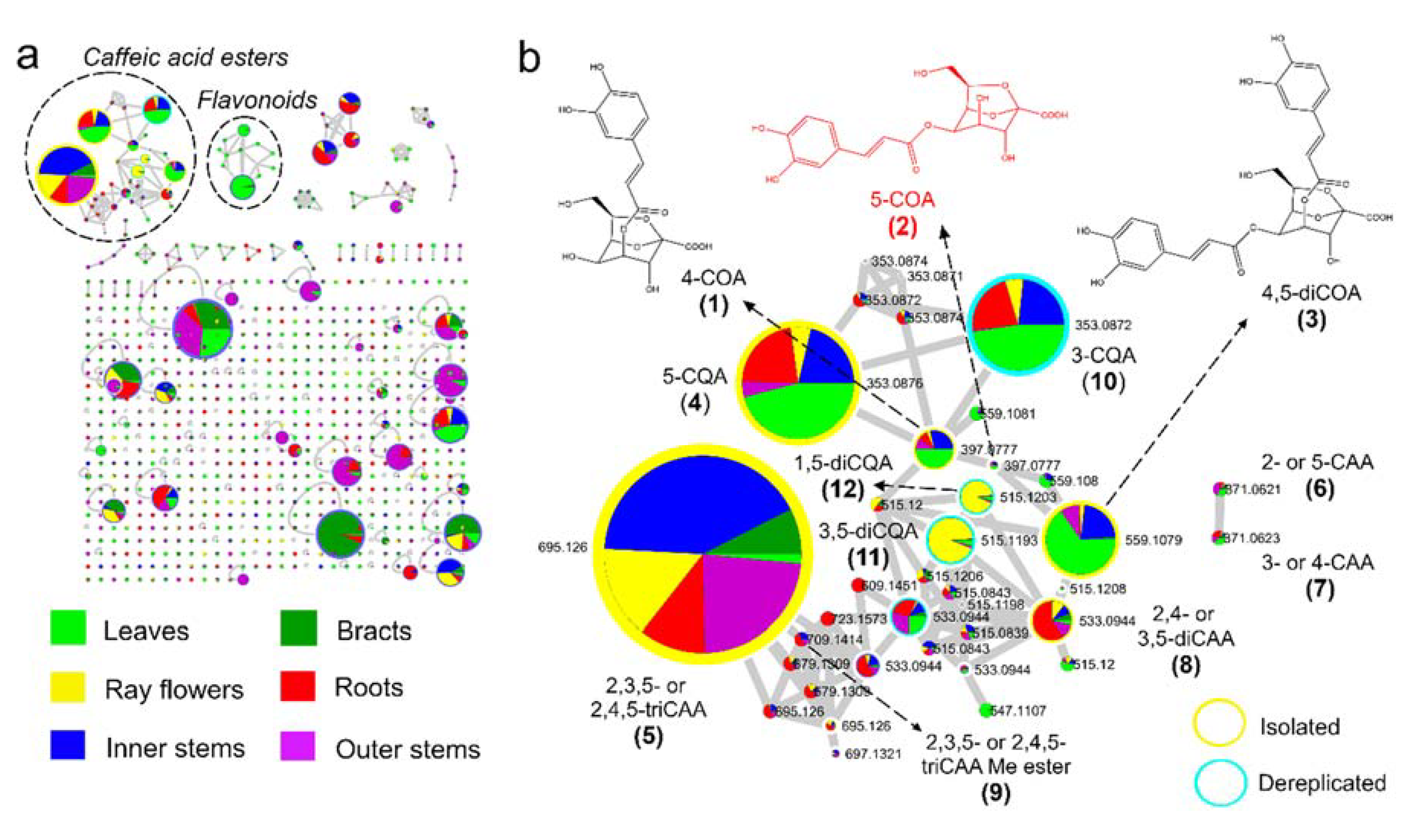
2.1. Feature-Based Molecular Networking of Different Organs
2.2. Isolation and Structural Elucidation of Caffeic Acid Esters from Yacon
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Metabolites and UHPLC-UV-HRMS/MS Analysis
3.3. Feature-Based Molecular Networking
3.4. Isolation of Caffeic Acid Esters
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grau, A.; Rea, J. Yacon. Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson. In Andean Roots and Tubers: Ahipa, Arracacha, Maca and Yacon. Promoting the Conservation and Use of Underutilized and Neglected Crops; Hermann, M., Heller, J., Eds.; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1997; p. 255. [Google Scholar]
- Oliveira, G.O.; Braga, C.P.; Fernandes, A.A.H. Improvement of biochemical parameters in type 1 diabetic rats after the roots aqueous extract of yacon [Smallanthus sonchifolius (Poepp.& Endl.)] treatment. Food Chem. Toxicol. 2013, 59, 256–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honoré, S.M.; Cabrera, W.M.; Genta, S.B.; Sánchez, S.S. Protective effect of yacon leaves decoction against early nephropathy in experimental diabetic rats. Food Chem. Toxicol. 2012, 50, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Genta, S.; Cabrera, W.; Habib, N.; Pons, J.; Carillo, I.M.; Grau, A.; Sánchez, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Lebeda, A.; Doležalová, I.; Jirovský, D.; Simonovska, B.; Vovk, I.; Kosina, P.; Gasmanová, N.; Dziechciarková, M.; Ulrichová, J. The biological and chemical variability of Yacon. J. Agric. Food Chem. 2006, 54, 1347–1352. [Google Scholar] [CrossRef]
- Causey, J.L.; Feirtag, J.M.; Gallaher, D.D.; Tungland, B.C.; Slavin, J.L. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr. Res. 2000, 20, 191–201. [Google Scholar] [CrossRef]
- Genta, S.B.; Cabrera, W.M.; Mercado, M.I.; Grau, A.; Catalán, C.A.; Sánchez, S.S. Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: Constituents of the most active fractions. Chem. Biol. Interact. 2010, 185, 143–152. [Google Scholar] [CrossRef]
- Park, J.-S.; Yang, J.-S.; Hwang, B.-Y.; Yoo, B.-K.; Han, K. Hypoglycemic effect of yacon tuber extract and its constituent, chlorogenic acid, in streptozotocin-induced diabetic rats. Biomol. Ther. 2009, 17, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Takenaka, M.; Yan, X.; Ono, H.; Yoshida, M.; Nagata, T.; Nakanishi, T. Caffeic acid derivatives in the roots of yacon (Smallanthus sonchifolius). J. Agric. Food Chem. 2003, 51, 793–796. [Google Scholar] [CrossRef]
- Takenaka, M.; Ono, H. Novel octulosonic acid derivatives in the composite Smallanthus sonchifolius. Tetrahedron Lett. 2003, 44, 999–1002. [Google Scholar] [CrossRef]
- Terada, S.; Itoh, K.; Noguchi, N.; Ishida, T. Alpha-Glucosidase Inhibitor for Blood Glucose Level Elevation and Functional Food Containing Tricaffeoylaldaric Acid and Method for Producing Tricaffeoylaldaric Acid. U.S. Patent 209649, 20 August 2009. [Google Scholar]
- Padilla-González, G.F.; Amrehn, E.; Frey, M.; Gómez-Zeledón, J.; Kaa, A.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression studies reveal the diversity, distribution and spatial regulation of the specialized metabolism of yacon (Smallanthus sonchifolius, Asteraceae). Int. J. Mol. Sci. 2020, 21, 4555. [Google Scholar] [CrossRef]
- Da Silva, M.D.F.G.; Dionísio, A.P.; de Abreu, F.A.P.; de Brito, E.S.; Wurlitzer, N.J.; Silva, L.M.A.; Ribeiro, P.R.V.; Rodrigues, S.; Taniguchi, C.A.K.; Pontes, D.F. Evaluation of nutritional and chemical composition of yacon syrup using 1H NMR and UPLC-ESI-Q-TOF-MSE. Food Chem. 2018, 245, 1239–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-González, G.F.; Frey, M.; Gómez-Zeledón, J.; Da Costa, F.B.; Spring, O. Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacon (Smallanthus sonchifolius, Asteraceae). Sci. Rep. 2019, 9, 13178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, P.; Qu, H.; Cheng, Y. Characterization of phenolic compounds in Erigeron breviscapus by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Q.; Ma, J.; Wu, B.; Zeng, X. Chemical characterization of phenolic compounds in Erigeron injection by rapid-resolution LC coupled with multi-stage and quadrupole-TOF-MS. Chromatographia 2010, 72, 651–658. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Diazgranados, M.; Da Costa, F.B. Biogeography shaped the metabolome of the genus Espeletia: A phytochemical perspective on an Andean adaptive radiation. Sci. Rep. 2017, 7, 8835. [Google Scholar] [CrossRef] [Green Version]
- Padilla-González, G.F.; Diazgranados, M.; Oliveira, T.B.; Chagas-Paula, D.A.; Da Costa, F.B. Chemistry of the subtribe Espeletiinae (Asteraceae) and its correlation with phylogenetic data: An in silico chemosystematic approach. Bot. J. Linn. Soc. 2018, 186, 18–46. [Google Scholar] [CrossRef]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef]
- Dudek, M.K.; Dudkowski, Ł.; Bazylko, A.; Kaźmierski, S.; Kiss, A.K. Caffeic acid derivatives isolated from the aerial parts of Galinsoga parviflora and their effect on inhibiting oxidative burst in human neutrophils. Phytochem. Lett. 2016, 16, 303–310. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, H.K.; Linthorst, H.J.M.; Hollander, J.G.; Lefeber, A.W.M.; Erkelens, C.; Nuzillard, J.-M.; Verpoorte, R. NMR Metabolomics to revisit the tobacco mosaic virus infection in Nicotiana tabacum leaves. J. Nat. Prod. 2006, 69, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]



| Compound 2 (5-COA) | Compound 1 (4-COA) | Compound 3 (4,5-diCOA) | |||||
|---|---|---|---|---|---|---|---|
| 13C | 1H | HMBC | 13C | 1H | 13C | 1H | |
| 1 | 170.3 | - | - | 170.6 | - | 170.2 | - |
| 2 | 105.3 | - | - | 104.9 | - | 115.1 | - |
| 3 | 75.1 | 4.07, bs | 4,5 | 73.0 | 4.05, br s | 73.6 | 4.12, brs |
| 4 | 70.7 | 4.16, bd (5.7) | 2,6,3,5 | 73.3 | 5.27 d (5.3) | 71.1 | 5.43, d (6.3) |
| 5 | 69.3 | 5.30, dd (5.7, 4.2) | 1’,11,12,13 | 65.4 | 4.34 dd (4.4, 5.3) | 66.6 | 5.56, dd (4.7, 6.3) |
| 6 | 76.6 | 4.66, dd (4.2, 3.9) | 2,4 | 78.8 | 4.46, dd (4.4, 3.7) | 76.2 | 4.70, dd (4.7, 3.7) |
| 7 | 82.4 | 4.27, ddd (3.9, 4.7, 8.2) | 8 | 82.5 | 4.28, ddd (3.7, 4.2, 8.2) | 82.3 | 4.34, ddd (3.7, 4.2, 7.8) |
| 8 | 60.6 | 4.09, dd (4.7, 11.8) | 2 | 60.8 | 4.37 dd (8.2, 12.1) | 60.7 | 4.40, dd (7.8, 11.4) |
| 4.37, dd (8.2, 11.8) | 7,6 | 4.04, dd (4.2, 12.1) | 4.06, dd (4.2, 11.4) | ||||
| 4-caffeoyl | |||||||
| 1′ | - | - | - | 168.4 | - | 167.8 | - |
| 2′ | - | - | - | 115.2 | 6.36, d (15.9) | 114.4 | 6.36, d (15.9) |
| 3′ | - | - | - | 147.4 | 7.63, d (15.9) | 148.4 | 7.63, d (15.9) |
| 1″ | - | - | - | 127.8 | - | 127.6 | - |
| 2″ | - | - | - | 115.2 | 7.09, d (1.9) | 115.3 | 7.09, d (2.1) |
| 3″ | - | - | - | 146.8 | - | 149.9 | - |
| 4″ | - | - | - | 149.6 | - | 149.9 | - |
| 5″ | - | - | - | 116.5 | 6.78, d (8.2) | 116.5 | 6.77, d (8.1) |
| 6″ | - | - | - | 123.1 | 6.99, dd (1.9, 8.2) | 123.5 | 6.99, dd (2.1, 8.1) |
| 5-caffeoyl | |||||||
| 1′ | 167.9 | - | - | - | - | 167.1 | - |
| 2′ | 114.6 | 6.32, d (15.9) | 1′,1″ | - | - | 113.9 | 6.14, d (15.9) |
| 3′ | 147.7 | 7.62, d (15.9) | 1′,1″,2″,2′6″ | - | - | 148.1 | 7.46, d (15.9) |
| 1″ | 127.7 | - | - | - | - | 127.4 | - |
| 2″ | 115.2 | 7.06, d (2.0) | 4″,6″ | - | - | 114.8 | 6.98, d (2.1) |
| 3″ | 146.8 | - | - | - | - | 146.9 | - |
| 4″ | 149.7 | - | - | - | - | 149.9 | - |
| 5″ | 116.5 | 6.78, d (8.1) | 1″,3″ | - | - | 116.4 | 6.65, d (8.2) |
| 6″ | 123.1 | 6.97, dd (2.0, 8.1) | 2″,3′ | - | - | 123.7 | 6.77, dd (2.1, 8.2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-González, G.F.; Sadgrove, N.J.; Ccana-Ccapatinta, G.V.; Leuner, O.; Fernandez-Cusimamani, E. Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae). Metabolites 2020, 10, 407. https://doi.org/10.3390/metabo10100407
Padilla-González GF, Sadgrove NJ, Ccana-Ccapatinta GV, Leuner O, Fernandez-Cusimamani E. Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae). Metabolites. 2020; 10(10):407. https://doi.org/10.3390/metabo10100407
Chicago/Turabian StylePadilla-González, Guillermo F., Nicholas J. Sadgrove, Gari V. Ccana-Ccapatinta, Olga Leuner, and Eloy Fernandez-Cusimamani. 2020. "Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae)" Metabolites 10, no. 10: 407. https://doi.org/10.3390/metabo10100407
APA StylePadilla-González, G. F., Sadgrove, N. J., Ccana-Ccapatinta, G. V., Leuner, O., & Fernandez-Cusimamani, E. (2020). Feature-Based Molecular Networking to Target the Isolation of New Caffeic Acid Esters from Yacon (Smallanthus sonchifolius, Asteraceae). Metabolites, 10(10), 407. https://doi.org/10.3390/metabo10100407






