Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development
Abstract
1. Introduction
2. Symptoms of Huanglongbing
2.1. Symptoms of HLB on Leaves
2.2. Symptoms of HLB on Citrus Fruits
2.3. Symptoms of HLB on Roots
2.4. Symptoms of HLB on the Whole Tree
3. Deciphering the Role of Citrus Metabolites in HLB Symptom Development
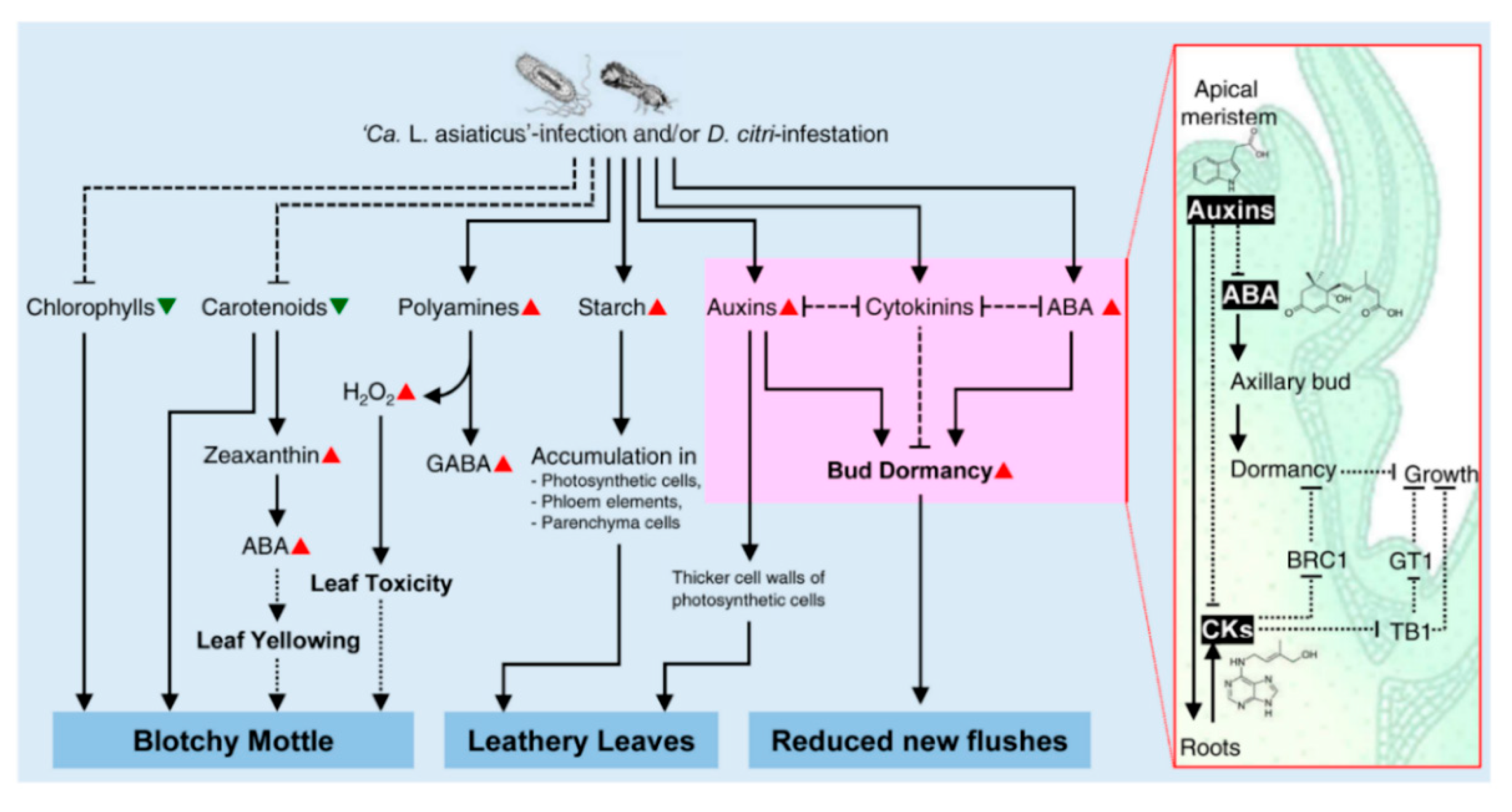
3.1. The Role of Citrus Metabolites in the Development of Blotchy Mottle Symptom
3.2. The Role of Citrus Metabolites in the Development of Leathery Leaf Symptom
3.3. The Role of Citrus Metabolites in the Reduction of New Flushes
3.4. The Role of Citrus Metabolites in the Development of Lopsided Fruits
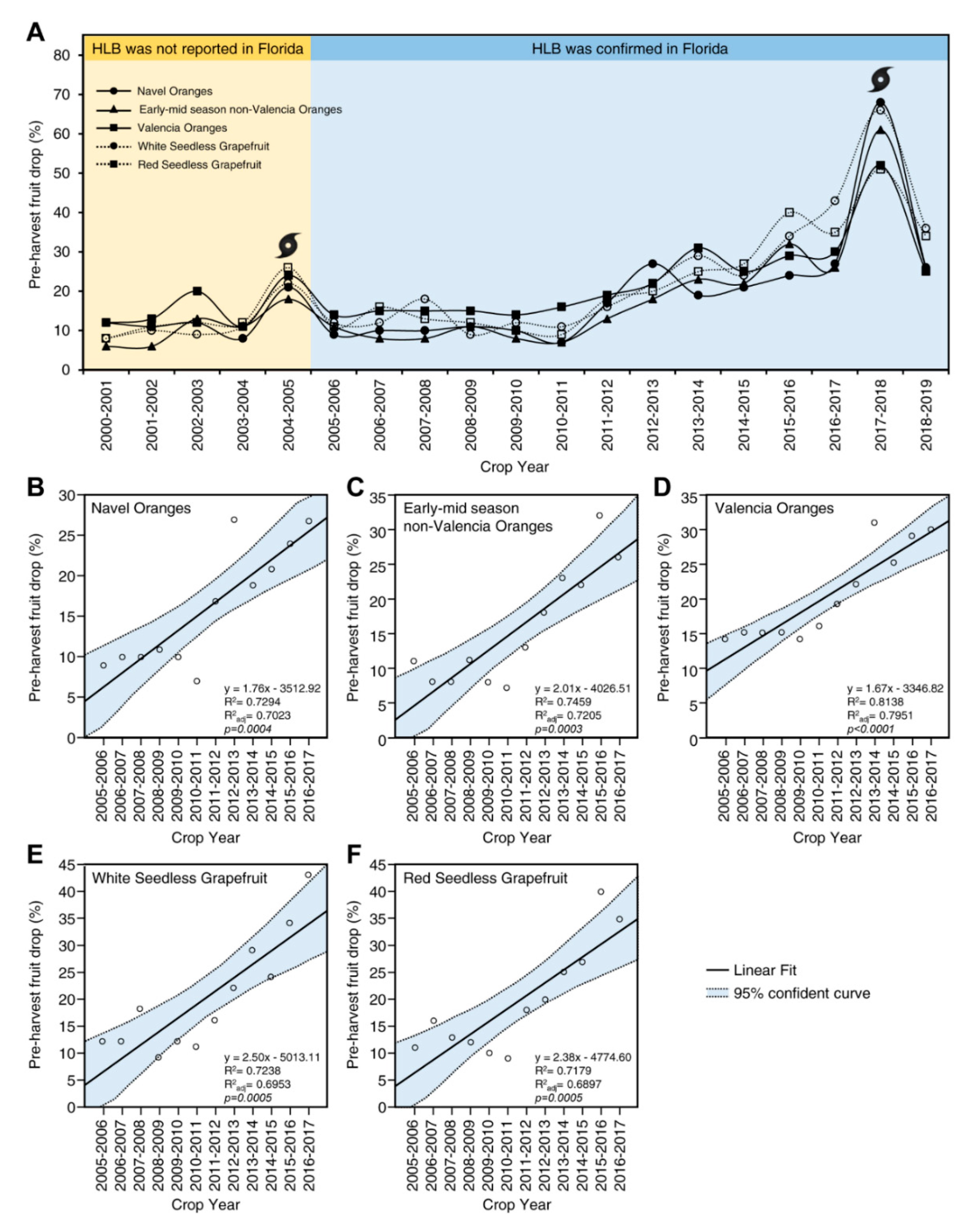
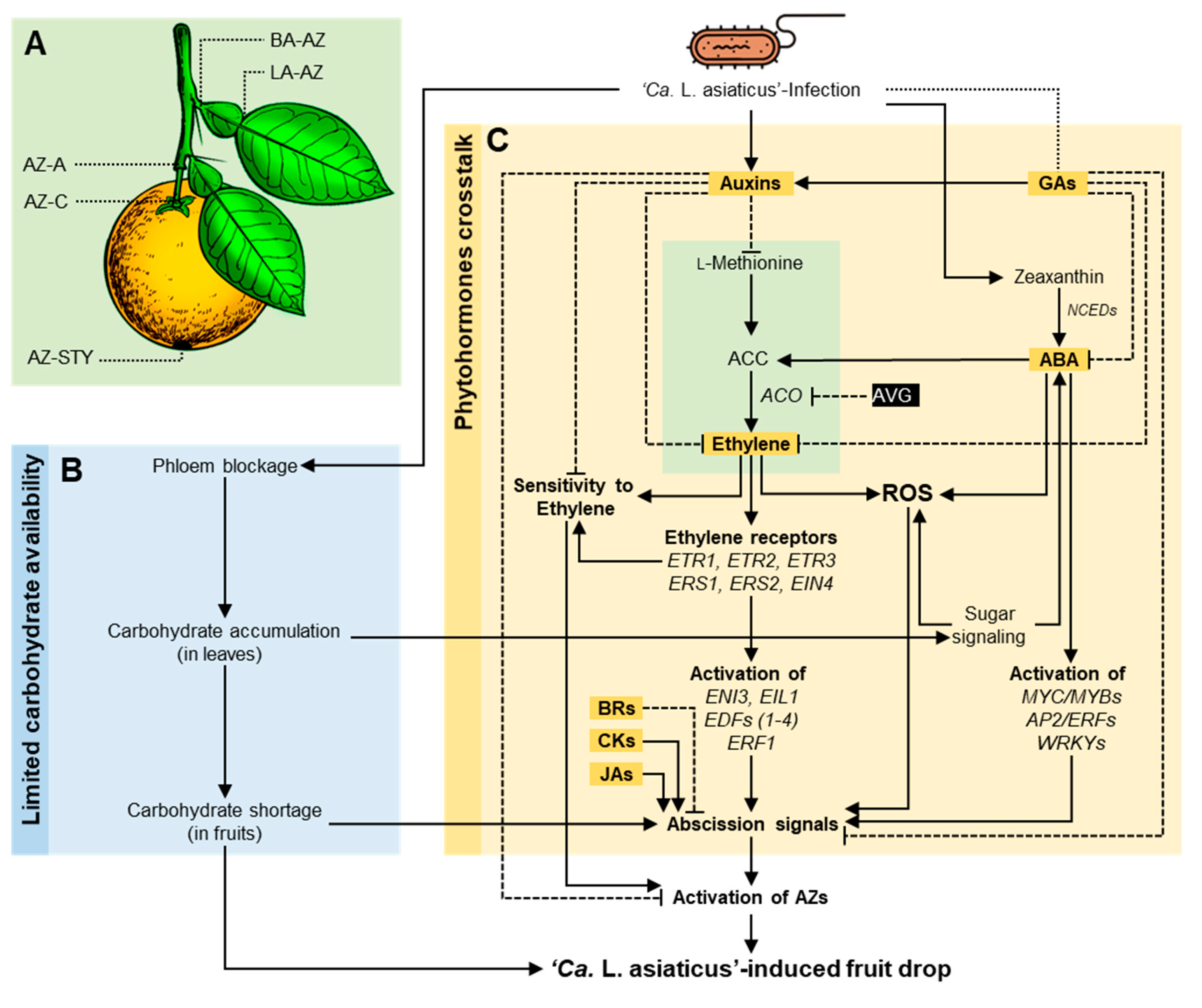
3.5. The Role of Citrus Metabolites in HLB-Induced Preharvest Fruit Drop
3.6. The Role of Citrus Metabolites in HLB-Associated Root Damage
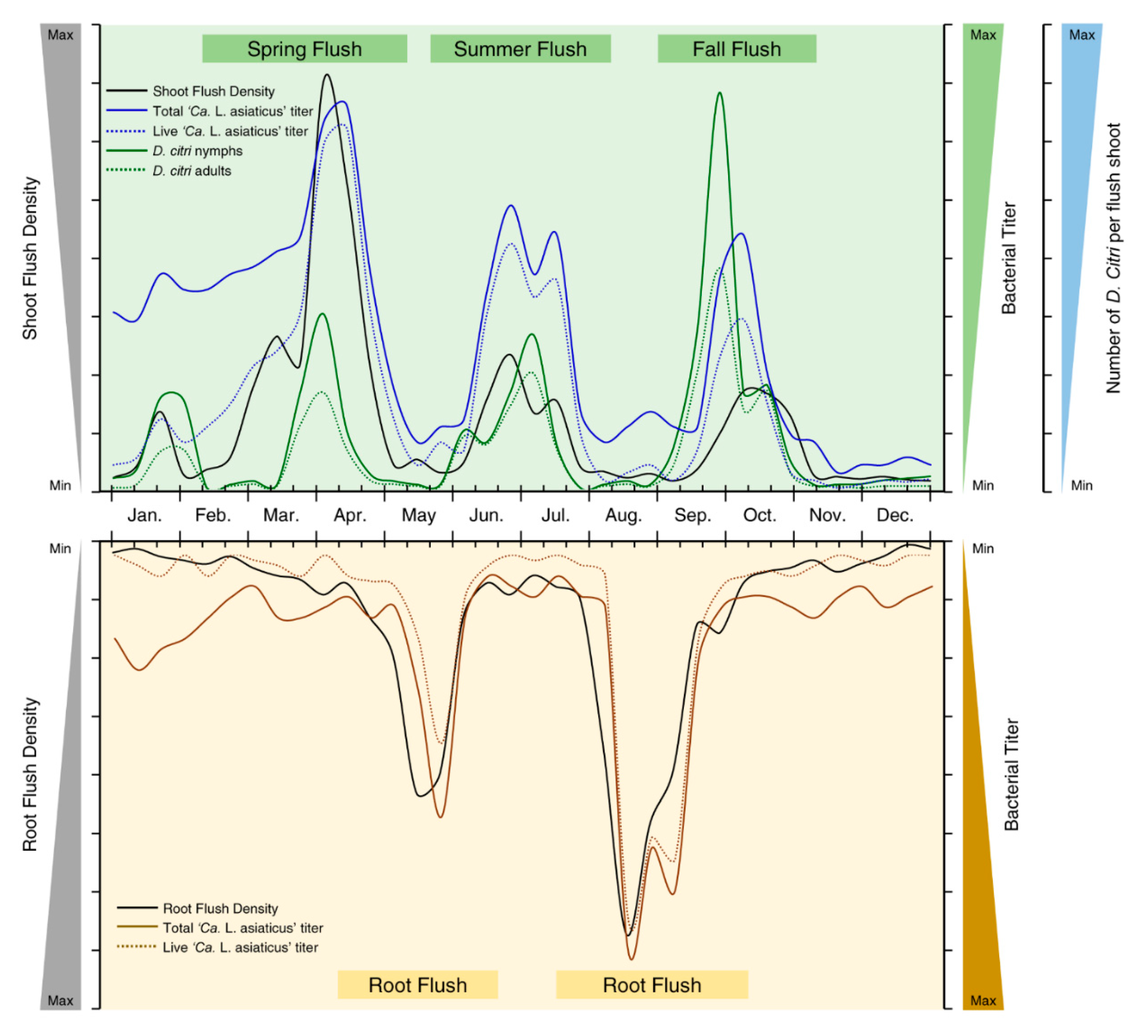
4. Association between Citrus Flushing Cycles and Population Dynamics of Both ‘Ca. L. Asiaticus’ and Its Vector, D. citri
5. Conclusion Remarks and Future Prospects
6. Summary Points
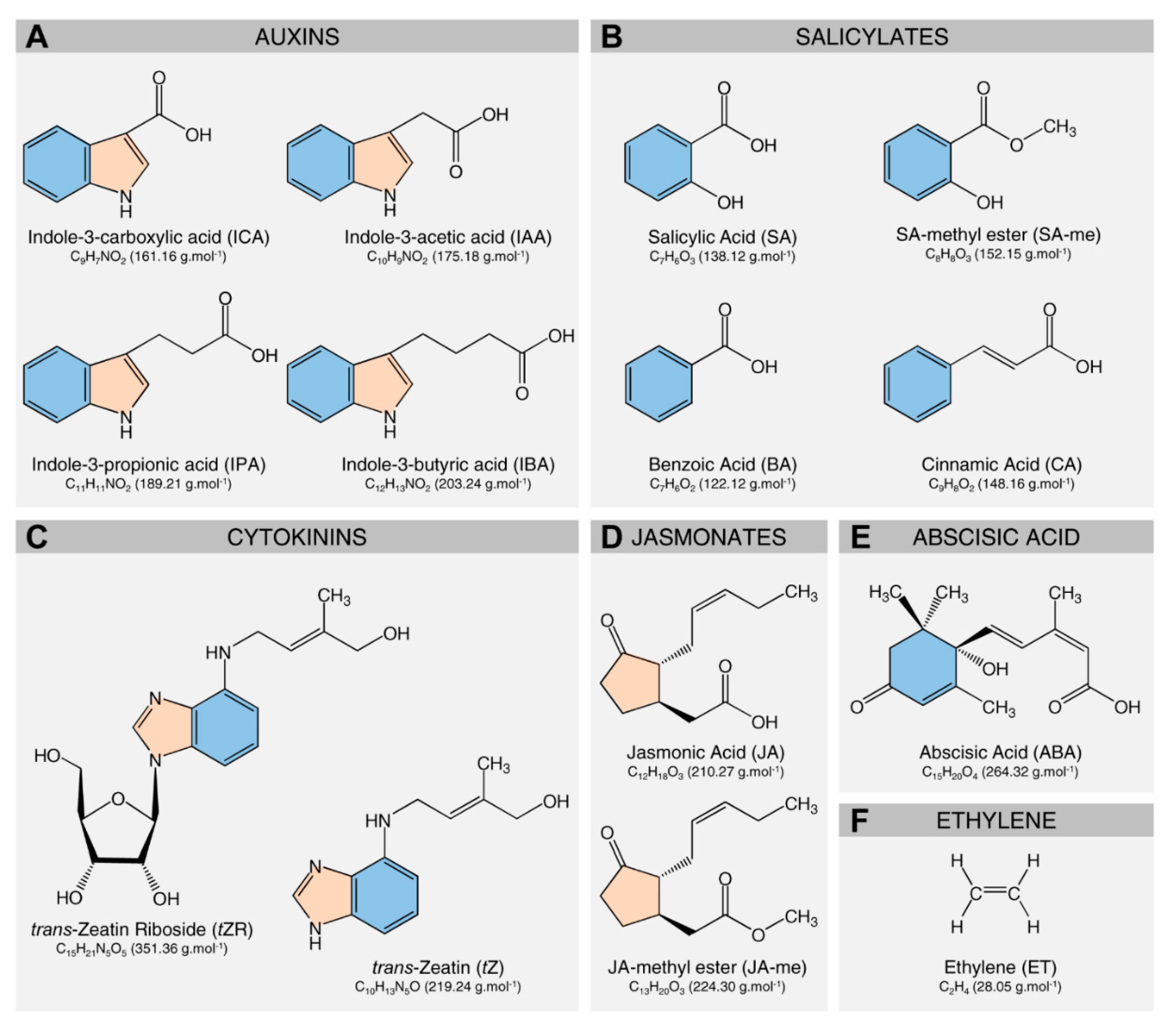
- HLB symptom development is a complicated process, reliant on a complex metabolic network that is mainly regulated by phytohormones. This complex network consisting of at least three major molecular mechanisms including:
- The disorder of carbohydrate metabolism is associated with source–sink disruption due to starch accumulation in leaves, but not in fruits, possibly due to the upregulation of glucose-phosphate transport (GPT).
- Imbalance of stress-associated phytohormones of citrus plants, particularly jasmonic-salicylic acid crosstalk.
- Activation of detoxification proteins, particularly glutathione-S-transferases (GSTs) and modulation of antioxidant pathways.
- The blotchy mottle symptom of HLB is not due to the degradation of chlorophylls and carotenoids only, but ABA may play a positive role in HLB symptom development via the induction of leaf yellowing. In addition, polyamines, GABA, and their catabolic genes (CsDAO and CsPAO) might be involved in blotchy mottle symptom via the production of H2O2. Interestingly, ‘Ca. L. asiaticus’ could survive the toxic effects of accumulated H2O2 using its own peroxidase. However, the detoxification system of citrus plants might not be sufficient to reduce the high H2O2 levels, which may eventually become toxic to the leaf tissue and cause the characteristic blotchy mottle symptom.
- The leathery leaf symptom of HLB might be due to the extensive accumulation of starch grains and/or auxins in the photosynthetic cells, phloem elements, vascular parenchyma, and all other parenchyma cells of the symptomatic leaves and petioles, causing thicker and leathery leaves.
- Development of the misshapen fruit symptom might be due to the spatial distribution of IAA and ABA in HLB-symptomatic fruits, abnormal growth, and cell enlargement in the subepidermal layers (hypodermis), differential expression of phytohormone-related genes, and/or differential transcription of auxin-responsive genes, particularly GH3-like proteins (GH3.1 and GH3.4) and ABA-related genes (GRAM-domain containing protein).
- HLB increases preharvest mature fruit drop, which results in a significant reduction in yield. HLB-induced fruit drop might be mainly due to the alteration in metabolic homeostasis at the abscission zone (AZ), particularly the limited carbohydrate availability to citrus fruits due to phloem blockage and phytohormonal imbalance, including of auxin and indole derivatives, ethylene and its precursors, ABA, gibberellins, cytokinins, brassinolide, and methyl-jasmonate.
- Populations of both ‘Ca. L. asiaticus’ and D. citri were boosted during the flush cycles and dropped thereafter due to the movement downward from mature shoots to new root flushes with phloem flow, where ‘Ca. L. asiaticus’ preferably spends the non-flushing periods either in citrus roots or within its insect vector. Due to the production of the new flush cycle, ‘Ca. L. asiaticus’, again, moves upward to new shoots within carbohydrate mobilization.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strange, R.N.; Scott, P.R. Plant Disease: A Threat to Global Food Security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Nicaise, V. Crop immunity against viruses: Outcomes and future challenges. Front. Plant Sci. 2014, 5, 660. [Google Scholar] [CrossRef]
- Killiny, N. Generous hosts: What makes Madagascar periwinkle (Catharanthus roseus) the perfect experimental host plant for fastidious bacteria? Plant Physiol. Biochem. 2016, 109, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.; Garnier, M. Phloem-and xylem-restricted plant pathogenic bacteria. Plant Sci. 2003, 164, 423–438. [Google Scholar] [CrossRef]
- Will, T.; Furch, A.C.U.; Zimmermann, M.R. How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 2013, 4, 336. [Google Scholar] [CrossRef]
- Perilla-Henao, L.M.; Casteel, C.L. Vector-Borne Bacterial Plant Pathogens: Interactions with Hemipteran Insects and Plants. Front. Plant Sci. 2016, 7, 1163. [Google Scholar] [CrossRef] [PubMed]
- Lough, T.J.; Lucas, W.J. Integrative Plant Biology: Role of Phloem Long-Distance Macromolecular Trafficking. Annu. Rev. Plant Biol. 2006, 57, 203–232. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-Borne Plant Pathogens and Their Vectors: Ecology, Evolution, and Complex Interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef]
- Purcell, A.H. Insect Vector Relationships with Procaryotic Plant Pathogens. Annu. Rev. Phytopathol. 1982, 20, 397–417. [Google Scholar] [CrossRef]
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Garnier, M.; Jagoueix-Eveillard, S.; Cronje, P.R.; Le Roux, H.F.; Bove, J.M. Genomic characterization of a Liberibacter present in an ornamental rutaceous tree, Calodendrum capense, in the Western Cape province of South Africa. Proposal of “Candidatus Liberibacter africanus subsp. capensis”. Int. J. Syst. Evol. Microbiol. 2000, 50, 2119–2125. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A Destructive, Newly-Emerging, Century-Old Diesease of Citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef]
- Wang, N.; Trivedi, P. Citrus huanglongbing: A newly relevant disease presents unprecedented challenges. Phytopathology 2013, 103, 652–665. [Google Scholar] [CrossRef]
- McClean, A.P.D.; Oberholzer, P.C.J. Citrus psylla, a vector of the greening disease of Sweet Orange. S. Afr. J. Agric. Sci. 1965, 8, 297–298. [Google Scholar]
- Capoor, S.P.; Rao, D.G.; Viswanath, S.M. Diaphorina citri Kuway, a vector of the greening disease of citrus in India. Indian J. Agric. Sci. 1967, 37, 572–576. [Google Scholar]
- Garnier, M.; Bové, J.M.; Jagoueix-Eveillard, S.; Cronje, C.P.R.; Sanders, G.M.; Korsten, L.; Roux, H.F. le Presence of ‘Candidatus Liberibacter africanus’ in the Western Cape Province of South Africa. In Proceedings of the 14th Conference International Organization of Citrus Virologists Campinas, São Paulo, Brazil, 13–18 September 1998; pp. 369–372. [Google Scholar]
- Do Teixeira, D.C.; Saillard, C.; Eveillard, S.; Danet, J.L.; da Costa, P.I.; Ayres, A.J.; Bové, J. “Candidatus Liberibacter americanus”, associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 2005, 55, 1857–1862. [Google Scholar] [CrossRef]
- Cen, Y.; Zhang, L.; Xia, Y.; Guo, J.; Deng, X.; Zhou, W.; Sequeira, R.; Gao, J.; Wang, Z.; Yue, J.; et al. Detection of ‘Candidatus Liberibacter Asiaticus’ in Cacopsylla (Psylla) citrisuga (Hemiptera: Psyllidae). Fla. Entomol. 2012, 95, 304–311. [Google Scholar] [CrossRef]
- Bové, J.M.; Ayres, A.J. Etiology of three recent diseases of citrus in São Paulo State: Sudden death, variegated chlorosis and huanglongbing. IUBMB Life 2007, 59, 346–354. [Google Scholar] [CrossRef]
- Tatineni, S.; Sagaram, U.S.; Gowda, S.; Robertson, C.J.; Dawson, W.O.; Iwanami, T.; Wang, N. In planta distribution of “Candidatus Liberibacter asiaticus” as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology 2008, 98, 592–599. [Google Scholar] [CrossRef]
- da Graça, J.V. Citrus Greening Disease. Annu. Rev. Phytopathol. 1991, 29, 109–136. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian Citrus Psyllids (Sternorrhyncha: Psyllidae) and Greening Disease of Citrus: A Literature Review and Assessment of Risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Martinez, A.L.; Wallace, J.M. Citrus leaf-mottle-yellows disease in the Philippines and transmission of the causal virus by a Psyllid, Diaphorina citri. Plant Dis. Rep. 1967, 51, 692–695. [Google Scholar]
- Cevallos-Cevallos, J.M.; Futch, D.B.; Shilts, T.; Folimonova, S.Y.; Reyes-De-Corcuera, J.I. GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol. Biochem. 2012, 53, 69–76. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Robertson, C.J.; Garnsey, S.M.; Gowda, S.; Dawson, W.O. Examination of the Responses of Different Genotypes of Citrus to Huanglongbing (Citrus Greening) Under Different Conditions. Phytopathology 2009, 99, 1346–1354. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Killiny, N.; Valim, M.F.; Jones, S.E.; Omar, A.A.; Hijaz, F.; Gmitter, F.G.; Grosser, J.W. Metabolically speaking: Possible reasons behind the tolerance of ‘Sugar Belle’ mandarin hybrid to huanglongbing. Plant Physiol. Biochem. 2017, 116, 36–47. [Google Scholar] [CrossRef]
- Killiny, N.; Jones, S.E.; Nehela, Y.; Hijaz, F.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. All roads lead to Rome: Towards understanding different avenues of tolerance to huanglongbing in citrus cultivars. Plant Physiol. Biochem. 2018, 129, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M.; Garnier, M. Citrus greening and psylla vectors of the disease in the Arabian Peninsula. In Proceedings of the 9th Conference of the International Organization of Citrus Virologists, IOCV, Riverside, CA, USA; 1984; pp. 109–114. [Google Scholar]
- Su, H.J.; Wu, R.Y. Preliminary study on the etiology of Wentan pomelo decline. In Proceedings of the R.O.C.—United States Cooperative Science Seminar on Mycoplasma Diseases of Plants, Taipei, Taiwan, 27–31 March 1978; Su, H.-J., McCoy, R.E., Eds.; National Science Council: Taipei, Taiwan, 1979; pp. 45–57. [Google Scholar]
- Sutton, B.D.; Duan, Y.-P.; Halbert, S.; Sun, X.-A.; Schubert, T.; Dixon, W. Detection and identification of citrus huanglongbing (greening) in Florida, USA. In Proceedings of the Second International Citrus Canker and Huanglongbing Research Workshop, Orlando, FL, USA, 7–11 November 2005. [Google Scholar]
- Lopes, S.A.; Frare, G.F.; Martins, E.C. Hosts of liberibacter in Brazil. In Proceedings of the Huanglongbing—Greening International Workshop, Ribeirão Preto, SP, Brazil, 14–21 July 2006; p. 5. [Google Scholar]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.B. Citrus Huanglongbing: The Pathogen and Its Impact. Plant Health Prog. 2007, 8, 31. [Google Scholar] [CrossRef]
- Manicom, B.Q.; van Vuuren, S.P. Symptoms of greening disease with special emphasis on African greening. In Proceedings of the Fourth International Asia Pacific Conference on Citrus Rehabilitation, Chang Mai, Thailand, 4–10 February 1990; Aubert, B., Tontyaporn, S., Buangsuwon, D., Eds.; FAO UNDP: Chang Mai, Thailand, 1990; pp. 127–131. [Google Scholar]
- Nariani, T.K.; Raychaudhuri, S.P.; Viswanath, S.M. Tolerance to greening in certain citrus species. Curr. Sci. 1973, 42, 513–514. [Google Scholar]
- McCollum, G.; Baldwin, E. Huanglongbing: Devastating disease of citrus. Hortic. Rev. 2016, 44, 315–361. [Google Scholar]
- Lin, K.-H. Observations on yellow shoot of Citrus. Etiological studies of yellow shoot of Citrus. Acta Phytopathol. Sin. 1956, 2, 1–42. [Google Scholar]
- Zhao, X.Y. Huanglongbing in China. In Proceedings of the Huanglongbing Greening International Workshop, Ribeirão Prêto, Brazil, 16–20 July 2006; p. 3. [Google Scholar]
- McClean, A.P.D.; Schwarz, R.E. Greening or blotchy-mottle disease of citrus. Phytophylactica 1970, 2, 177–194. [Google Scholar]
- Albrecht, U.; Fiehn, O.; Bowman, K.D. Metabolic variations in different citrus rootstock cultivars associated with different responses to Huanglongbing. Plant Physiol. Biochem. 2016, 107, 33–44. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Bai, J.; Manthey, J.; Zhao, W.; Raithore, S.; Irey, M. Effect of Abscission Zone Formation on Orange (Citrus sinensis) Fruit/Juice Quality for Trees Affected by Huanglongbing (HLB). J. Agric. Food Chem. 2018, 66, 2877–2890. [Google Scholar] [CrossRef]
- Batool, A.; Iftikhar, Y.; Mughal, S.M.; Khan, M.M.; Jaskani, M.J.; Abbas, M.; Khan, I.A. Citrus greening disease—A major cause of citrus decline in the world—A review. Hortic. Sci. 2007, 34, 159–166. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Montesino, L.H.; Gasparoto, M.C.G.; Bergamin Filho, A.; Amorim, L. Yield loss caused by huanglongbing in different sweet orange cultivars in São Paulo, Brazil. Eur. J. Plant Pathol. 2011, 130, 577–586. [Google Scholar] [CrossRef]
- Liao, H.-L.; Burns, J.K. Gene expression in Citrus sinensis fruit tissues harvested from huanglongbing-infected trees: Comparison with girdled fruit. J. Exp. Bot. 2012, 63, 3307–3319. [Google Scholar] [CrossRef]
- Dandekar, A.M.; Martinelli, F.; Davis, C.E.; Bhushan, A.; Zhao, W.; Fiehn, O.; Skogerson, K.; Wohlgemuth, G.; D’Souza, R.; Roy, S.; et al. Analysis of Early Host Responses for Asymptomatic Disease Detection and Management of Specialty Crops. Crit. Rev. Immunol. 2010, 30, 277–289. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Giovino, A.; Marino, P.; Aksenov, A.A.; Pasamontes, A.; Peirano, D.J.; Davis, C.E.; Dandekar, A. Proposal of a Citrus translational genomic approach for early and infield detection of Flavescence dorée in Vitis. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2016, 150, 43–53. [Google Scholar]
- Nehela, Y.; Killiny, N. ‘Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri, Augment the Tricarboxylic Acid Cycle of Their Host via the g-Aminobutyric Acid Shunt and Polyamines Pathway. Mol. Plant-Microbe Interact. 2019, 32, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Cevallos-Cevallos, J.M.; García-Torres, R.; Etxeberria, E.; Reyes-De-Corcuera, J.I. GC-MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus Huanglongbing and zinc deficiency in leaves of “Valencia” sweet orange from commercial groves. Phytochem. Anal. 2011, 22, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Hijaz, F. Amino acids implicated in plant defense are higher in Candidatus Liberibacter asiaticus-tolerant citrus varieties. Plant Signal. Behav. 2016, 11, e1171449. [Google Scholar] [CrossRef]
- Kushad, M.M.; Yelenosky, G. Evaluation of Polyamine and Proline Levels during Low Temperature Acclimation of Citrus. Plant Physiol. 1987, 84, 692–695. [Google Scholar] [CrossRef]
- Ali, A.G.; Lovatt, C.J. Relationship of polyamines to low-temperature stress-induced flowering of the ‘Washington’ navel orange (Citrus sinensis L. Osbeck). J. Hortic. Sci. 1995, 70, 491–498. [Google Scholar] [CrossRef]
- Fu, X.Z.; Huang, Y.; Xing, F.; Chun, C.P.; Ling, L.L.; Cao, L.; Peng, L.Z. Changes in free polyamines and expression of polyamine metabolic genes under drought and high-temperature in Citrus sinensis. Biol. Plant. 2016, 60, 793–798. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. Metabolomic Response to Huanglongbing: Role of Carboxylic Compounds in Citrus sinensis Response to ‘Candidatus Liberibacter asiaticus’ and Its Vector, Diaphorina citri. Mol. Plant-Microbe Interact. 2017, 30, 666–678. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. One Target, Two Mechanisms: The Impact of “Candidatus Liberibacter asiaticus” and Its Vector, Diaphorina citri, on Citrus Leaf Pigments. Mol. Plant-Microbe Interact. 2017, 30, 543–556. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Citrus phytohormonal response to Candidatus Liberibacter asiaticus and its vector Diaphorina citri. Physiol. Mol. Plant Pathol. 2018, 102, 24–35. [Google Scholar] [CrossRef]
- Rosales, R.; Burns, J.K. Phytohormone Changes and Carbohydrate Status in Sweet Orange Fruit from Huanglongbing-infected Trees. J. Plant Growth Regul. 2011, 30, 312–321. [Google Scholar] [CrossRef]
- Martinelli, F.; Uratsu, S.L.; Albrecht, U.; Reagan, R.L.; Phu, M.L.; Britton, M.; Buffalo, V.; Fass, J.; Leicht, E.; Zhao, W.; et al. Transcriptome Profiling of Citrus Fruit Response to Huanglongbing Disease. PLoS ONE 2012, 7, e38039. [Google Scholar] [CrossRef] [PubMed]
- Slisz, A.M.; Breksa, A.P.; Mishchuk, D.O.; McCollum, G.; Slupsky, C.M. Metabolomic analysis of citrus infection by “Candidatus Liberibacter” reveals insight into pathogenicity. J. Proteome Res. 2012, 11, 4223–4230. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Perez, J.L.; Kunta, M.; Patt, J.M.; Mangan, R.L. Changes in free amino acids and polyamine levels in Satsuma leaves in response to Asian citrus psyllid infestation and water stress. Insect Sci. 2014, 21, 707–716. [Google Scholar] [CrossRef]
- Chin, E.L.; Mishchuk, D.O.; Breksa, A.P.; Slupsky, C.M. Metabolite signature of Candidatus liberibacter asiaticus infection in two citrus varieties. J. Agric. Food Chem. 2014, 62, 6585–6591. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, C.; Albrecht, U.; Shimizu, R.; Wang, G.; Bowman, K.D. Overexpression of a citrus NDR1 ortholog increases disease resistance in Arabidopsis. Front. Plant Sci. 2013, 4, 157. [Google Scholar] [CrossRef]
- Martinelli, F.; Dandekar, A.M. Genetic Mechanisms of the Devious Intruder Candidatus Liberibacter in Citrus. Front. Plant Sci. 2017, 8, 904. [Google Scholar] [CrossRef]
- Schneider, H. Anatomy of greening diseased sweet orange shoots. Phytopathology 1968, 58, 1155–1160. [Google Scholar]
- Wei, X.; Chen, C.; Yu, Q.; Gady, A.; Yu, Y.; Liang, G.; Gmitter, F.G. Comparison of carotenoid accumulation and biosynthetic gene expression between Valencia and Rohde Red Valencia sweet oranges. Plant Sci. 2014, 227, 28–36. [Google Scholar] [CrossRef]
- Wei, X.; Chen, C.; Yu, Q.; Gady, A.; Yu, Y.; Liang, G.; Gmitter, F.G. Novel expression patterns of carotenoid pathway-related genes in citrus leaves and maturing fruits. Tree Genet. Genomes 2014, 10, 439–448. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid Synthesis and Response. Arab. Book Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef] [PubMed]
- Jaschke, W.D.; Peuke, A.D.; Pate, J.S.; Hartung, W. Transport, synthesis and catabolism of abscisic acid (ABA) in intact plants of castor bean (Ricinus communis L.) under phosphate deficiency and moderate salinity. J. Exp. Bot. 1997, 48, 1737–1747. [Google Scholar] [CrossRef]
- Milborrow, B.V. The pathway of biosynthesis of abscisic acid in vascular plants: A review of the present state of knowledge of ABA biosynthesis. J. Exp. Bot. 2001, 52, 1145–1164. [Google Scholar] [CrossRef] [PubMed]
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Nehela, Y.; Killiny, N. Multiple phytohormonal signaling mediates citrus response to the bacterial pathogen Candidatus Liberibacter asiaticus. In Proceedings of the International Congress of Plant Pathology (ICPP) 2018, Boston, MA, USA, 29 July–3 August 2018; American Phytopathological Society (APS): St Paul, MN, USA, 2018; Volume 108. [Google Scholar]
- Ferrante, A.; Vernieri, P.; Serra, G.; Tognoni, F. Changes in Abscisic Acid During Leaf Yellowing of Cut Stock Flowers. Plant Growth Regul. 2004, 43, 127–134. [Google Scholar] [CrossRef]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M.; et al. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar] [CrossRef]
- Walters, D.R. Polyamines in plant–microbe interactions. Physiol. Mol. Plant Pathol. 2000, 57, 137–146. [Google Scholar] [CrossRef]
- Walters, D. Resistance to plant pathogens: Possible roles for free polyamines and polyamine catabolism. New Phytol. 2003, 159, 109–115. [Google Scholar] [CrossRef]
- Pitino, M.; Armstrong, C.M.; Duan, Y. Molecular mechanisms behind the accumulation of ATP and H2O2 in citrus plants in response to ‘Candidatus Liberibacter asiaticus’ infection. Hortic. Res. 2017, 4, 17040. [Google Scholar] [CrossRef]
- Jain, M.; Fleites, L.A.; Gabriel, D.W. Prophage-Encoded Peroxidase in ‘Candidatus Liberibacter asiaticus’ Is a Secreted Effector That Suppresses Plant Defenses. Mol. Plant-Microbe Interact. 2015, 28, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, E.; Gonzalez, P.; Achor, D.; Albrigo, G. Anatomical distribution of abnormally high levels of starch in HLB-affected Valencia orange trees. Physiol. Mol. Plant Pathol. 2009, 74, 76–83. [Google Scholar] [CrossRef]
- Whitaker, D.C.; Giurcanu, M.C.; Young, L.J.; Gonzalez, P.; Etxeberria, E.; Roberts, P.; Hendricks, K.; Roman, F. Starch Content of Citrus Leaves Permits Diagnosis of Huanglongbing in the Warm Season but Not Cool Season. HortScience 2014, 49, 757–762. [Google Scholar] [CrossRef]
- Singh, S.; Greulach, V.A. Effects of Alpha-Naphthaleneacetic Acid and Alpha-Naphthaleneacetamide on the Development of the Cotton Plant. Am. J. Bot. 1949, 36, 646–651. [Google Scholar] [CrossRef]
- Gómez, H.D. Experiences on Hlb (Huanglongbing) Symptoms Detection in Florida. In El Ier Taller Internacional de Plagas Cuarentenarias de Los Cítricos Sobre Huanglongbing de Los Cítricos (Candidatus Liberibacter Spp.) y el Psílido Asiático de Los Cítricos (Diaphorina Citri); Concitver: Hermosillo, Sonora, Mexico, 2008; pp. 1–7. [Google Scholar]
- Dewdney, M. Huanglongbing. Citrus Ind. 2012, 93, 40–41. [Google Scholar]
- Ongaro, V.; Leyser, O. Hormonal control of shoot branching. J. Exp. Bot. 2007, 59, 67–74. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Fennell, A.Y.; Schlauch, K.A.; Gouthu, S.; Deluc, L.G.; Khadka, V.; Sreekantan, L.; Grimplet, J.; Cramer, G.R.; Mathiason, K.L. Short day transcriptomic programming during induction of dormancy in grapevine. Front. Plant Sci. 2015, 6, 834. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Du, P.; Xiao, W.; Tan, Q.; Chen, X.; Li, L.; Gao, D. Expression of ABA Metabolism-Related Genes Suggests Similarities and Differences Between Seed Dormancy and Bud Dormancy of Peach (Prunus persica). Front. Plant Sci. 2016, 6, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.S.; Doğramacı, M.; Horvath, D.P.; Anderson, J.V.; Foley, M.E. Comparison of phytohormone levels and transcript profiles during seasonal dormancy transitions in underground adventitious buds of leafy spurge. Plant Mol. Biol. 2017, 94, 281–302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic Acid (ABA) Promotes the Induction and Maintenance of Pear (Pyrus pyrifolia White Pear Group) Flower Bud Endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of Liberibacter Infection (Huanglongbing Disease) of Citrus on Orange Fruit Physiology and Fruit/Fruit Juice Quality: Chemical and Physical Analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Montesino, L.H.; Stuchi, E.S. Effects of huanglongbing on fruit quality of sweet orange cultivars in Brazil. Eur. J. Plant Pathol. 2009, 125, 565–572. [Google Scholar] [CrossRef]
- Dagulo, L.; Danyluk, M.D.; Spann, T.M.; Valim, M.F.; Goodrich-Schneider, R.; Sims, C.; Rouseff, R. Chemical Characterization of Orange Juice from Trees Infected with Citrus Greening (Huanglongbing). J. Food Sci. 2010, 75, C199–C207. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef]
- Martinelli, F.; Reagan, R.L.; Uratsu, S.L.; Phu, M.L.; Albrecht, U.; Zhao, W.; Davis, C.E.; Bowman, K.D.; Dandekar, A.M. Gene Regulatory Networks Elucidating Huanglongbing Disease Mechanisms. PLoS ONE 2013, 8, e74256. [Google Scholar] [CrossRef]
- Talon, M.; Tadeo, F.R.; Ben-Cheikh, W.; Gomez-Cadenas, A.; Mehouachi, J.; Pérez-Botella, J.; Primo-Millo, E. Hormonal regulation of fruit set and abscission in citrus: Classical concepts and new Evidence. Acta Hortic. 1998, 463, 209–217. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- NASS USDA—National Agricultural Statistics Service—Florida—Citrus Production Forecasts. Available online: https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Forecast/history.php (accessed on 10 October 2019).
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef]
- Miles, G.P.; Stover, E.; Ramadugu, C.; Keremane, M.L.; Lee, R.F. Apparent Tolerance to Huanglongbing in Citrus and Citrus-related Germplasm. HortScience 2017, 52, 31–39. [Google Scholar] [CrossRef]
- Kim, J.-S.; Sagaram, U.S.; Burns, J.K.; Li, J.-L.; Wang, N. Response of Sweet Orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ Infection: Microscopy and Microarray Analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef]
- Aritua, V.; Achor, D.; Gmitter, F.G.; Albrigo, G.; Wang, N. Transcriptional and Microscopic Analyses of Citrus Stem and Root Responses to Candidatus Liberibacter asiaticus Infection. PLoS ONE 2013, 8, e73742. [Google Scholar] [CrossRef]
- Fu, S.M.; Hartung, J.; Zhou, C.Y.; Su, H.N.; Tan, J.; Li, Z.A. Ultrastructural Changes and Putative Phage Particles Observed in Sweet Orange Leaves Infected with ‘Candidatus Liberibacter asiaticus’. Plant Dis. 2015, 99, 320–324. [Google Scholar] [CrossRef]
- Addicott, F. Abscission; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1982. [Google Scholar]
- Goren, R. Anatomical, Physiological, and Hormonal Aspects of Abscission in Citrus. Hortic. Rev. 1993, 145–182. [Google Scholar] [CrossRef]
- Sawicki, M.; Aït Barka, E.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–1719. [Google Scholar] [CrossRef]
- Chung, K.R.; Shilts, T.; Ertürk, Ü.; Timmer, L.W.; Ueng, P.P. Indole derivatives produced by the fungus Colletotrichum acutatum causing lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2003, 226, 23–30. [Google Scholar] [CrossRef]
- Mesejo, C.; Rosito, S.; Reig, C.; Martínez-Fuentes, A.; Agustí, M. Synthetic Auxin 3,5,6-TPA Provokes Citrus clementina (Hort. ex Tan) Fruitlet Abscission by Reducing Photosynthate Availability. J. Plant Growth Regul. 2012, 31, 186–194. [Google Scholar] [CrossRef]
- Tudela, D.; Primo-Millo, E. 1-Aminocyclopropane-1-Carboxylic Acid Transported from Roots to Shoots Promotes Leaf Abscission in Cleopatra Mandarin (Citrus reshni Hort. ex Tan.) Seedlings Rehydrated after Water Stress. Plant Physiol. 1992, 100, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sipes, D.L.; Einset, J.W. Role of ethylene in stimulating stylar abscission in pistil explants of lemons. Physiol. Plant. 1982, 56, 6–10. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees 2006, 20, 348–355. [Google Scholar] [CrossRef]
- Merelo, P.; Agustí, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gómez-Cadenas, A.; Coimbra, S.; Gómez, M.D.; Pérez-Amador, M.A.; Domingo, C.; et al. Cell Wall Remodeling in Abscission Zone Cells during Ethylene-Promoted Fruit Abscission in Citrus. Front. Plant Sci. 2017, 8, 126. [Google Scholar]
- Agustí, J.; Zapater, M.; Iglesias, D.J.; Cercós, M.; Tadeo, F.R.; Talón, M. Differential expression of putative 9-cis-epoxycarotenoid dioxygenases and abscisic acid accumulation in water stressed vegetative and reproductive tissues of citrus. Plant Sci. 2007, 172, 85–94. [Google Scholar] [CrossRef]
- Ben-Cheikh, W.; Perez-Botella, J.; Tadeo, F.R.; Talon, M.; Primo-Millo, E. Pollination Increases Gibberellin Levels in Developing Ovaries of Seeded Varieties of Citrus. Plant Physiol. 1997, 114, 557–564. [Google Scholar] [CrossRef]
- Mahouachi, J.; Iglesias, D.J.; Agustí, M.; Talon, M. Delay of early fruitlet abscission by branch girdling in citrus coincides with previous increases in carbohydrate and gibberellin concentrations. Plant Growth Regul. 2009, 58, 15–23. [Google Scholar] [CrossRef]
- Sipes, D.L.; Einset, J.W. Cytokinin stimulation of abscission in lemon pistil explants. J. Plant Growth Regul. 1983, 2, 73–80. [Google Scholar] [CrossRef]
- Iwahori, S.; Tominaga, S.; Higuchi, S. Retardation of abscission of citrus leaf and fruitlet explants by brassinolide. Plant Growth Regul. 1990, 9, 119–125. [Google Scholar] [CrossRef]
- Hartmond, U.; Yuan, R.; Burns, J.K.; Grant, A.; Kender, W.J. Citrus fruit abscission induced by methyl-jasmonate. J. Am. Soc. Hortic. Sci. 2000, 125, 547–552. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Liao, H.-L.; Chen, H.; Chung, K.-R. Plant hormone inhibitors for reducing postbloom fruit drop (PFD) of citrus. In Proceedings of the 119th Annual Meeting of the Florida State Horticultural Society, Tampa, FL, USA, 4–6 June 2006; Volume 119, pp. 78–81. [Google Scholar]
- Zacarias, L.; Talon, M.; Ben-Cheikh, W.; Lafuente, M.T.; Primo-Millo, E. Abscisic acid increases in non-growing and paclobutrazol-treated fruits of seedless mandarins. Physiol. Plant. 1995, 95, 613–619. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Tadeo, F.R.; Talon, M.; Primo-Millo, E. Leaf abscission induced by ethylene in water-stressed intact seedlings of Cleopatra mandarin requires previous abscisic acid accumulation in roots. Plant Physiol. 1996, 112, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Guinn, G. Fruit Age and Changes in Abscisic Acid Content, Ethylene Production, and Abscission Rate of Cotton Fruits. Plant Physiol. 1982, 69, 349–352. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Mehouachi, J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 2000, 210, 636–643. [Google Scholar] [CrossRef]
- Agustí, M.; Zaragoza, S.; Iglesias, D.J.; Almela, V.; Primo-Millo, E.; Talón, M. The synthetic auxin 3,5,6-TPA stimulates carbohydrate accumulation and growth in citrus fruit. Plant Growth Regul. 2002, 36, 141–147. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiol. 2003, 23, 199–204. [Google Scholar] [CrossRef]
- Veloso Dos Santos, J.D.; Murata, M.; Gerberich, K.; Bright, D.; Johnson, E. Limited movement of Candidatus Liberibacter asiaticus in split-root citrus provides a model system for local and systemic effects of Huanglongbing. Phytopathology 2018, 108, 127. [Google Scholar]
- Aubert, B. Le Greening, Une Maladie Infectieuse Des Agrumes, D’origine Bactérienne, Transmise Par Des Homoptères Psyllidés, CIRAD-IRFA. Ph.D. Thesis, Université de Bordeaux II, Bordeaux, France, 1987. [Google Scholar]
- Maust, B.E.; Espadas, F.; Talavera, C.; Aguilar, M.; Santamaría, J.M.; Oropeza, C. Changes in Carbohydrate Metabolism in Coconut Palms Infected with the Lethal Yellowing Phytoplasma. Phytopathology 2003, 93, 976–981. [Google Scholar] [CrossRef]
- Bertaccini, A.; Duduk, B. Phytoplasma and phytoplasma diseases: A review of recent research. Phytopathol. Mediterr. 2009, 48, 355–378. [Google Scholar]
- Li, W.; Levy, L.; Hartung, J.S. Quantitative distribution of “Candidatus Liberibacter asiaticus” in citrus plants with citrus huanglongbing. Phytopathology 2009, 99, 139–144. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.R.; Dai, Z.H.; Deng, X.L. Distribution pattern and titer of Candidatus Liberibacter asiaticus in periwinkle (Catharanthus roseus). J. Integr. Agric. 2018, 17, 2501–2508. [Google Scholar] [CrossRef]
- Folimonova, S.Y.; Achor, D.S. Early Events of Citrus Greening (Huanglongbing) Disease Development at the Ultrastructural Level. Phytopathology 2010, 100, 949–958. [Google Scholar] [CrossRef]
- Shi, Q.; Pitino, M.; Zhang, S.; Krystel, J.; Cano, L.M.; Shatters, R.G.; Hall, D.G.; Stover, E. Temporal and spatial detection of Candidatus Liberibacter asiaticus putative effector transcripts during interaction with Huanglongbing-susceptible, −tolerant, and -resistant citrus hosts. BMC Plant Biol. 2019, 19, 122. [Google Scholar] [CrossRef]
- Hu, H.; Roy, A.; Brlansky, R.H. Live Population Dynamics of ‘Candidatus Liberibacter asiaticus’, the Bacterial Agent Associated with Citrus Huanglongbing, in Citrus and Non-Citrus Hosts. Plant Dis. 2014, 98, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Lewis-Rosenblum, H.; Pelz-Stelinski, K.; Stelinski, L.L. Incidence of Candidatus Liberibacter asiaticus Infection in Abandoned Citrus Occurring in Proximity to Commercially Managed Groves. J. Econ. Entomol. 2010, 103, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.F.; Keremane, M.L.; Ramadugu, C.; Roose, M.; Khan, I.A.; Lee, R.F. Detection of Citrus Huanglongbing-Associated ‘Candidatus Liberibacter asiaticus’ in Citrus and Diaphorina citri in Pakistan, Seasonal Variability, and Implications for Disease Management. Phytopathology 2014, 104, 257–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopes, S.A.; Luiz, F.Q.B.F.; Oliveira, H.T.; Cifuentes-Arenas, J.C.; Raiol-Junior, L.L. Seasonal Variation of ‘Candidatus Liberibacter asiaticus’ Titers in New Shoots of Citrus in Distinct Environments. Plant Dis. 2017, 101, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Attaran, E.; Killiny, N.; Beyenal, H.; Gang, D.; Omsland, A. Controlled replication of “Candidatus Liberibacter asiaticus” DNA in citrus leaf discs. Microb. Biotechnol. 2020, 3, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G. Incidence of “Candidatus Liberibacter asiaticus” in a Florida population of Asian citrus psyllid. J. Appl. Entomol. 2018, 142, 97–103. [Google Scholar] [CrossRef]
- Catling, H.D. Distribution of the psyllid vectors of Citrus greening disease, with notes on the biology and bionomics of Diaphorina citri. FAO Plant Prot. Bull. 1970, 18, 8–15. [Google Scholar]
- Pluke, R.W.H.; Qureshi, J.A.; Stansly, P.A. Citrus flushing patterns, Diaphorina citri (Hemiptera: Psyllidae) populations and parasitism by Tamarixia radiata (Hymenoptera: Eulophidae) in Puerto Rico. Fla. Entomol. 2008, 91, 36–42. [Google Scholar] [CrossRef]
- Chavan, V.M.; Summanwar, A.S. Population dynamics and aspects of the biology of citrus psylla, Diaphorina citri Kuw. in Maharashtra. In Proceedings of the 12th Conference of the International Organization of Citrus Virologists, IOCV, Riverside, CA, USA, 15–18 November 1993; Volume 12, pp. 244–249. [Google Scholar]
- Hall, D.G.; Albrigo, L.G. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar] [CrossRef]
- Monzo, C.; Qureshi, J.A.; Stansly, P.A. Insecticide sprays, natural enemy assemblages and predation on Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Bull. Entomol. Res. 2014, 104, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Sétamou, M.; Bartels, D.W. Living on the Edges: Spatial Niche Occupation of Asian Citrus Psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Citrus Groves. PLoS ONE 2015, 10, e0131917. [Google Scholar] [CrossRef]
- Sétamou, M.; Simpson, C.R.; Alabi, O.J.; Nelson, S.D.; Telagamsetty, S.; Jifon, J.L. Quality Matters: Influences of Citrus Flush Physicochemical Characteristics on Population Dynamics of the Asian Citrus Psyllid (Hemiptera: Liviidae). PLoS ONE 2016, 11, e0168997. [Google Scholar] [CrossRef]
- Laranjeira, F.F.; Santos, T.T.C.; Moreira, A.S.; Sanches, I.; Nascimento, A.S.; Silva, S.X.B.; Andrade, E.C.; Almeida, D.O. Association Between Citrus Flushing Cycles and Asian Citrus Psyllid Demography in Huanglongbing-Free Area in Brazil. Neotrop. Entomol. 2019, 48, 503–514. [Google Scholar] [CrossRef]
- Bevington, K.B.; Castle, W.S. Annual root growth pattern of young citrus trees in relation to shoot growth, soil temperature, and soil water content. J. Am. Soc. Hortic. Sci. 1985, 110, 840–845. [Google Scholar]
- Catling, H.D. The bionomics of the South African citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae) I. The influence of the flushing rhythm of citrus and factors which regulate flushing. J. Entomol. Soc. S. Afr. 1969, 32, 191–208. [Google Scholar]
- Cook, G.; Maqutu, V.Z.; Van Vuuren, S.P. Population dynamics and seasonal fluctuation in the percentage infection of Trioza erytreae with “Candidatus” Liberibacter africanus, the African citrus greening pathogen, in an orchard severely infected with African greening and transmission by field-collected Trioza erytreae. Afr. Entomol. 2014, 22, 127–135. [Google Scholar]
- Knapp, J.L.; Albrigo, L.G.; Browning, H.W.; Bullock, R.C.; Heppner, J.B.; Hall, D.G.; Hoy, M.A.; Nguyen, R.; Peña, J.E.; Stansly, P.A. Citrus Leafminer, Phyllocnistis Citrella Stainton: Current Status in Florida—1995; Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 1995. [Google Scholar]
- Goldschmidt, E.E.; Koch, K.E. Citrus. In Photoassimilate Distribution in Plants and Crops: Source Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 797–824. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nehela, Y.; Killiny, N. Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development. Metabolites 2020, 10, 409. https://doi.org/10.3390/metabo10100409
Nehela Y, Killiny N. Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development. Metabolites. 2020; 10(10):409. https://doi.org/10.3390/metabo10100409
Chicago/Turabian StyleNehela, Yasser, and Nabil Killiny. 2020. "Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development" Metabolites 10, no. 10: 409. https://doi.org/10.3390/metabo10100409
APA StyleNehela, Y., & Killiny, N. (2020). Revisiting the Complex Pathosystem of Huanglongbing: Deciphering the Role of Citrus Metabolites in Symptom Development. Metabolites, 10(10), 409. https://doi.org/10.3390/metabo10100409






