A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner
Abstract
1. Introduction
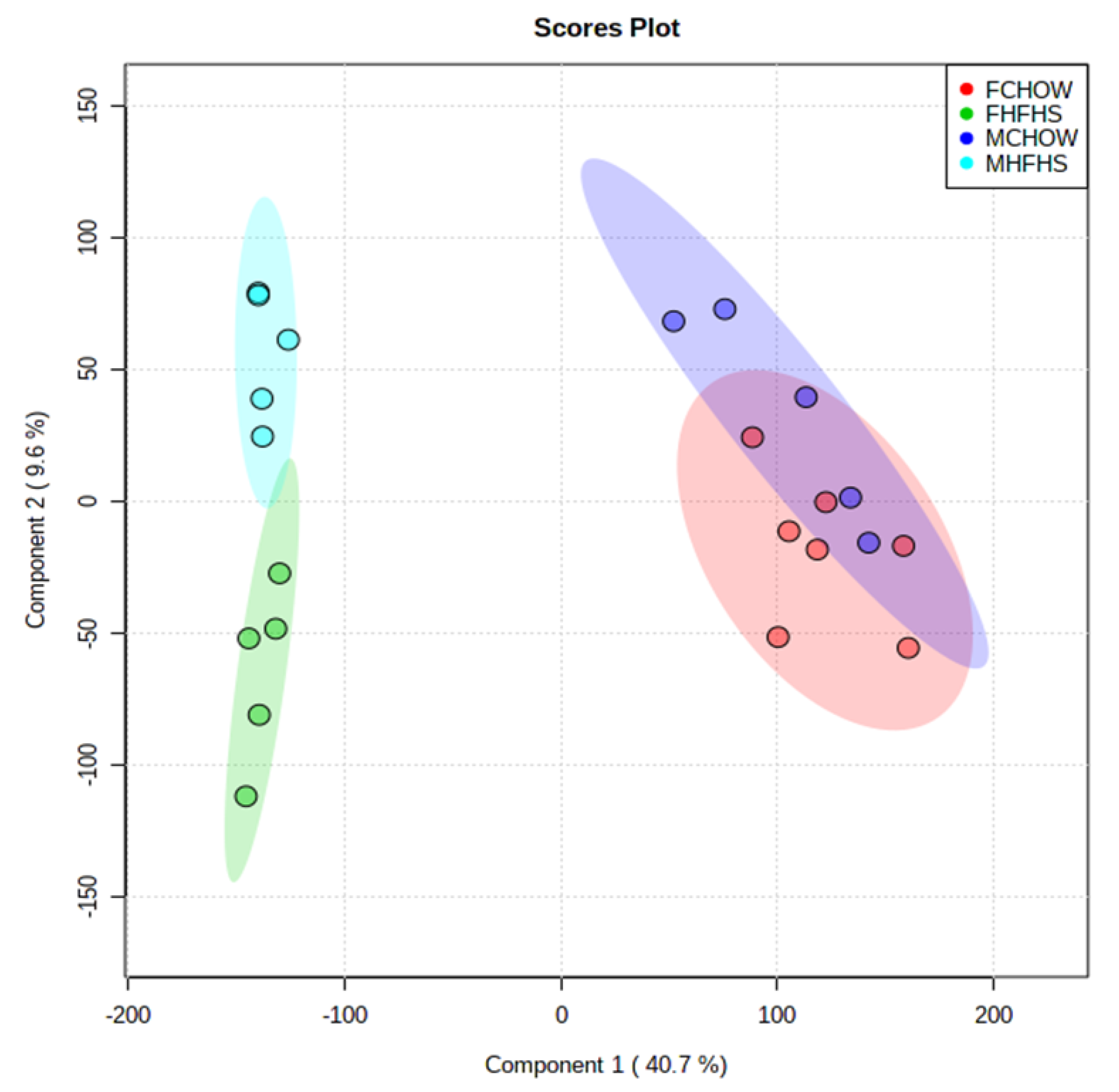
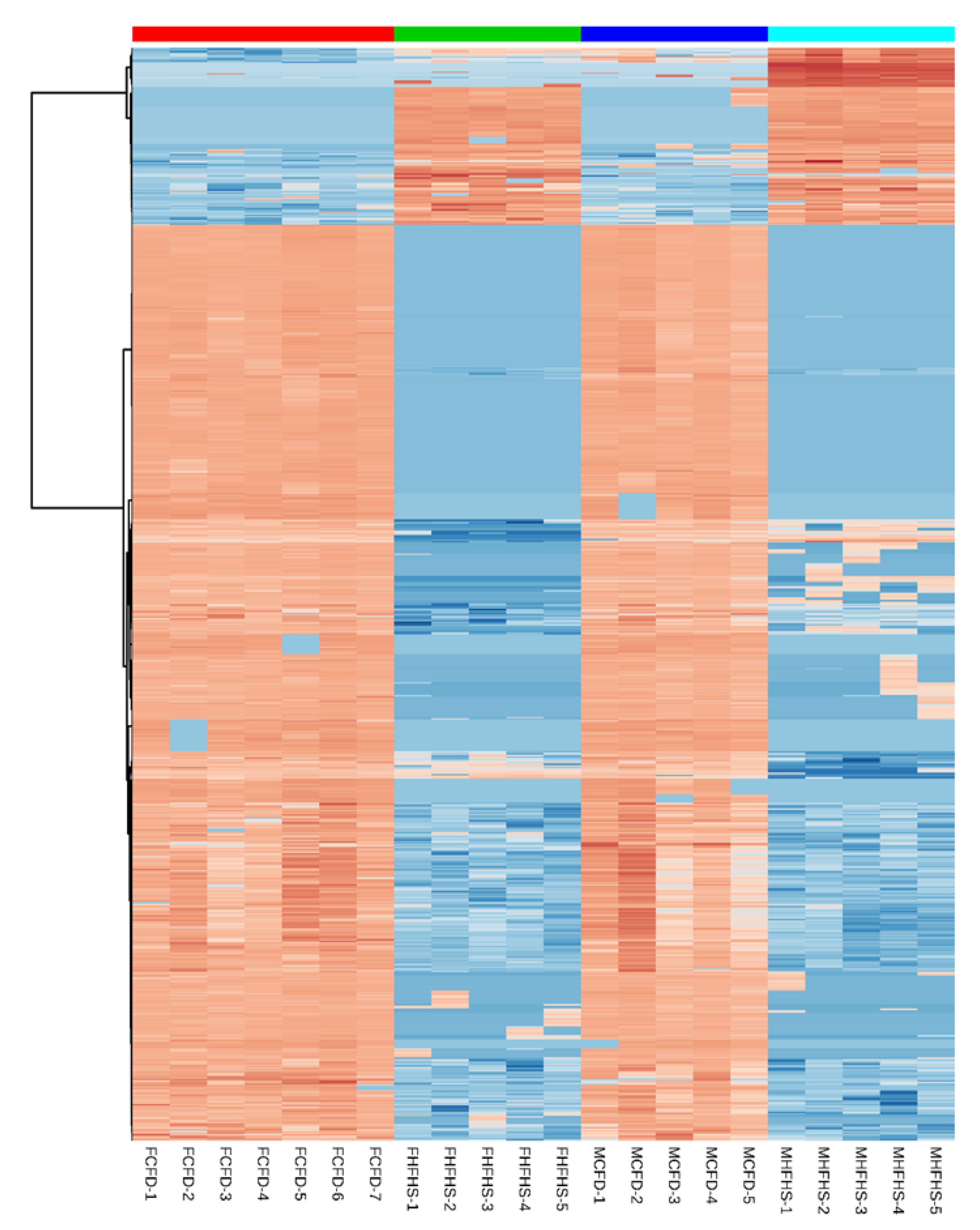
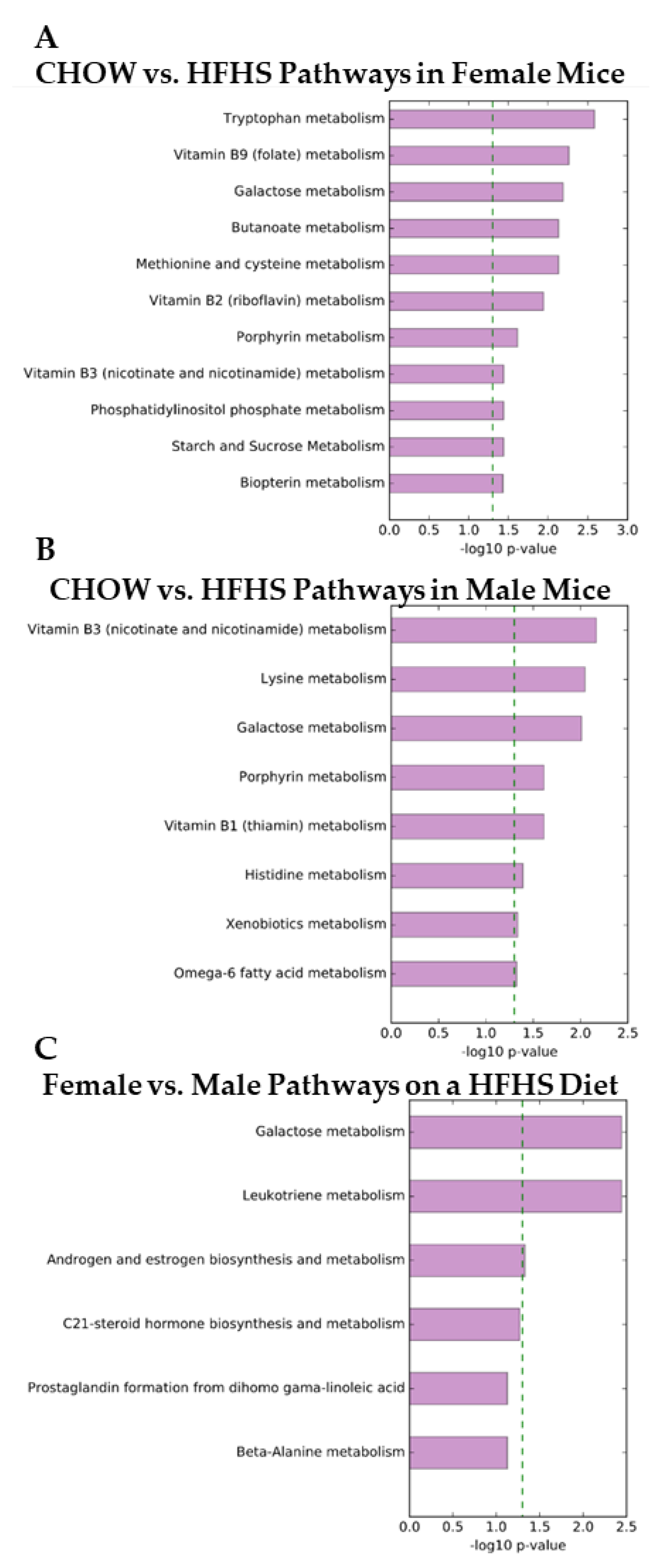
2. Results
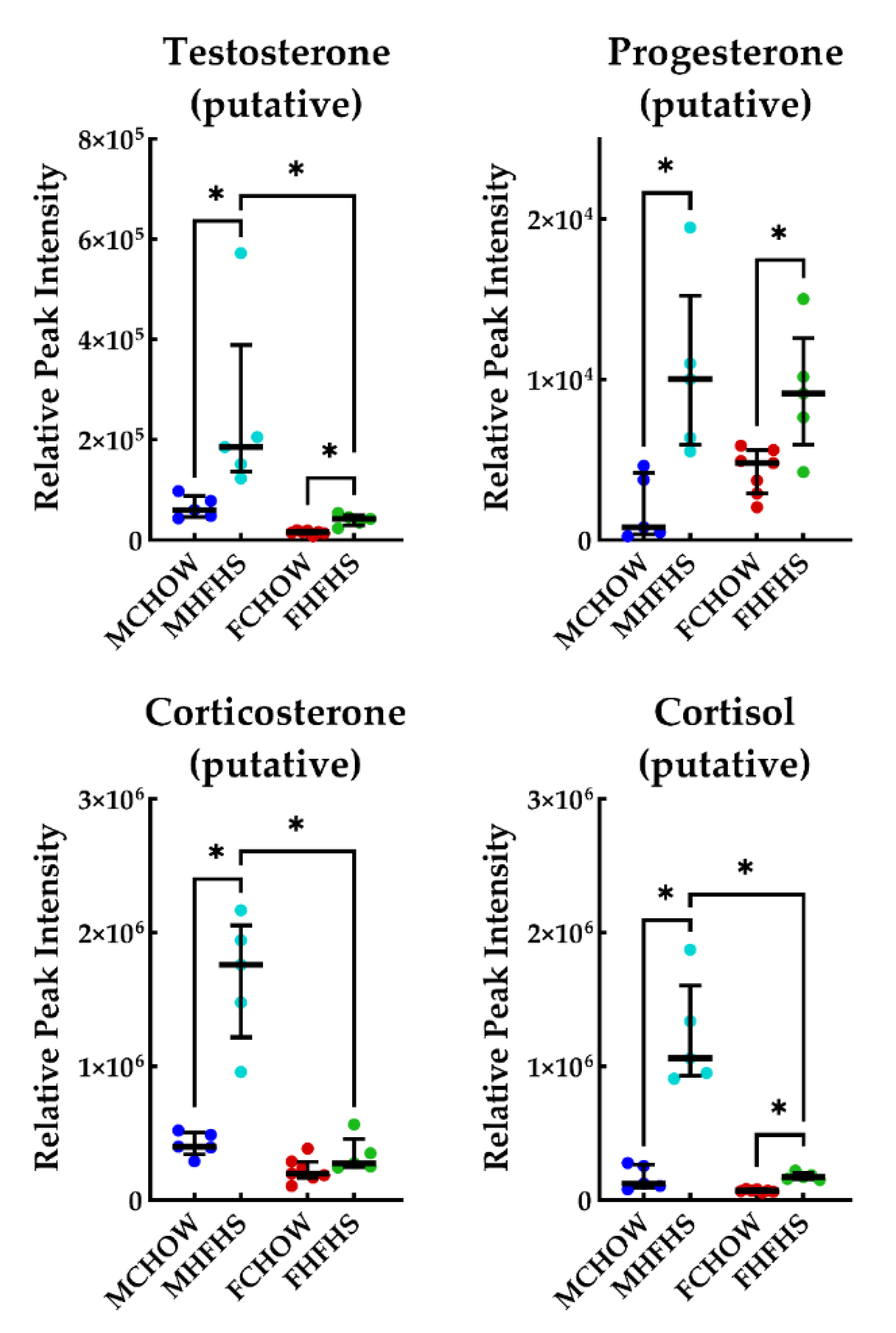
3. Discussion
3.1. Inflammation
3.2. Androgen Biosynthesis and Metabolism
3.3. Neurotransmitter Biosynthesis and Metabolism
4. Materials and Methods
4.1. Experimental Timeline
4.2. Metabolite Extraction
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; Ferrari, A.J.; et al. The State of US Health, 1990–2016. JAMA 2018, 319, 1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Li, Y.; Afshin, A.; Springmann, M.; Mozaffarian, D.; Stampfer, M.J.; Hu, F.B.; Murray, C.J.L.; Willett, W.C. Global Improvement in Dietary Quality Could Lead to Substantial Reduction in Premature Death. J. Nutr. 2019, 149, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Gene-Diet Interaction and Precision Nutrition in Obesity. Int. J. Mol. Sci. 2017, 18, 787. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.A.; Schoenfeld, P.; Locke, G.R. The Epidemiology of Irritable Bowel Syndrome in North America: A Systematic Review. Am. J. Gastroenterol. 2002, 97, 1910–1915. [Google Scholar] [CrossRef]
- Loftus, E.V. Clinical Epidemiology of Inflammatory Bowel Disease: Incidence, Prevalence, and Environmental Influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.; Boushey, R. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Lilienfeld, A.M. Practical Limitations of Epidemiologic Methods. Environ. Health Perspect. 1983, 52, 3–8. [Google Scholar] [CrossRef]
- Loos, R.J.F. From Nutrigenomics to Personalizing Diets: Are We Ready for Precision Medicine? Am. J. Clin. Nutr. 2019, 109, 1–2. [Google Scholar] [CrossRef]
- Vellers, H.L.; Letsinger, A.C.; Walker, N.R.; Granados, J.Z.; Lightfoot, J.T. High Fat High Sugar Diet Reduces Voluntary Wheel Running in Mice Independent of Sex Hormone Involvement. Front. Physiol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Knab, A.M.; Bowen, R.S.; Hamilton, A.T.; Gulledge, A.A.; Lightfoot, J.T. Altered Dopaminergic Profiles: Implications for the Regulation of Voluntary Physical Activity. Behav. Brain Res. 2009, 204, 147–152. [Google Scholar] [CrossRef]
- Ferguson, D.P.; Dangott, L.J.; Vellers, H.L.; Schmitt, E.E.; Lightfoot, J.T. Differential Protein Expression in the Nucleus Accumbens of High and Low Active Mice. Behav. Brain Res. 2015, 291, 283–288. [Google Scholar] [CrossRef]
- Arneth, B.M. Gut-Brain Axis Biochemical Signalling from the Gastrointestinal Tract to the Central Nervous System: Gut Dysbiosis and Altered Brain Function. Postgrad. Med. J. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting Network Activity from High Throughput Metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From Genomics to Chemical Genomics: New Developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Duarte, N.C.; Becker, S.A.; Jamshidi, N.; Thiele, I.; Mo, M.L.; Vo, T.D.; Srivas, R.; Palsson, B. Global Reconstruction of the Human Metabolic Network Based on Genomic and Bibliomic Data. Proc. Natl. Acad. Sci. USA 2007, 104, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sorokin, A.; Mazein, A.; Selkov, A.; Selkov, E.; Demin, O.; Goryanin, I. The Edinburgh Human Metabolic Network Reconstruction and Its Functional Analysis. Mol. Syst. Biol. 2007, 3, 135. [Google Scholar] [CrossRef]
- Sridharan, G.V.; Choi, K.; Klemashevich, C.; Wu, C.; Prabakaran, D.; Pan, L.B.; Steinmeyer, S.; Mueller, C.; Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and Quantification of Bioactive Microbiota Metabolites in the Mouse Gut. Nat. Commun. 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Sellmann, C.; Priebs, J.; Landmann, M.; Degen, C.; Engstler, A.J.; Jin, C.J.; Gärttner, S.; Spruss, A.; Huber, O.; Bergheim, I. Diets Rich in Fructose, Fat or Fructose and Fat Alter Intestinal Barrier Function and Lead to the Development of Nonalcoholic Fatty Liver Disease over Time. J. Nutr. Biochem. 2015, 26, 1183–1192. [Google Scholar] [CrossRef]
- Derikx, J.P. Non-Invasive Markers of Gut Wall Integrity in Health and Disease. World J. Gastroenterol. 2010, 16, 5272. [Google Scholar] [CrossRef] [PubMed]
- Schippa, S.; Conte, M. Dysbiotic Events in Gut Microbiota: Impact on Human Health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.H.; DiBaise, J.K.; McClain, C.J. Gut Microbiota, Intestinal Permeability, Obesity-Induced Inflammation, and Liver Injury. J. Parenter. Enter. Nutr. 2011, 35 (Suppl. 5), 14S–20S. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Cargile, C.M.; Cohen, N.D.; Chapkin, R.S.; Weeks, B.R.; Davidson, L.A.; Goldsby, J.S.; Hunt, C.L.; Steinmeyer, S.H.; Menon, R.; Suchodolski, J.S.; et al. The Microbiota-Derived Metabolite Indole Decreases Mucosal Inflammation and Injury in a Murine Model of NSAID Enteropathy. Gut Microbes 2016, 7, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Cho, J.R.; Zhou, C.; Treweek, J.B.; Chan, K.; McKinney, S.L.; Yang, B.; Gradinaru, V. Cholinergic Mesopontine Signals Govern Locomotion and Reward through Dissociable Midbrain Pathways. Neuron 2016, 90, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Myers, J.; Faselis, C.; Panagiotakos, D.B.; Doumas, M.; Pittaras, A.; Manolis, A.; Kokkinos, J.P.; Karasik, P.; Greenberg, M.; et al. Exercise Capacity and Mortality in Older Men. Circulation 2010, 122, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; McPhee, N.; Pearce, K.; Benson, S.; Schedlowski, M.; Engler, H. Endotoxin-Initiated Inflammation Reduces Testosterone Production in Men of Reproductive Age. Am. J. Physiol. Metab. 2018, 314, E206–E213. [Google Scholar] [CrossRef]
- Palme, R.; Rettenbacher, S.; Touma, C.; El-Bahr, S.M.; Mostle, E. Stress Hormones in Mammals and Birds: Comparative Aspects Regarding Metabolism, Excretion, and Noninvasive Measurement in Fecal Samples. Ann. N. Y. Acad. Sci. 2005, 1040, 162–171. [Google Scholar] [CrossRef]
- Morris, D.J.; Brem, A.S. Role of Gut Metabolism of Adrenal Corticosteroids and Hypertension: Clues Gut-Cleansing Antibiotics Give Us. Physiol. Genom. 2019, 51, 83–89. [Google Scholar] [CrossRef]
- Johnson, K.V.; Foster, K.R. Why Does the Microbiome Affect Behaviour? Nat. Rev. Microbiol. 2018. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.J. Epithelial Acetylcholine—A New Paradigm for Cholinergic Regulation of Intestinal Fluid and Electrolyte Transport. J. Physiol. 2011, 589, 771–772. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and Immunology of the Cholinergic Antiinflammatory Pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef]
- Ford, E.S.; Dietz, W.H. Trends in Energy Intake among Adults in the United States: Findings from NHANES. HHS Public Access 2015, 97, 848–853. [Google Scholar] [CrossRef]
- Vos, M.B.; Kimmons, J.E.; Gillespie, C.; Welsh, J.; Blanck, H.M. Dietary Fructose Consumption among US Children and Adults: The Third National Health and Nutrition Examination Survey. Medscape J. Med. 2008, 10, 160. [Google Scholar] [PubMed]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic Vulnerability to Diet-Induced Obesity in the C57BL/6J Mouse: Physiological and Molecular Characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Breinager, L.; Kyrillou, E.; Lacuna, K.; Rocha, R.; Sclafani, A. Differential Effects of Sucrose and Fructose on Dietary Obesity in Four Mouse Strains. Physiol. Behav. 2010, 101, 331–343. [Google Scholar] [CrossRef]
- Knab, A.M.; Bowen, R.S.; Moore-harrison, T.; Hamilton, A.T.; Turner, J.; Lightfoot, J.T. Repeatability of Exercise Behaviors in Mice. Physiol. Behav. 2010, 98, 433–440. [Google Scholar] [CrossRef]
- Lightfoot, J.T.; Turner, M.J.; Pomp, D.; Kleeberger, S.R.; Leamy, L.J. Quantitative Trait Loci for Physical Activity Traits in Mice. Physiol. Genom. 2008, 32, 401–408. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letsinger, A.C.; Menon, R.; Iyer, A.R.; Vellers, H.L.; Granados, J.Z.; Jayaraman, A.; Lightfoot, J.T. A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner. Metabolites 2020, 10, 421. https://doi.org/10.3390/metabo10100421
Letsinger AC, Menon R, Iyer AR, Vellers HL, Granados JZ, Jayaraman A, Lightfoot JT. A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner. Metabolites. 2020; 10(10):421. https://doi.org/10.3390/metabo10100421
Chicago/Turabian StyleLetsinger, Ayland C., Rani Menon, Anjushree R. Iyer, Heather L. Vellers, Jorge Z. Granados, Arul Jayaraman, and J. Timothy Lightfoot. 2020. "A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner" Metabolites 10, no. 10: 421. https://doi.org/10.3390/metabo10100421
APA StyleLetsinger, A. C., Menon, R., Iyer, A. R., Vellers, H. L., Granados, J. Z., Jayaraman, A., & Lightfoot, J. T. (2020). A High Fat/High Sugar Diet Alters the Gastrointestinal Metabolome in a Sex Dependent Manner. Metabolites, 10(10), 421. https://doi.org/10.3390/metabo10100421




