Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues
Abstract
:1. Introduction
2. Results
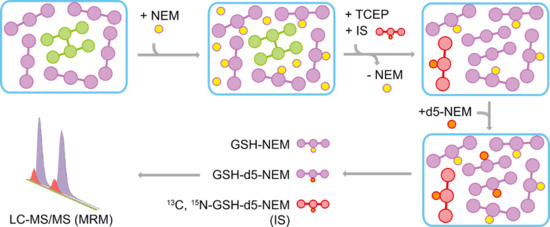
2.1. Method Overview
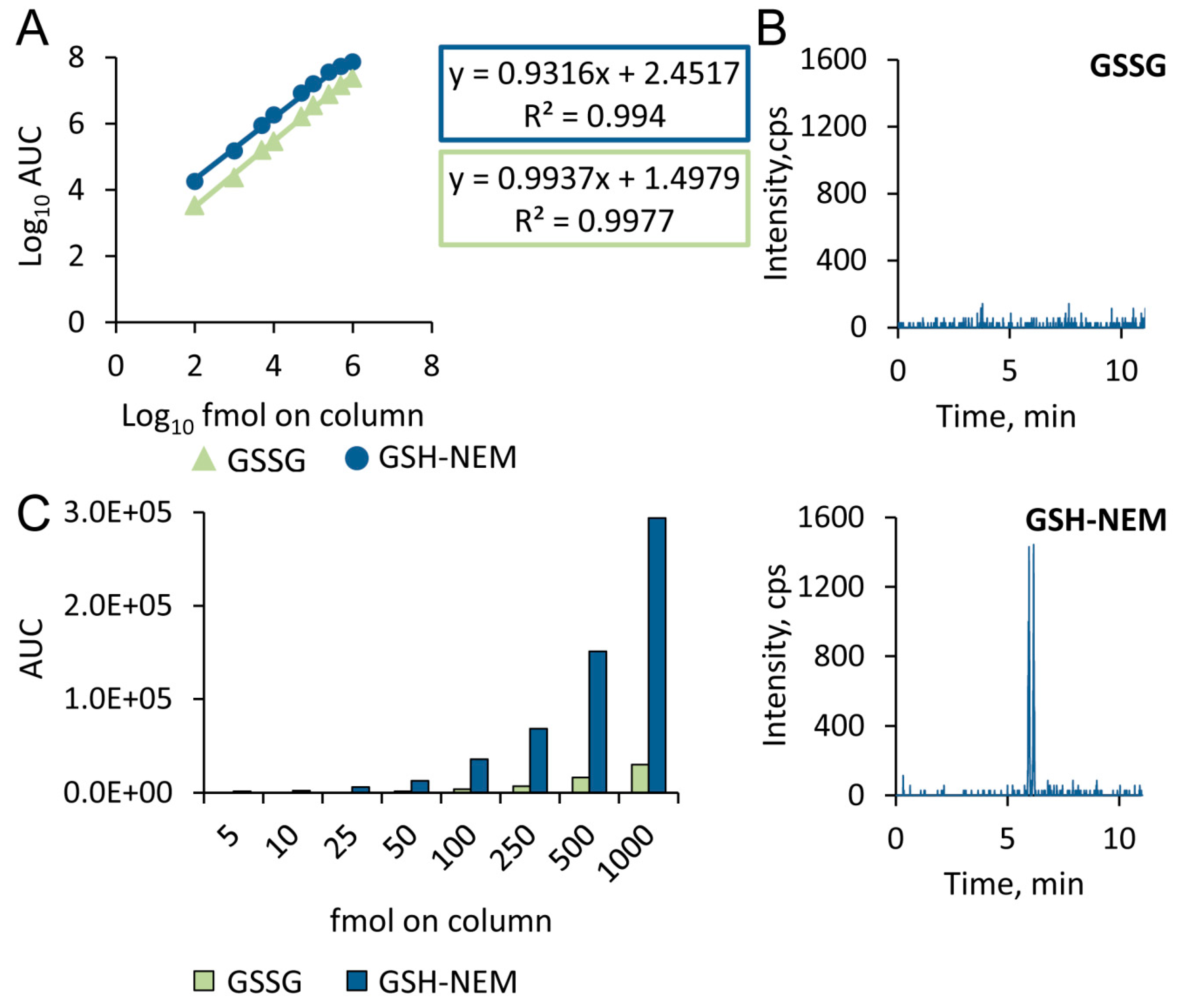
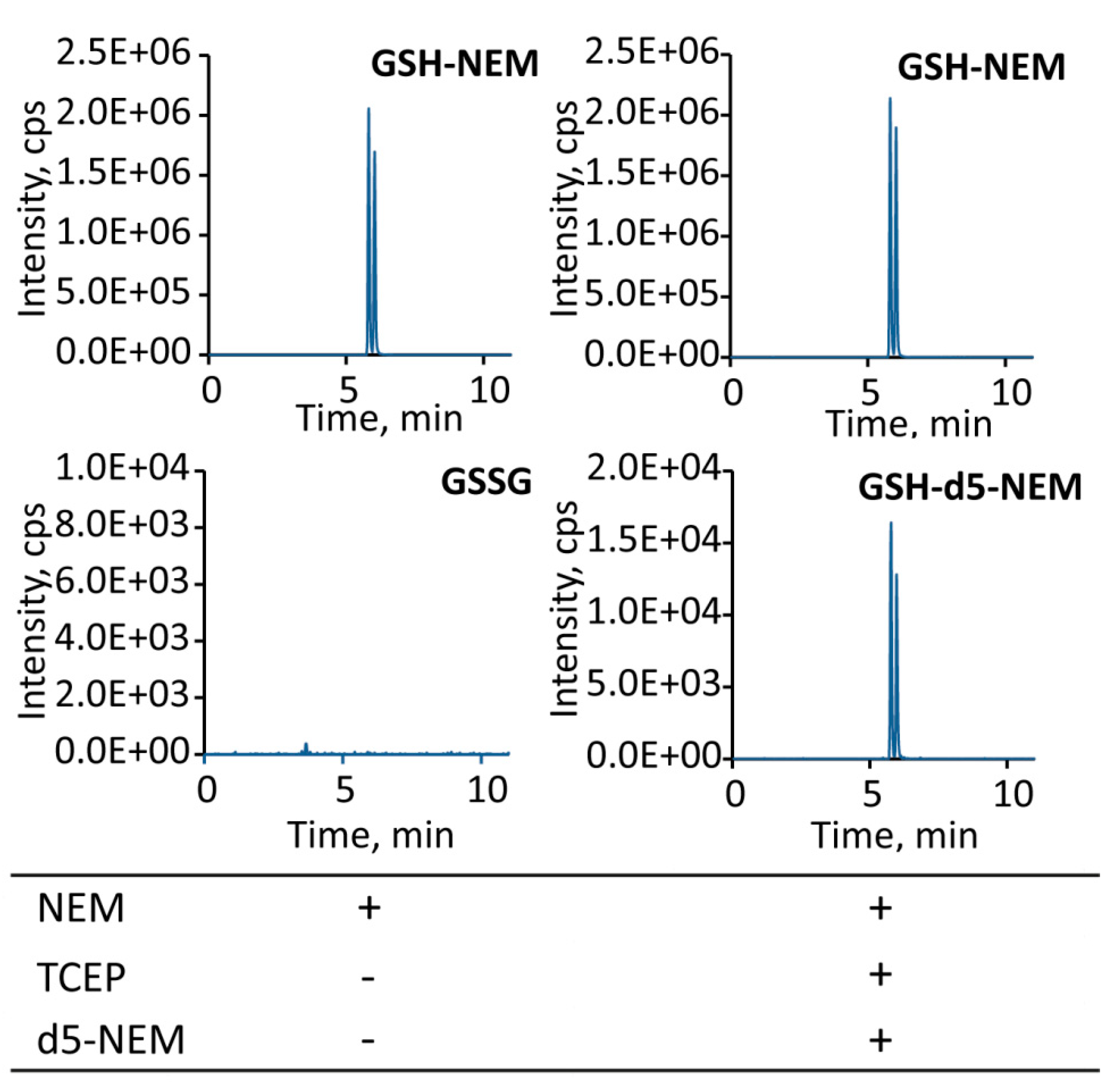
2.2. Sensitivity of the Approach
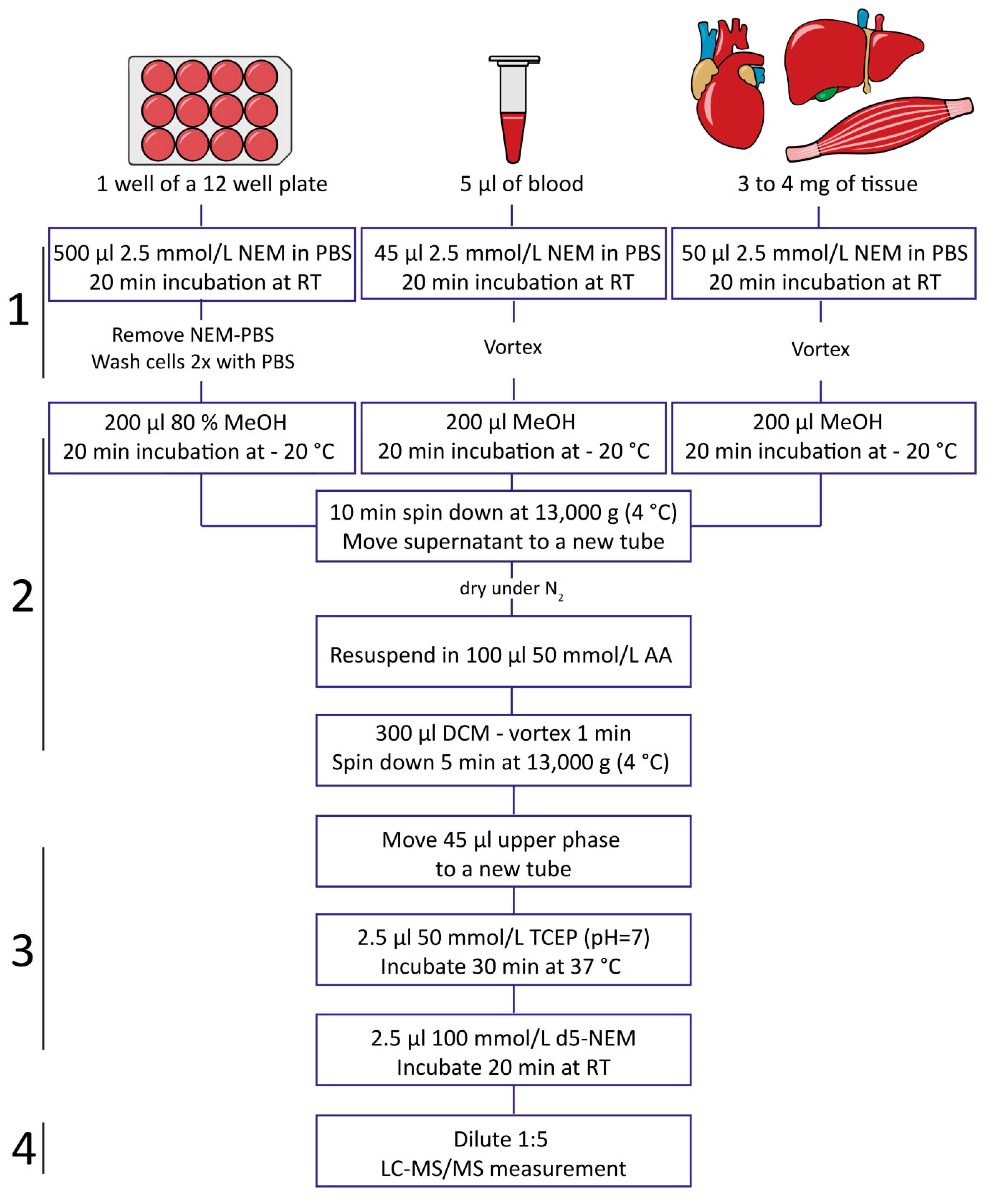
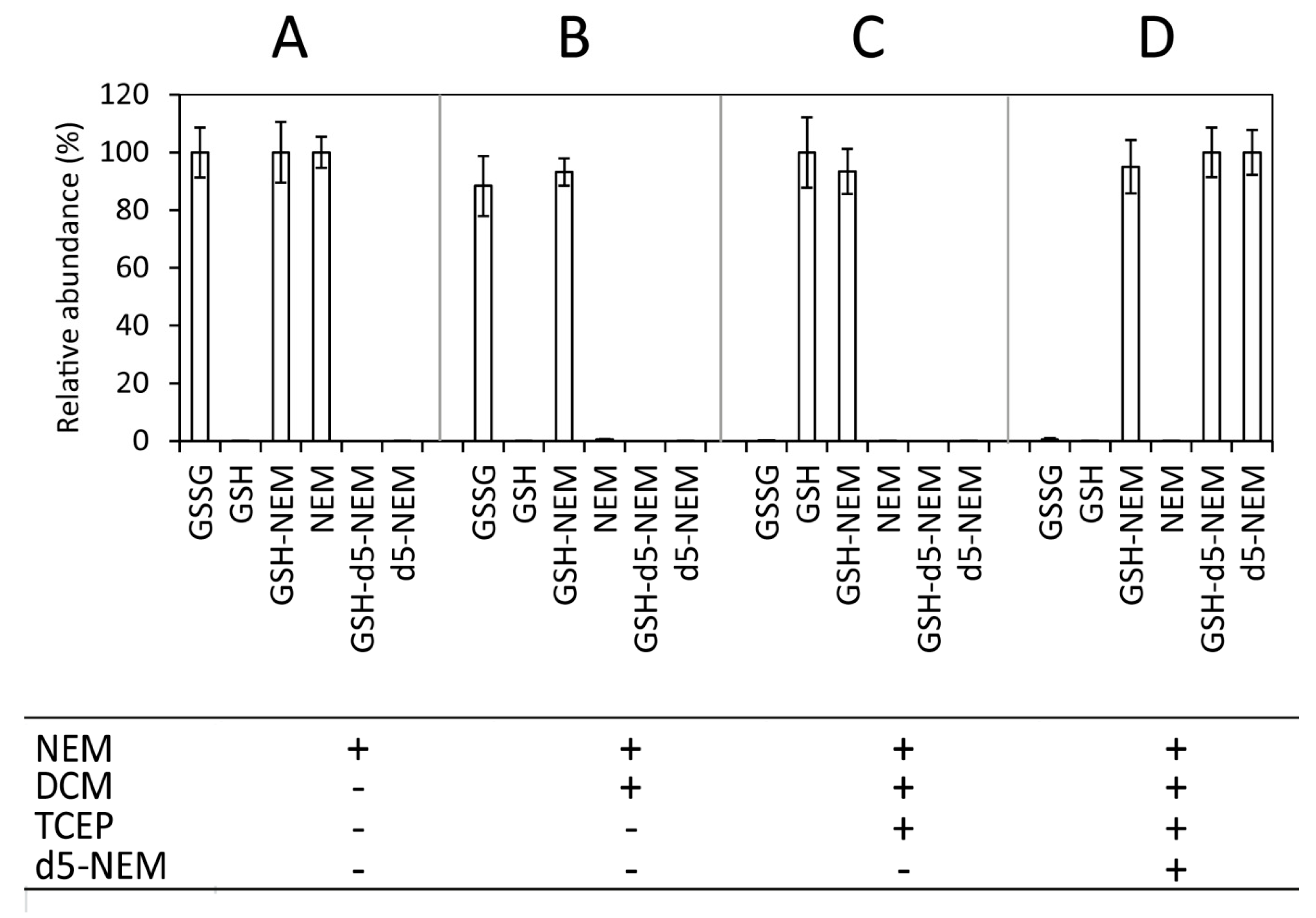
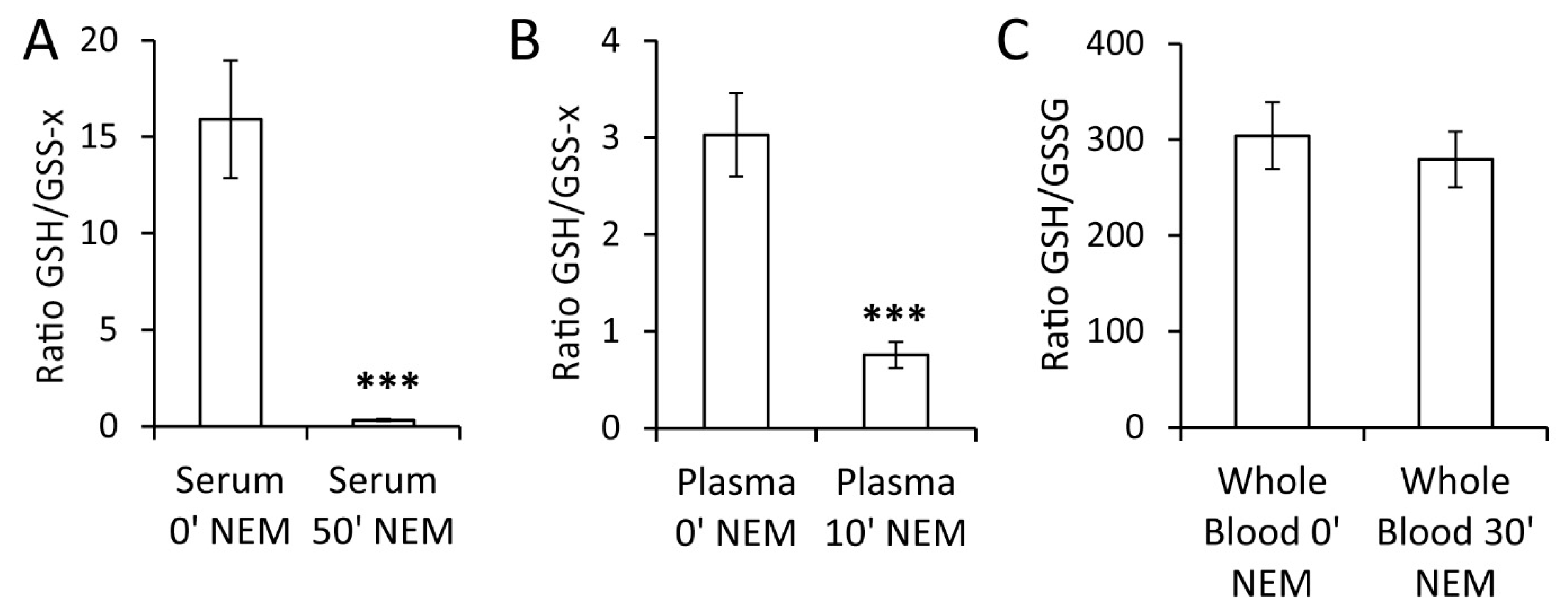
2.3. Impact of Sample Processing on GSH/GSSG Determination in Blood
3. Discussion
4. Materials and Methods
4.1. Standards
4.2. Liquid Chromatography Coupled with Tandem Mass Spectrometry
4.3. Blood, Plasma, and Serum Sample Collection and Processing
5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustarini, D.; Tsikas, D.; Colombo, G.; Milzani, A.; Dalle-Donne, I.; Fanti, P.; Rossi, R. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: An elephant in the room. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1019, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Kanaan, G.N.; Harper, M.-E. Cellular redox dysfunction in the development of cardiovascular diseases. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D.; Tapiero, H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003, 57, 145–155. [Google Scholar] [CrossRef]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Fanti, P.; Rossi, R. Analysis of GSH and GSSG after derivatization with N-ethylmaleimide. Nat. Protoc. 2013, 8, 1660–1669. [Google Scholar] [CrossRef]
- Sutton, T.R.; Minnion, M.; Barbarino, F.; Koster, G.; Fernandez, B.O.; Cumpstey, A.F.; Wischmann, P.; Madhani, M.; Frenneaux, M.P.; Postle, A.D.; et al. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol. 2018, 16, 359–380. [Google Scholar] [CrossRef]
- Rajer, M.; Kmet, M. Quantitative analysis of fine needle aspiration biopsy samples. Radiol. Oncol. 2005, 39, 269–272. [Google Scholar]
- Paulech, J.; Solis, N.; Cordwell, S.J. Characterization of reaction conditions providing rapid and specific cysteine alkylation for peptide-based mass spectrometry. Biochim. Biophys. Acta 2013, 1834, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Han, G.Y. A procedure for quantitative determination of tris(2-carboxyethyl)phosphine, an odorless reducing agent more stable and effective than dithiothreitol. Anal. Biochem. 1994, 220, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Shafer, D.E.; Inman, J.K.; Lees, A. Reaction of Tris(2-carboxyethyl)phosphine (TCEP) with maleimide and alpha-haloacyl groups: Anomalous elution of TCEP by gel filtration. Anal. Biochem. 2000, 282, 161–164. [Google Scholar] [CrossRef] [Green Version]
- Giustarini, D.; Colombo, G.; Garavaglia, M.L.; Astori, E.; Portinaro, N.M.; Reggiani, F.; Badalamenti, S.; Aloisi, A.M.; Santucci, A.; Rossi, R.; et al. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017, 112, 360–375. [Google Scholar] [CrossRef]
- Giustarini, D.; Galvagni, F.; Orlandini, M.; Fanti, P.; Rossi, R. Immediate stabilization of human blood for delayed quantification of endogenous thiols and disulfides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1019, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Rossi, R.; Milzani, A.; Dalle-Donne, I.; Giustarini, D.; Lusini, L.; Colombo, R.; Di Simplicio, P. Blood glutathione disulfide: In vivo factor or in vitro artifact? Clin. Chem. 2002, 48, 742–753. [Google Scholar] [CrossRef] [Green Version]
- Tuck, M.K.; Chan, D.W.; Chia, D.; Godwin, A.K.; Grizzle, W.E.; Krueger, K.E.; Rom, W.; Sanda, M.; Sorbara, L.; Stass, S.; et al. Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. J. Proteome Res. 2009, 8, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Blanco, R.A.; Ziegler, T.R.; Carlson, B.A.; Cheng, P.-Y.; Park, Y.; Cotsonis, G.A.; Accardi, C.J.; Jones, D.P. Diurnal variation in glutathione and cysteine redox states in human plasma. Am. J. Clin. Nutr. 2007, 86, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, W.A.; Richie, J.P. Status of glutathione and other thiols and disulfides in human plasma. Biochem. Pharmacol. 2000, 60, 19–29. [Google Scholar] [CrossRef]
- Fu, X.; Cate, S.A.; Dominguez, M.; Osborn, W.; Özpolat, T.; Konkle, B.A.; Chen, J.; López, J.A. Cysteine Disulfides (Cys-ss-X) as Sensitive Plasma Biomarkers of Oxidative Stress. Sci. Rep. 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, F.G. On the isolation of glutathione. J. Biol. Chem. 1927, 72, 185–187. [Google Scholar]
- Monostori, P.; Wittmann, G.; Karg, E.; Túri, S. Determination of glutathione and glutathione disulfide in biological samples: An in-depth review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3331–3346. [Google Scholar] [CrossRef] [PubMed]
- Reinbold, J.; Koehler, P.; Rychlik, M. Quantitation of glutathione and its oxidation products in erythrocytes by multiple-label stable-isotope dilution. Anal. Biochem. 2014, 445, 41–48. [Google Scholar] [CrossRef]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. In Vitro 2014, 28, 1006–1015. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. An improved HPLC measurement for GSH and GSSG in human blood. Free Radic. Biol. Med. 2003, 35, 1365–1372. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [Green Version]
| Analyte Name | Q1 Mass (amu) | Q3 Mass (amu) | CE (V) | CXP (V) |
|---|---|---|---|---|
| GSSG (Transition 1) | 613.2 | 355.3 | 33 | 14 |
| GSSG (Transition 2) | 613.2 | 484.2 | 25 | 12 |
| GSH (Transition 1) | 308 | 179 | 19 | 15 |
| GSH (Transition 2) | 308 | 76.23 | 47 | 15 |
| GSH-NEM (Transition 1) | 433 | 201 | 35 | 15 |
| GSH-NEM (Transition 2) | 433 | 84.2 | 59 | 15 |
| GSH-d5-NEM (Transition 1) | 438 | 206 | 35 | 15 |
| GSH-d5-NEM (Transition 2) | 438 | 84.2 | 59 | 15 |
| NEM (Transition 1) | 126 | 80 | 25 | 12 |
| NEM (Transition 2) | 126 | 98 | 19 | 16 |
| d5-NEM (Transition 1) | 131 | 80 | 25 | 12 |
| d5-NEM (Transition 2) | 131 | 98 | 19 | 16 |
| 13C2,15N-GSH-d5-NEM (IS) | 441 | 206 | 35 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomin, T.; Schittmayer, M.; Birner-Gruenberger, R. Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues. Metabolites 2020, 10, 71. https://doi.org/10.3390/metabo10020071
Tomin T, Schittmayer M, Birner-Gruenberger R. Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues. Metabolites. 2020; 10(2):71. https://doi.org/10.3390/metabo10020071
Chicago/Turabian StyleTomin, Tamara, Matthias Schittmayer, and Ruth Birner-Gruenberger. 2020. "Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues" Metabolites 10, no. 2: 71. https://doi.org/10.3390/metabo10020071
APA StyleTomin, T., Schittmayer, M., & Birner-Gruenberger, R. (2020). Addressing Glutathione Redox Status in Clinical Samples by Two-Step Alkylation with N-ethylmaleimide Isotopologues. Metabolites, 10(2), 71. https://doi.org/10.3390/metabo10020071