Nidulantes of Aspergillus (Formerly Emericella): A Treasure Trove of Chemical Diversity and Biological Activities
Abstract
:1. Introduction
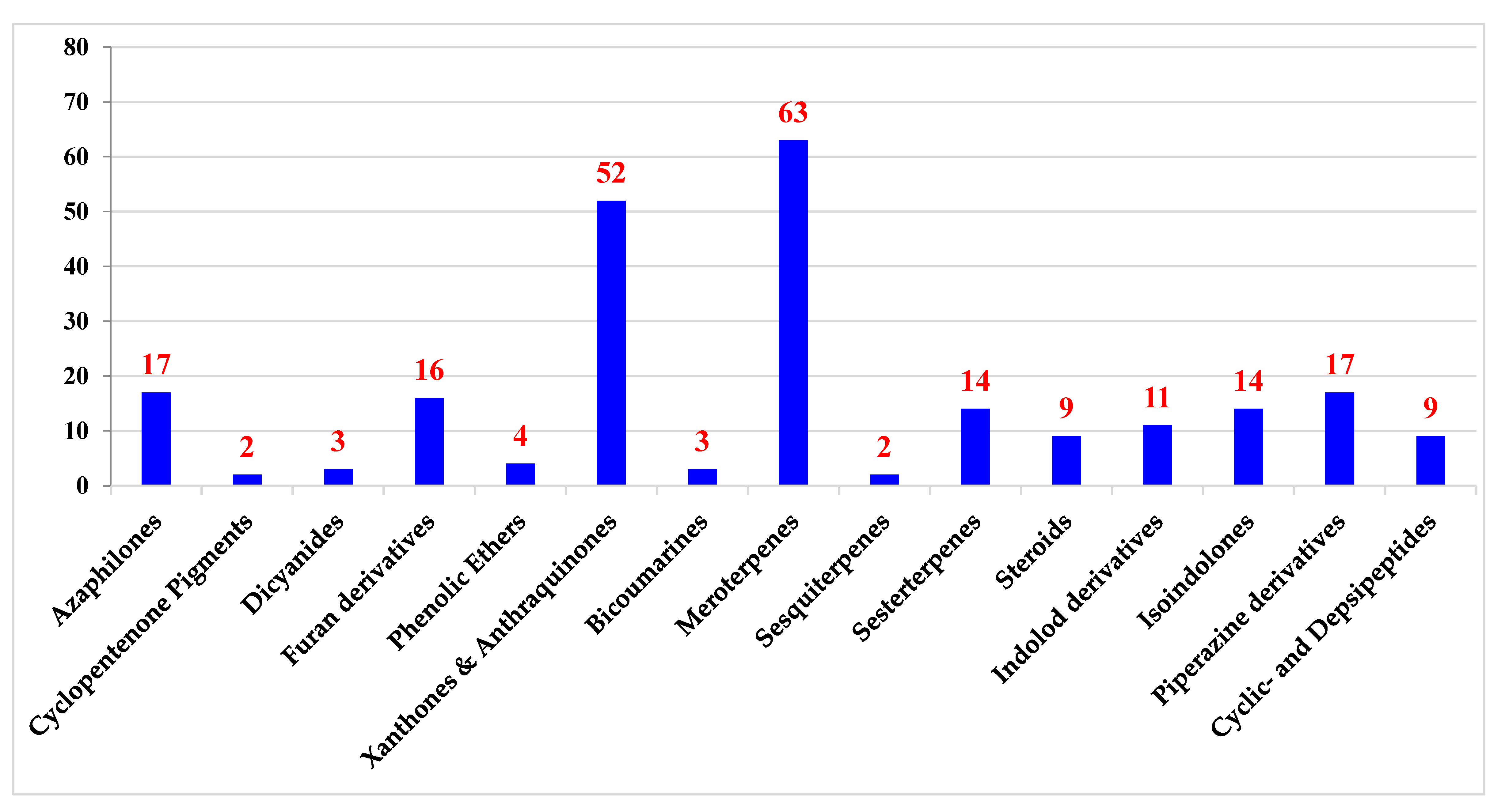
2. Polyketides
2.1. Azaphilones
2.2. Cyclopentenone Pigments
2.3. 1,4-diphenyl-2,3-dicyano-1,3-butadienes (Dicyanides)
2.4. Furan Derivatives
2.5. Phenolic Ethers
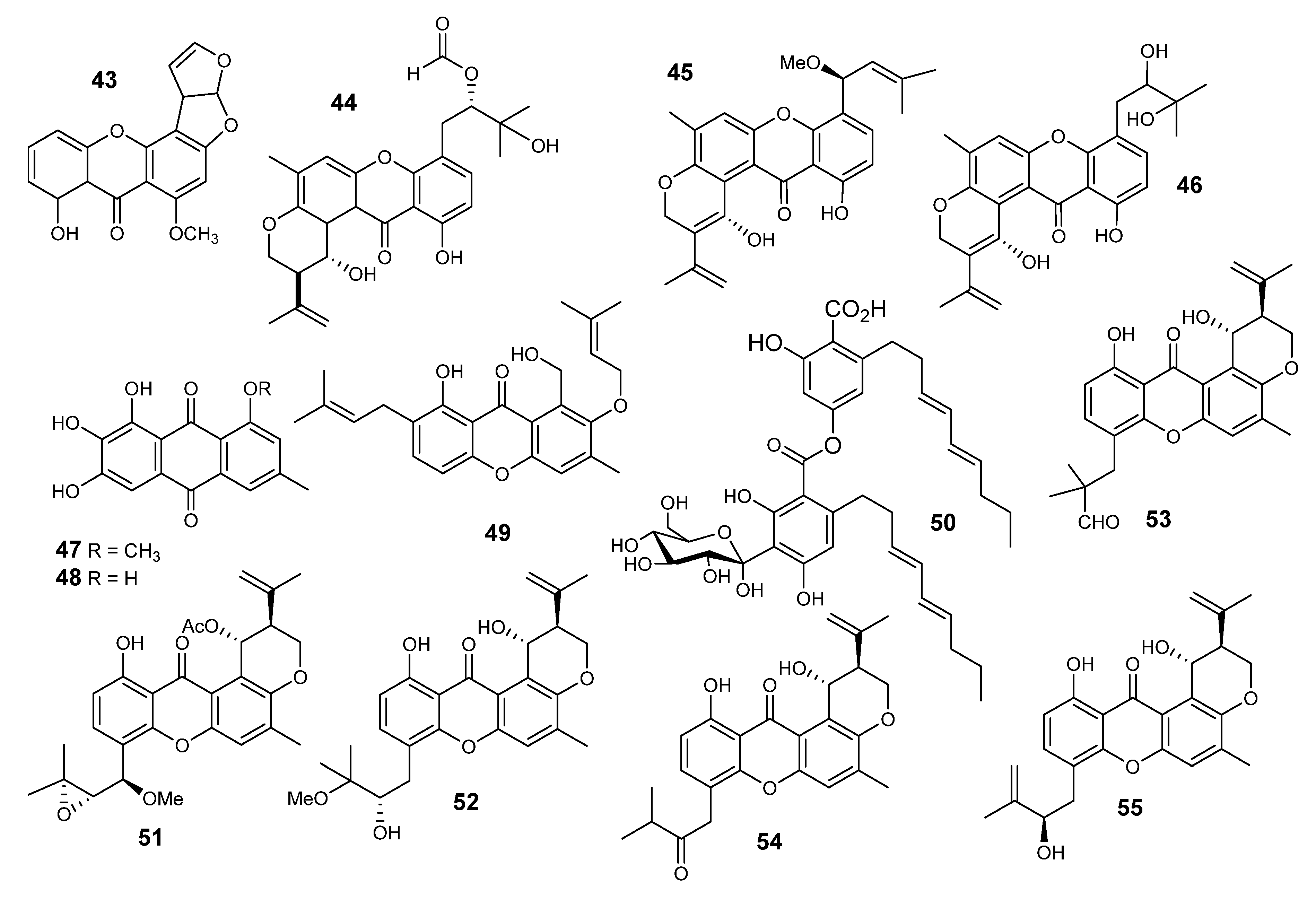
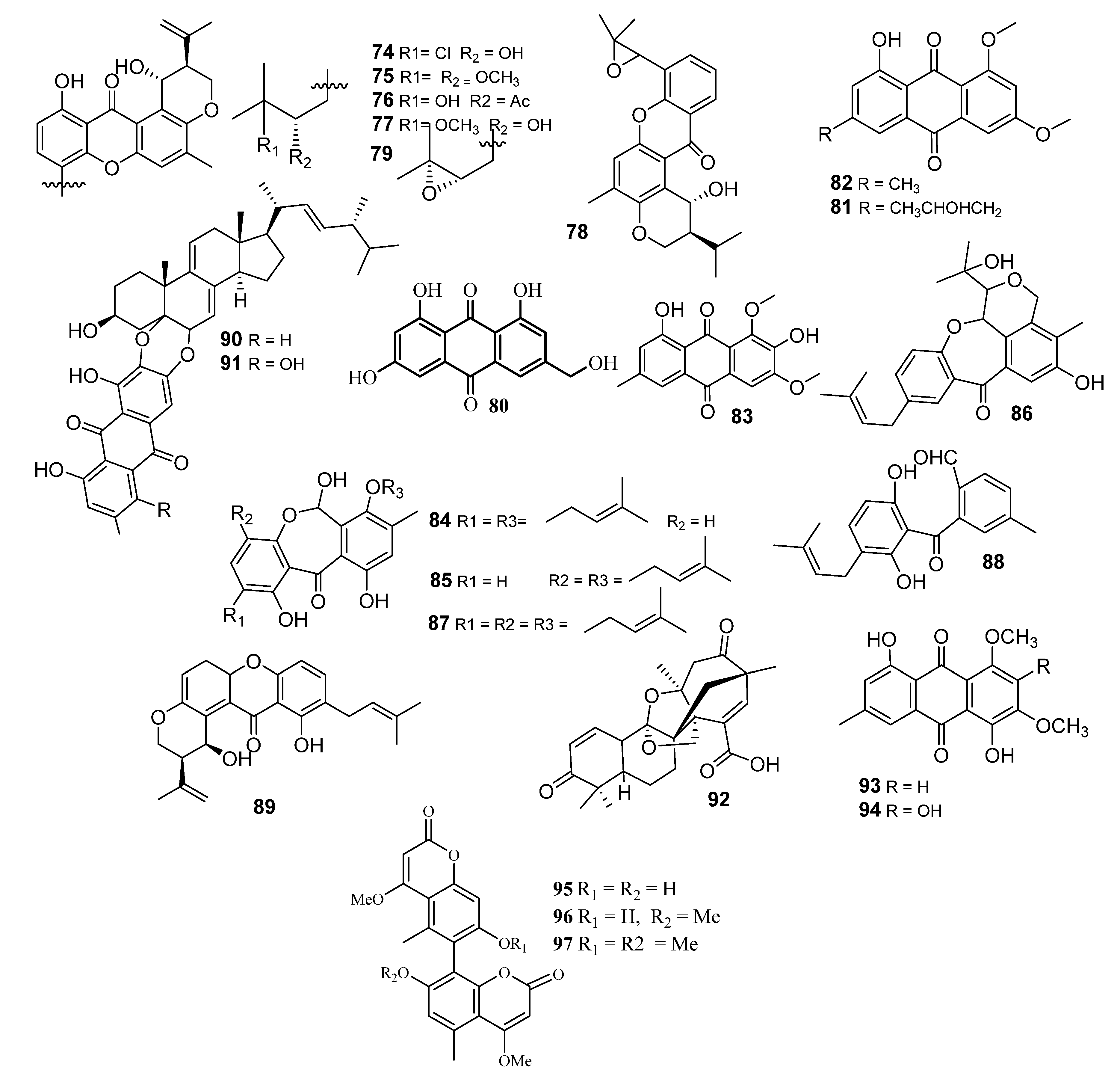
2.6. Xanthones and Anthraquinones
3. Shikimates
Bicoumarins
4. Mevalonates
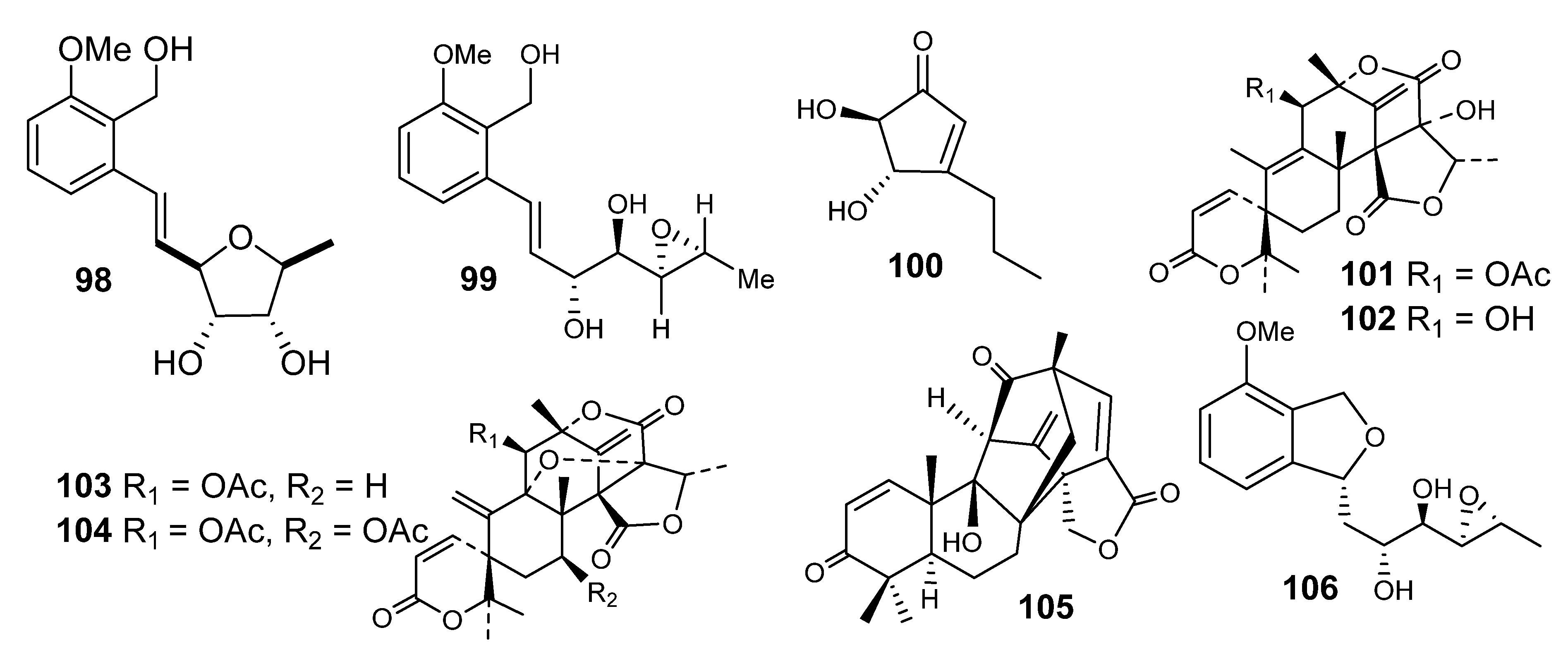
4.1. Meroterpenes
4.2. Sesquiterpenes
4.3. Sesterterpenes
4.4. Steroids
5. Amino Acids Derivatives
5.1. Alkaloids
5.1.1. Indole-Derivatives
5.1.2. Isoindolones
5.1.3. Piperazines
5.2. Cyclic Peptides and Depsipeptides
6. Conclusions and Future Prospective
Funding
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spect. 2017, 5, 79–95. [Google Scholar]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, S.; Ramawat, K.G.; Mérillon, J.M. Different Shades of Fungal Metabolites: An Overview, in Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing Switzerland: Basel, Switzerland, 2017; pp. 1–29. [Google Scholar]
- Kogel, K.H.; Franken, P.; Hueckelhoven, R. Endophyte or parasite-what decides? Current Opin. Plant. Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Branco, S. Fungal diversity from communities to genes. Fung. Biology Rev. 2019, 33, 225–237. [Google Scholar] [CrossRef]
- Cuadros-Orellana, S.; Leite, L.R.; Smith, A.; Medeiros, J.D.; Badotti, F.; Fonseca, P.L.C.; Vaz, A.B.M.; Oliveira, G.; Góes-Neto, A. Assessment of Fungal Diversity in the Environment using Metagenomics: A Decade in Review. Fung. Gen. Biol. 2013, 3, 1–13. [Google Scholar]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth and Bisby’s Dictionary of the Fungi, 10th ed.; CABI International: Wallingford, CT, USA, 2008. [Google Scholar]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 10, 463–471. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- Schulz, B.; Boyle, C.; Draeger, S.; Roemmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Shafi, S.; Shameem, N.; Bandh, S.A. Fungal endophytes as prolific source of phytochemicals and other bioactive natural products: A review. Microb. Pathog. 2015, 82, 50–59. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.-Y.; Sun, S.-F.; Li, Y.; Liu, Y.-B. Fungi: outstanding source of novel chemical scaffolds. J. Asian Nat. Prod. Res. 2018, 22, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Naganoa, Y.; Miura, T.; Nishia, S.; Lima, A.O.; Nakayama, C.; Pellizari, V.H.; Fujikura, K. Fungal diversity in deep-sea sediments associated with Asphalt Seeps at Sao Paulo Plateau. Deep Sea Res. 2017, 146, 59–67. [Google Scholar] [CrossRef]
- Beekman, A.M.; Barrow, R.A. Fungal Metabolites as Pharmaceuticals. Aust. J. Chem. 2014, 67, 827–843. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Mayer, A.M.S.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: a Current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M. Sir Edward Abraham’s contribution to the development of the cephalosporins: A reassessment. Int. J. Antimicrob. Agents. 2000, 15, 179–184. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Triggering cryptic natural product biosynthesis in microorganisms. Org. Biomol. Chem. 2009, 7, 1753–1760. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhao, H.; Zhao, X.; Xu, X.; Di, Y.; Jiang, C.; Shi, J.; Shao, D.; Huang, Q.; Yang, H.; et al. Production of bioproducts by endophytic fungi: chemical ecology, biotechnological applications, bottlenecks, and solutions. Appl. Microbiol. Biot. 2018, 102, 6279–6298. [Google Scholar] [CrossRef]
- Egbuta, M.A.; Mwanza, M.; Babalola1, O.O. A review of the ubiquity of ascomycetes filamentous fungi in relation to their economic and medical importance. Adv. Microbiol. 2016, 6, 1140–1158. [Google Scholar] [CrossRef] [Green Version]
- Geiser, D.M. Sexual structures in Aspergillus: Morphology, importance and genomics. Medical. Mycol. 2009, 47, S21–S26. [Google Scholar] [CrossRef]
- Thongkantha, S.; Lumyong, S.; McKenzie, E.H.C.; Hyde, K.D. Fungal saprobes and pathogens occurrence on tissues of Dracaena loureiri and Pandanus spp. Fungal Divers. 2008, 30, 149–179. [Google Scholar]
- Chen, A.J.; Frisvad, J.C.; Sun, B.D.; Varga, J.; Kocsube, S.; Dijksterhuis, J.; Kim, D.H.; Hong, S.B.; Houbraken, J.; Samson, R.A. Aspergillus section Nidulantes (formerly Emericella): polyphasic taxonomy, chemistry and biology. Stud. Mycol. 2016, 84, 1–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, T.; Awa, K.N.; Miyaji, M.; Udagawa, S.I.; Nakajima, S.; Kawai, K.I. Falconensins A, B, C, and D, new compounds related to azaphilone, from Emericella falconensis. Chem. Pharm. Bull. 1992, 40, 3142–3144. [Google Scholar] [CrossRef] [Green Version]
- Itabashi, T.; Ogasawara, N.; Nozawa, K.; Kawai, K.I. Isolation and structures of new azaphilone derivatives, falconensins E-G, from Emericella falconensis and absolute configurations of falconensins A-G. Chem. Pharm. Bull. 1996, 44, 2213–2217. [Google Scholar] [CrossRef] [Green Version]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef]
- Itabashi, T.; Nozawa, K.; Nakajima, S.; Kawai, K.I. A new azaphilone, falconensin H, from Emericella falconensis. Chem. Pharm. Bull. 1993, 41, 2040–2041. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, N.; Kawai, K.I. Hydrogenated azaphilones from Emericella falconensis and Emericella Fruticulosa. Phytochem. 1998, 47, 1131–1135. [Google Scholar] [CrossRef]
- Ogasawara, N.; Mizuno, R.; Kawai, K.I. Structures of a new type of yellow pigments, falconensones A and B, from Emericella falconensis. J. Chem. Soc. Perkin. Trans. I 1987, 17, 1735–1738. [Google Scholar] [CrossRef]
- Takahashi, N.; Kubo, Y.; Iwahori, A.; Kawai, K.I.; Fukui, T. Induction of apoptosis in the human promyelocytic leukemia cell line HL60 by falconensone A and its derivatives, new polyenes. Biol. Pharm. Bull. 2000, 23, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musso, L.; Dallavalle, S.; Merlini, L.; Bava, A.; Nasini, G.; Penco, S.; Giannini, G.; Giommarelli, C.; de Cesare, A.; Zuco, V.; et al. Natural and semisynthetic azaphilones as a new scaffold for Hsp90 inhibitors. Bioorg. Med. Chem. 2010, 18, 6031–6043. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Tamagawa, K.; Kawai, K.I.; Fukui, T. Antioxidant properties of a new type of polyene, falconensone A and its derivatives. Biol. Pharm. Bull. 2000, 23, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, K.; Itabashi, T.; Kawai, K.I.; Takido, M. Inhibitory effects of falconensins on 12-O-tetradecanoylphorbol- 13-acetate-induced inflammatory ear edema in mice. J. Nat. Med. 2008, 62, 384–386. [Google Scholar] [CrossRef]
- Takahashi, H.; Nozawa, K.; Kawai, K.I. Isolation and Structures of Dicyanide Derivatives, Epurpurins A to C, from Emericella purpurea. Chem. Pharm. Bull. 1996, 44, 2227–2230. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Pang, W.X.J.; Xu, L.L.; Zhao, T.; Long, X.Y.; Zhang, Q.Y.; Qin, H.L.; Yang, D.F.; Yang, X.L. Two new alkylated furan derivatives with antifungal and antibacterial activities from the plant endophytic fungus Emericella sp. XL029. Nat. Prod. Res. 2018, 32, 2625–2631. [Google Scholar] [CrossRef]
- Saito, T.; Itabashi, T.; Wakana, D.; Takeda, H.; Yaguchi, T.; Kawai, K.-I.; Hosoe, T. Isolation and structure elucidation of new phthalides and phthalane derivatives, isolated as antimicrobial agents from Emericella sp. IFM57991. J. Antibiot. 2016, 69, 89–96. [Google Scholar] [CrossRef]
- Rizzacasa, M.A.; Sargent, M.V. The synthesis of desertorin C, a bicoumarin from the fungus Emericella desertorum. J. Chem. Soc. Perkin. Trans. I 1988, 8, 2425–2428. [Google Scholar] [CrossRef]
- Yamazaki, M.; Maebayashi, Y. The isolation and structure determination of violaceic acid, a new biphenyl ether type metabolite from Emericella violacea. Chem. Pharm. Bull. 1982, 30, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, M.; Maebayashi, Y. Structure determination of violaceol-1 and -ii, new fungal metabolites from a strain of Emericella violacea. Chem. Pharm. Bull. 1982, 30, 514–518. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S.J. Xanthone derivatives: New insights in biological activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally Occurring Xanthones: Chemistry and Biology. J. Appl. Chem. 2013, 621459. [Google Scholar] [CrossRef] [Green Version]
- Frisvad, J.; Samson, R.A. Emericella venezulensis, a new species with stellate ascospores producing sterigmatocystin and aflatoxin. Systematic Appl. Microbiol. 2004, 27, 672–680. [Google Scholar] [CrossRef]
- Park, H.-S.; Jun, S.-C.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Diversity, application, and synthetic biology of industrially important aspergillus fungi. Adv. Appl. Microbiol. 2017, 100, 161–202. [Google Scholar]
- Malmstrøm, J.; Christophersen, C.; Barrero, A.F.; Oltra, J.E.; Justicia, J.; Rosales, A. Bioactive metabolites from a marine-derived strain of the fungus Emericella variecolor. J. Nat. Prod. 2002, 65, 364–367. [Google Scholar]
- Chexal, K.K.; Fouweather, C.; Holker, J.S.E.; Simpson, T.J.; Young, K. The Biosynthesis of Fungal Metabolites. Part 111.l Structure of Shamixanthone and Tajixanthone, Metabolites of Aspergillus variecolor. J. Chem. Soc. Perkin I 1974, 1584–1593. [Google Scholar] [CrossRef]
- Chexal, K.K.; Holker, J.S.E.; Simpson, T.J. The biosynthesis of fungal metabolites. Part v1.l structures and biosynthesis of some minor metabolites from variant strains of Aspergillus variecolor. J. Chem. Soc. Perkin I 1975, 6, 549–554. [Google Scholar] [CrossRef]
- Bringmann, G.; Lang, G.; Steffens, S.; Gunther, E.; Schaumann, K. Evariquinone, isoemericellin, and stromemycin from a sponge derived strain of the fungus Emericella variecolor. Phytochemistry 2003, 63, 437–443. [Google Scholar] [CrossRef]
- Hopmann, C.; Knauf, M.A.; Weithmann, K.; Wink, J. Preparation of stromemycins as stromelysin inhibitors; Pct international patent application No. WO 01/44264 A2; Aventis Pharma Deutschland G.m.b.H.: Eiterfeld, Germany, 2001. [Google Scholar]
- Pornpakakul, S.; Liangsakul, J.; Ngamrojanavanich, N.; Roengsumran, S.; Sihanonth, P.; Piapukiew, J.; Sangvichien, E.; Puthong, S.; Petsom, A. cytotoxic activity of four xanthones from Emericella variecolor, an endophytic fungus isolated from Croton oblongifolius Arch. Pharm. Res. 2006, 29, 140–144. [Google Scholar]
- Moosophon, P.; Kanokmedhakul, S.; Kanokmedhakul, K.; Soytong, K. Prenylxanthones and a bicyclo[3.3.1]nona-2,6-diene derivative from the fungus Emericella rugulosa. J. Nat. Prod. 2009, 72, 1442–1446. [Google Scholar]
- Fujimoto, H.; Asai, T.; Kim, Y.P.; Ishibashi, M. Nine Constituents Including Six xanthone-Related Compounds Isolated from Two Ascomycetes, Gelasinospora santiflorii and Emericella quadrilineata, Found in a Screening Study Focused on Immunomodulatory Activity. Chem. Pharm. Bull. 2006, 54, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Fukuda, Y.; Akagawa, R.N.; Tsun, T.; Umemura, K.; Andoh, T. Inhibition of DNA topoisomerases by nidulalin A derivatives. Biol. Pharm. Bull. 2000, 23, 511–512. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, M.; del Carmen González, M.; Rodríguez-Sotres, R.; Sosa-Peinado, A.; González-Andrade, M.; Cerda-García-Rojas, C.M.; Mata, R. Calmodulin inhibitors from the fungus Emericella sp. Bioorg. Med. Chem. 2009, 17, 2167–2174. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, R.A.N.; Kawahara, B.K.N.; Awa, A.M.Y.; Shoichi, N.A.; Kawai, K.I. Stereochemistry of an 1 8,22-Cyclosterol, Mer-NF8054X, from Emericella heterothallica and Aspergillus ustus. Chem. Pharm. Bull. 1995, 43, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Fang, L.-Z.; Liu, F.-L.; Pang, X.-J.; Qin, H.; Li, T.; Zhao, L.-L.; Xu, Y.D.-F.; Yang, X.-L. New prenylxanthones, polyketide hemiterpenoid pigments from the endophytic fungus Emericella sp. XL029 and their anti-agricultural pathogenic fungal and antibacterial activities†. RSC Adv. 2017, 7, 31115. [Google Scholar] [CrossRef] [Green Version]
- Fredimoses, M.; Zhou, X.; Wen, A.; Wang, J.; Liao, S.; Liu, J.; Yang, B.; Yang, X.; Liu, Y. new prenylxanthones from the deep-sea derived fungus Emericella sp. SCSIO 05240. Mar. Drugs 2014, 12, 3190–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredimoses, M.; Zhou, X.; Ai, W.; Tian, X.; Yang, B.; Lin, X.; Liu, J.; Liu, Y. Emerixanthone E, a new xanthone derivative from deep sea fungus Emericella sp. SCSIO 05240. Nat. Prod. Res. 2019, 33, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Karlj, A.; Kehraus, S.; Krick, A.; Eguereua, E.; Kelter, G.; Maurer, M.; Wortmann, A.; Fiebig, H.H.; Konig, G.M. Arugosins G and H: prenylated polyketides from the marine-derived fungus Emericella niduans var acristata. J. Nat. Prod. 2006, 69, 995–1000. [Google Scholar] [CrossRef]
- Ballantine, J.A.; Francis, D.J.; Hassal, C.H.; Wright, J.I.C. The biosynthesis of phenols. Part XXI. The molecular structure of arugosin, a metabolite of a wild-type strain of Aspergillus rugulosus. J. Chem. Soc. 1970, 9, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Liangsakul, J.; Srisurichan, S.; Pornpakakul, S. Anthraquinone–steroids, evanthrasterol A and B, and a meroterpenoid, emericellic acid, from endophytic fungus, Emericella variecolor. Steroids 2016, 106, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Joshi, H. Coumarin: chemical and pharmacological profile. J. Appl. Pharm. Sci. 2012, 2, 236–240. [Google Scholar]
- Nozawa, K.; Seyea, H.; Nakajima, S.; Udagawar, S.I.; Kawa, K.I. Studies on fungal products. Part 10. Isolation and structures of novel bicoumarins, desertorins A, B, and C, from Emericella desertorum. J. Chem. Soc. Perkin. Trans. I 1987, 1735–1738. [Google Scholar] [CrossRef]
- Menna, M.; Imperatore, C.; D’Aniello, F.; Aiello, A. meroterpenes from marine invertebrates: structures, occurrence, and ecological implications. Mar. Drugs. 2013, 11, 1602–1643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Sun, S.; Zhu, T.; Lin, Z.; Gu, J.; Li, D.; Gu, Q. Antiviral isoindolone derivatives from an endophytic fungus Emericella sp. associated with Aegiceras corniculatum. Phytochemistry 2011, 72, 1436–1442. [Google Scholar] [CrossRef]
- Liangsakul, J.; Pornpakakul, S.; Sangvichien, E.; Muangsin, N.; Sihanonth, P. Emervaridione and varioxiranediol, two new metabolites from the endophytic fungus, Emericella variecolor. Tetrahedron Lett. 2011, 52, 6427–6430. [Google Scholar] [CrossRef]
- Tarawneh, A.H.; León, F.; Radwan, M.M.; Rosa, L.H.; Cutler, S.J. Secondary Metabolites from the Fungus Emericella nidulans. Nat. Prod. Commun. 2013, 8, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Wu, C.; Long, H.; Chen, R.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Varioxiranols A−G and 19-O-Methyl-22-methoxypre-shamixanthone, PKS and hybrid PKS-derived metabolites from a sponge-associated Emericella variecolor fungus. J. Nat. Prod. 2015, 78, 2461–2470. [Google Scholar] [CrossRef]
- Long, H.; Cheng, Z.; Huang, W.; Wu, Q.; Li, X.; Cui, J.; Proksch, P.; Lin, W. Diasteltoxins A–C, asteltoxin-based dimers from a mutant of the sponge-associated Emericella variecolor fungus. Org. Lett. 2016, 18, 4678–4681. [Google Scholar] [CrossRef]
- Hashimoto, M.; Ichijo, H.; Fujiwara, K.; Sugasawa, H.; Abo, S.; Matsudo, K.; Uchigawa, N.; Goda, Y.; Fujii, I. Functional expression of a highly-reducing polyketide synthase of Emericella variecolor IFM42010, an asteltoxin-producing strain, resulted in production of two polyenoic β-ketolactones with opposite stereochemistry. Bioorg. Med. Chem. Lett. 2019, 29, 126686. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Li, Q.; Huang, J.; Li, X.-N.; Zhu, H.; Liu, J.; Wang, J.; Xue, Y.; Zhang, Y. Bioassay-guided isolation of antibacterial metabolites from Emericella sp. TJ29. J. Nat. Prod. 2017, 80, 2399–2405. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.-J.; Zhang, S.-B.; Chen, H.-L.; Zhao, W.-T.; Yang, D.-F.; Xian, P.-J.; Xu, L.-L.; Tao, Y.-D.; Fu, H.-Y.; Yang, X.-L. Emericelactones A-D: four novel polyketides produced by Emericella sp. XL 029, a fungus associated the leaves of Panax notoginseng. Tetrahedron Lett. 2018, 59, 4566–4570. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Cheng, L.; Wei, M.; Dai, C.; He, Y.; Gong, J.; Zhu, R.; Li, X.-N.; Junjun, L.; et al. Emeridones A-F, a series of 3,5-demethylorsellinic acid-based meroterpenoids with rearranged skeletons from an endophytic fungus Emericella sp. TJ29. J. Org. Chem. 2019, 84, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Sun, W.; Li, Q.; Li, X.N.; Zhu, H.; Huang, J.; Liu, J.; Wang, J.; Xue, Y.; et al. Spiroaspertrione A, a bridged spirocyclic meroterpenoid as a Potent Potentiator of Oxacillin against Methicillin-Resistant Staphylococcus aureus from Aspergillus sp. TJ 23. J. Org. Chem. 2017, 82, 3125–3131. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, T.K.; Yang, X.L. Emerones A-C: Three novel merosesquiterpenoids with unprecedented skeletons from Emericella sp. XL029. Org. Biomolec. Chem 2019. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; He, Y.; Guan, D.; Cheng, L.; Hao, X.; Wei, M.; Zheng, Y.; Liu, C.; Li, X.N.; et al. Emeriones A−C: Three highly methylated polyketides with bicyclo[4.2.0]octene and 3,6-Dioxabicyclo[3.1.0]hexane functionalities from Emericella nidulans. Org. Lett. 2019, 21, 5091–5095. [Google Scholar] [CrossRef]
- Pang, X.J.; Zhang, S.B.; Xian, P.J.; Wu, X.; Yang, D.F.; Fu, H.Y.; Yang, X.L. Emericellins A and B: two sesquiterpenoids with an unprecedented tricycle [4,4,2,1]hendecane scaffold from the liquid cultures of endophytic fungus Emericella sp. XL 029. Fitoterapia 2018, 131, 55–58. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Hung, J.H.; Zhang, S. Sesterterpenoids. Nat. Prod. Rep. 2007, 24, 1401–1429. [Google Scholar] [CrossRef]
- Xu, Y.M.; Espinosa-Artiles, P.; Liu, M.X.; Arnold, E.A.A.; Gunatilaka, A.A.L. Secoemestrin D, a cytotoxic epitetrathiodioxopiperizine, and emericellenes A−E, five sesterterpenoids from Emericella sp. AST0036, a fungal endophyte of Astragalus lentiginosus. J. Nat. Prod. 2013, 76, 2330–2336. [Google Scholar] [CrossRef] [Green Version]
- Hensens, O.D.; Zink, D.; Williamson, J.M.; Lotti, V.J.; Chang, R.S.L.; Goetz, M.A. Variecolin, a sesterterpenoid of novel skeleton from Aspergillus variecolor MF138. J. Org. Chem. 1991, 56, 3399–3403. [Google Scholar] [CrossRef]
- Takahashi, H.; Hosoe, T.; Nozawa, K.; Kawai, K.-I. Two new sesterterpenes from the ascomycetous fungus Emericella purpurea. J. Nat. Prod. 1999, 62, 1712–1713. [Google Scholar] [CrossRef]
- Yoganathan, K.; Rossant, C.; Glover, R.P.; Cao, S.; Vittal, J.J.; Ng, S.; Huang, Y.; Buss, A.D.; Butler, M.S. Inhibition of the human chemokine receptor ccr5 by variecolin and variecolol and isolation of four new variecolin analogues, emericolins A-D, from Emericella aurantiobrunnea. J. Nat. Prod. 2004, 67, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Nakamura, E.; Ishibashi, M. Immunomodulatory constituents from an ascomycete, Emericeella aurantiobrunnea. Chem. Pharm. Bull. 2000, 48, 1436–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, K.; Chiba, H.; Kaneto, R.; Sakkamoto, M.; Okamura, K.; Tone, H. Mer-NF8054A and X, novel antifungal steroids, isolated from Aspergillus sp. J. Antibiot. 1994, 47, 591. [Google Scholar] [CrossRef] [PubMed]
- Hosoe, T.; Sameshima, T.; Dobashi, K.; Kawai, K.-I. Structures of two new 18,22-cyclosterols, emesterones A and B, from Emericella heterothallica. Chem. Pharm. Bull. 1998, 46, 850–852. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Fukui, T.; Iwahori, A.; Kubo, Y.; Hosoe, T.; Kawai, K.-I. Induction of differentiation and apoptosis in human promyelocytic leukemia hl60 cell line by a new type of steroids. Exp. Cell Res. 1998, 245, 313–320. [Google Scholar] [CrossRef]
- Sichaem, J.; Nguyen, H.H.; Diong, T.H. Hopane-6α,16α,22-triol: a New hopane triterpenoid from the lichen Parmotrema sancti-angelii. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, N.; Iitaka, Y.; Iwasaki, S.; Okuda, S. Stellatic acid: A new class of sesterterpenoids; X-ray crystal structure. Tetrahedron Lett. 1980, 21, 1961–1962. [Google Scholar]
- Nozawa, K.; Nakajima, S.; Kawai, K.I. Isolation and structures of lndoloditerpenes, possible biosynthetic intermediates to the tremorgenic mycotoxin, paxilline, from Emericella striata. J. Chem. Soc. Perkin Trans. I 1988, 9, 2607–2610. [Google Scholar] [CrossRef]
- Nozawa, K.; Horie, Y.; Udagawa, S.I.; Kawai, K.I.; Yamazak, M. Isolation of a new tremorgenic indoloditerpene, 1’-0-acetylpaxilline, from Emericella striata and distribution of paxilline in Emericella spp. Chem. Pharm. Bull. 1989, 37, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Nozawa, K.; Udagawa, S.; Nakajima, S.; Kawai, K. Studies on fungal products. Part 17: isolation and structures of novel indoloditerpenes, emindoles DA and DB, from Emericella desertorum: X-ray molecular structure of emindole DA acetate. J. Chem. Soc. Perkin Trans. 1988, 7, 1689–1694. [Google Scholar] [CrossRef]
- Nozawa, K.; Udagawa, S.; Nakajima, S.; Kawai, K. Structures of two stereoisomers of a new type of lndoloditerpene related to the tremorgenic mycotoxin paxilline, from Emericella desertorurn and Emericella striata. J. Chem. Soc. Chem. Commun. 1987, 15, 1157–1159. [Google Scholar] [CrossRef]
- Nozawa, K.; Yuyama, M.; Nakajima, S.; Kawai, K.; Udagawa, S. Studies on fungal products. Part 19. isolation and structure of a novel indoloditerpene, emindole SA, from Emericella striata. J. Chem. Soc. Perkin Trans. I 1988, 8, 2155–2160. [Google Scholar] [CrossRef]
- Rainier, J.D.; Smith, A.B. Polyene cyclizations to indole diterpenes. The first synthesis of (+)-emindole SA using a biomimetic approach. Tetrahedron Lett. 2000, 41, 9419–9423. [Google Scholar] [CrossRef]
- Fueki, S.; Tokiwano, T.; Toshima, H.; Oikawa, H. Biosynthesis of indole diterpenes, emindole, and paxilline: Involvement of a common intermediate. Org. Let. 2004, 6, 2697–2700. [Google Scholar] [CrossRef]
- Hosoe, T.; Itabashi, T.; Kobayashi, N.; Udagawa, S.I.; Kawai, K.-I. Three new types of indoloditerpenes, emindole PA—PC, from Emericella purpurea, revision of the structure of emindole PA. Chem. Pharm. Bull. 2006, 54, 185–187. [Google Scholar] [CrossRef] [Green Version]
- Mcleay, L.M. Tremorgenic mycotoxins paxilline, penitrem and lolitrem B, the non-tremorgenic 31-epilolitrem B and electromyographic activity of the reticulum and rumen of sheep. Res. Veter. Sci. 1999, 66, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Sun, X.; Li, N.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Isoindolone-containing meroterpenoids from the endophytic fungus Emericella nidulans HDN12-249. Org. Lett. 2016, 18, 4670–4673. [Google Scholar] [CrossRef]
- Patil, M.; Poyil, A.N.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Bugarin, A. Design, synthesis, and molecular docking study of new piperazine derivativeas potential antimicrobial agents. Bioorg. Chem. 2019, 92, 103217. [Google Scholar] [CrossRef]
- Welch, T.R.; Williams, R.M. Epidithiodioxopiperazines occurrence synthesis and biogenesis. Nat. Prod. Rep. 2014, 31, 1376–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seya, H.; Nakajima, S.; Kawa, K.I.; Udagawab, S.I. Structure and absolute configuration of emestrin, a new macrocyclic epidithiodioxopiperazine from Emericella striata. J. Chem. Soc. Chem. Commun. 1985, 10, 657–658. [Google Scholar] [CrossRef]
- Seya, H.; Nozawa, K.; Nakajima, S.; Kawai, K.I.; Udagawa, S.I. Studies on fungal products. Part 8. Isolation and structure of emestrin, a novel antifungal macrocyclic epidithiodioxopiperazine from Emericella striata. X-Ray molecular structure of emestrin. J. Chem. Soc. Perkin. Trans. I 1986, 109–116. [Google Scholar] [CrossRef]
- Kawahara, N.; Nakajima, S.; Yamazaki, M.; Kawai, K.I. Structure of a novel epidithiodioxopiperazine, emethallicin A, a potent inhibitor of histamine release from Emericella heterothallica. Chem. Pharm. Bull. 1989, 37, 2592–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, N.; Nozawa, K.; Nakajima, S.; Kawai, K. Emeheterone, a pyrazinone derivative from Emericella heterothallica. Phytochemistry 1988, 27, 3022–3024. [Google Scholar] [CrossRef]
- Kawahara, N.; Nozawa, K.; Nakajima, S.; Yamazaki, M.; Kawaia, K.I. Sulfur-containing dioxopiperazine derivatives from Emericella heterothallica. Heterocycles 1989, 29, 397–402. [Google Scholar]
- Kawahara, N.; Nozawa, K.; Yamazaki, M.; Nakajima, S.; Kawaia, K.I. Structures of novel epipolythiodioxopiperazines, emethallicins B, C, and D. Potent inhibitors of histamine release from Emericella heterothallica. Chem. Pharm. Bull. 1990, 38, 73–78. [Google Scholar] [CrossRef]
- Kawahara, N.; Nozawa, K.; Yamazaki, M.; Nakajim, S.; Kawai, K.-I. Novel epidithiodioxopiperazines, emethallicins E and F, from Emericella heterothallica. Heterocycles 1990, 30, 507–515. [Google Scholar]
- Seya, H.; Nozawa, K.; Udagawa, S.I.; Nakajima, S.; Kawai, K.I. Studies on fungal products. IX.1 dethiosecoemestrin, a new metabolite related to emestrin, from Emericella striata. C hem. Pharm. Bull. 1986, 34, 2411–2416. [Google Scholar] [CrossRef] [Green Version]
- Hamada, Y.; Shiori, T. Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides. Chem. Rev. 2005, 105, 4441–4482. [Google Scholar] [CrossRef]
- Malmstrøm, J. Unguisins A and B: New cyclic peptides from the marine -derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, J.; Ryager, A.; Anthoni, U.; Nielsen, P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 2002, 60, 869–872. [Google Scholar]
- Ariawan, A.D.; Webb, J.E.A.; Howe, E.N.W.; Gale, P.A.; Thordarson, P.; Hunter, L. Cyclic peptide unguisin A is an anion receptor with high affinity for phosphate and pyrophosphate. Org. Biomol. Chem. 2017, 15, 1–6. [Google Scholar]
- Oh, D.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [PubMed]
- Ma, Y.; Xu, L.-F.; Huang, W.-F.; Wei, B.-G.; Lin, G.-Q. Total Synthesis of emericellamide A: a secondary metabolite of marine cyclic depsipeptides with antimicrobial properties. Synlett 2009, 8, 1307–1310. [Google Scholar]
- Ghosh, S.; Pradhan, T.K. The first total synthesis of emericellamide A. Tetrahedron Lett. 2008, 49, 3697–3700. [Google Scholar] [CrossRef]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [Green Version]
- De la Cruza, M.; Martin, J.; Gonzalez-Menendez, V.; Perez-Victoria, I.; Moreno, C.; Tormo, J.R.; El Aouad, N.; Guarro, J.; Vicente, F.; Reyes, F.; et al. Chemical and physical modulation of antibiotic activity in Emericella species. Chem. Biodiver. 2012, 9, 1095–1113. [Google Scholar] [CrossRef]
- Thiericke, R.; Rohrf, J. BiologicaI variation of microbial metabolites by precursor- directed biosynthesis. Nat. Prod. Report. 2011, 28, 196–268. [Google Scholar]
- Cacho, R.A.; Jiang, W.; Chooi, Y.H.; Walsh, C.T.; Tang, Y. Identification and characterization of the echinocandin B. biosynthetic gene cluster from Emericella rugulosa NRRL 11440. J. Am. Chem. Soc. 2012, 134, 16781–16790. [Google Scholar] [CrossRef] [Green Version]
- Quy, T.N.; Xuan, T.D.; Andriana, Y.; Tran, H.D.; Khanh, T.D.; Teschke, R. Cordycepin isolated from Cordyceps militaris: Its newly discovered herbicidal property and potential plant-based novel alternative to glyphosate. Molecules 2019, 24, 2901. [Google Scholar] [CrossRef] [Green Version]
- Xuan, T.D.; Tawata, S.; Khanh, T.D.; Chung, I.M. Decomposition of allelopathic plants in soil. J. Agron. Crop. Sci. 2005, 191, 162–171. [Google Scholar] [CrossRef]
- Trezzi, M.M.; Vidal, R.A.; Junior, A.A.B.; Bittencourt, H.V.H.; Filho, A.P.D.S.S. Allelopathy: Driving mechanisms governing its activity in agriculture. J. Plant Interact. 2016, 11, 53–60. [Google Scholar] [CrossRef]
- Yousef, F.S.; Ashour, M.L.; Singab, A.B.; Wink, M.A. Comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alburae, N.A.; Mohammed, A.E.; Alorfi, H.S.; Turki, A.J.; Asfour, H.Z.; Alarif, W.M.; Abdel-Lateff, A. Nidulantes of Aspergillus (Formerly Emericella): A Treasure Trove of Chemical Diversity and Biological Activities. Metabolites 2020, 10, 73. https://doi.org/10.3390/metabo10020073
Alburae NA, Mohammed AE, Alorfi HS, Turki AJ, Asfour HZ, Alarif WM, Abdel-Lateff A. Nidulantes of Aspergillus (Formerly Emericella): A Treasure Trove of Chemical Diversity and Biological Activities. Metabolites. 2020; 10(2):73. https://doi.org/10.3390/metabo10020073
Chicago/Turabian StyleAlburae, Najla Ali, Afrah E. Mohammed, Hajer Saeed Alorfi, Adnan Jaman Turki, Hani Zakaria Asfour, Walied Mohamed Alarif, and Ahmed Abdel-Lateff. 2020. "Nidulantes of Aspergillus (Formerly Emericella): A Treasure Trove of Chemical Diversity and Biological Activities" Metabolites 10, no. 2: 73. https://doi.org/10.3390/metabo10020073
APA StyleAlburae, N. A., Mohammed, A. E., Alorfi, H. S., Turki, A. J., Asfour, H. Z., Alarif, W. M., & Abdel-Lateff, A. (2020). Nidulantes of Aspergillus (Formerly Emericella): A Treasure Trove of Chemical Diversity and Biological Activities. Metabolites, 10(2), 73. https://doi.org/10.3390/metabo10020073




