Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease
Abstract
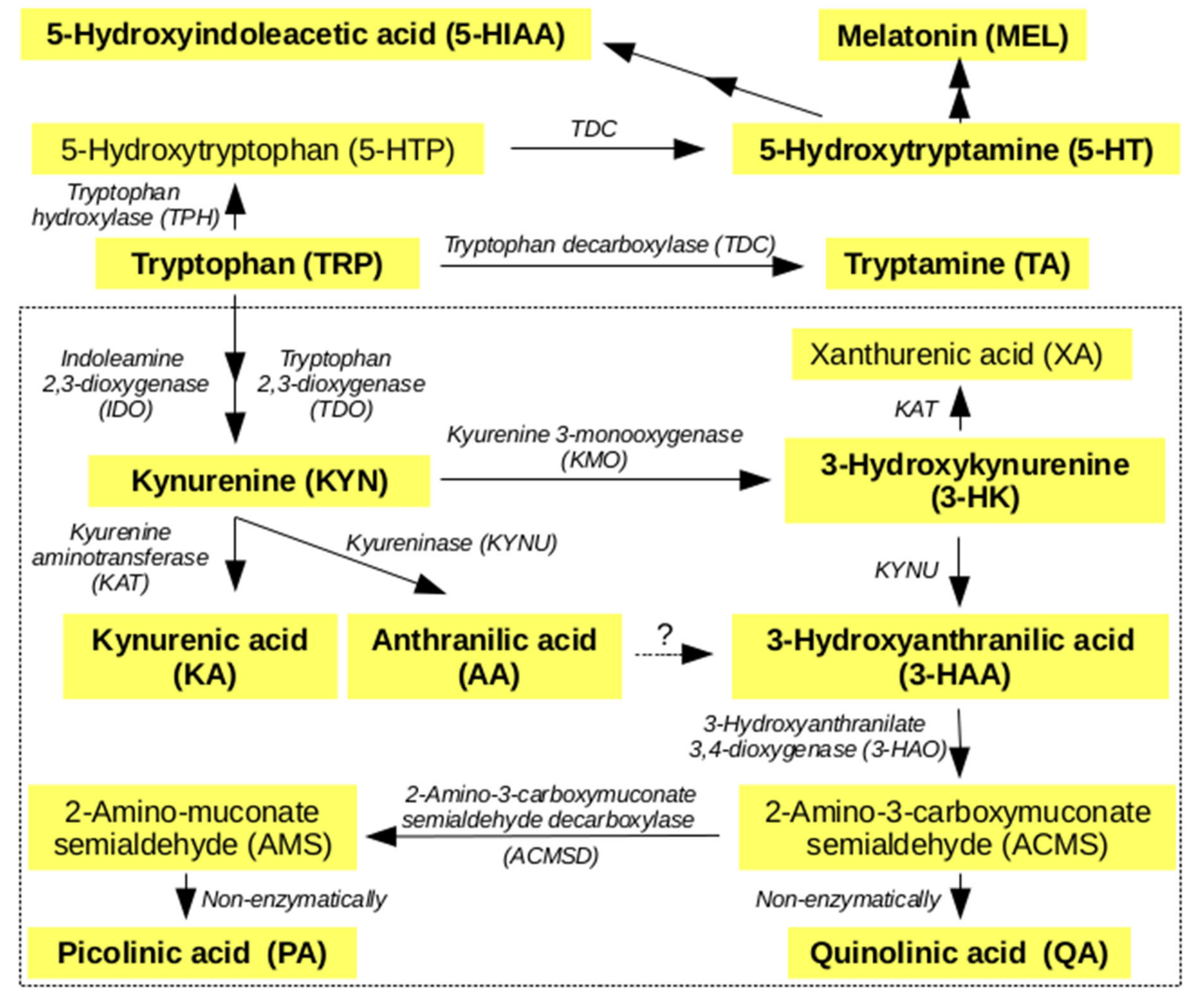
:1. Introduction
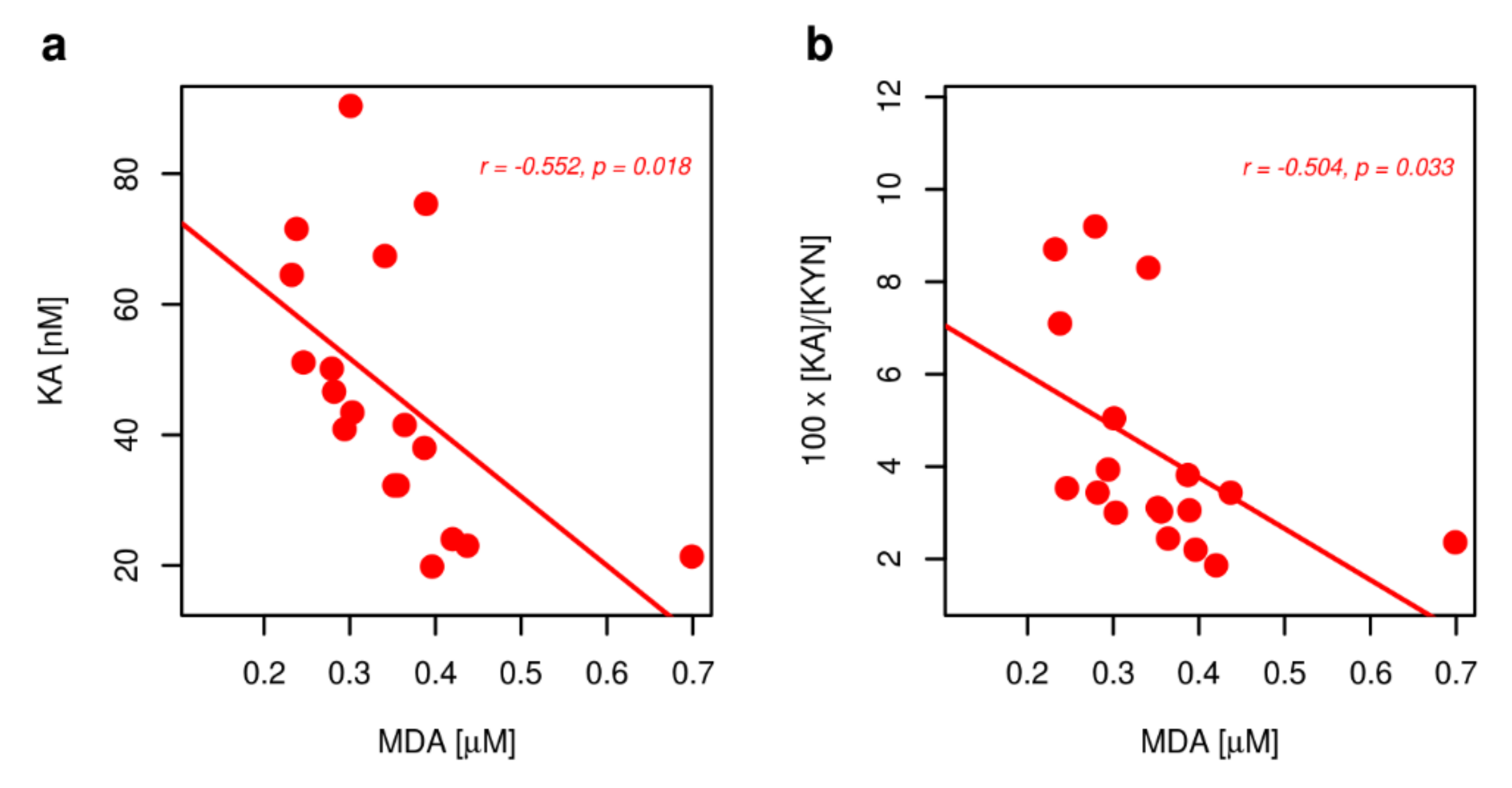
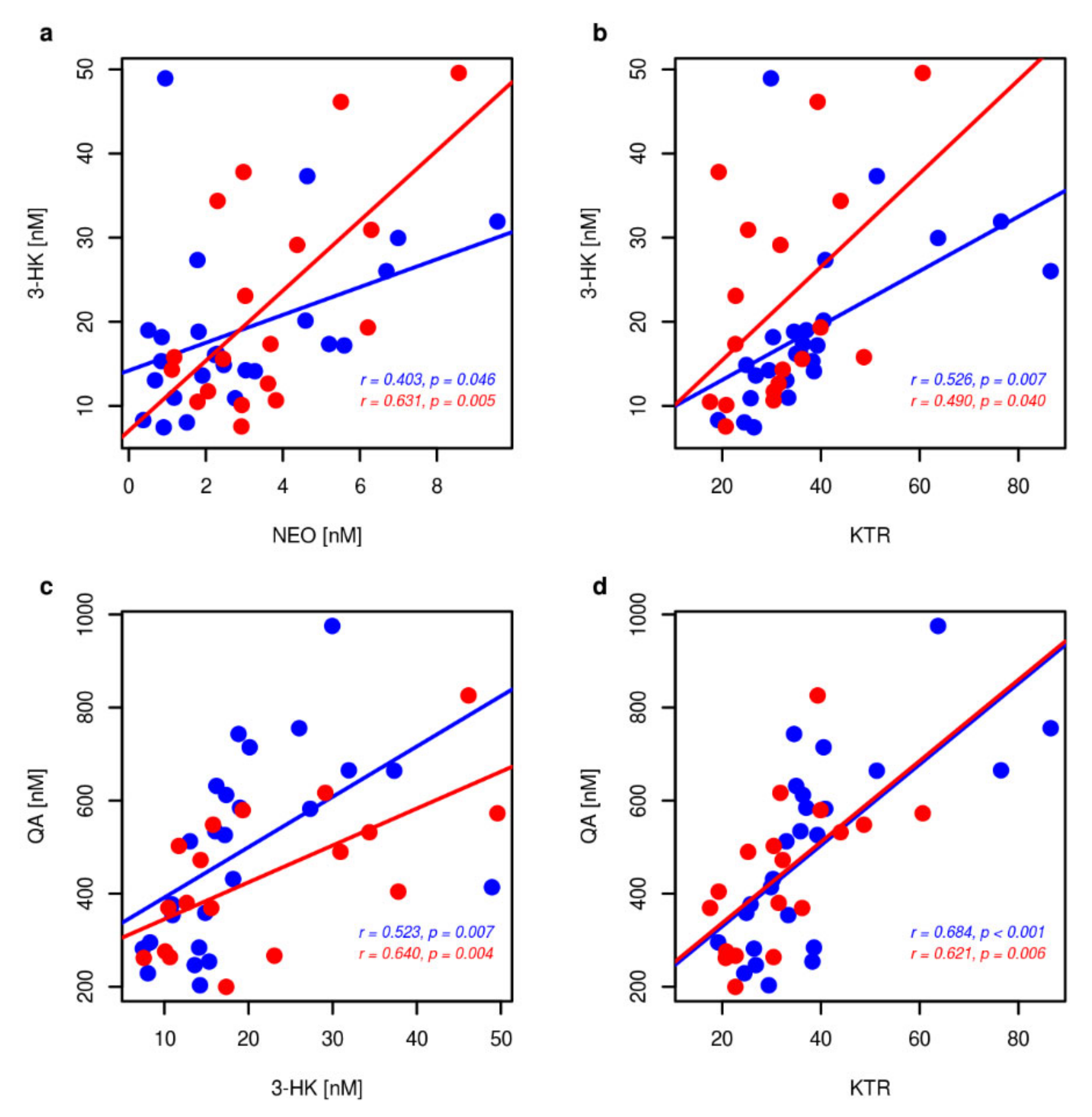
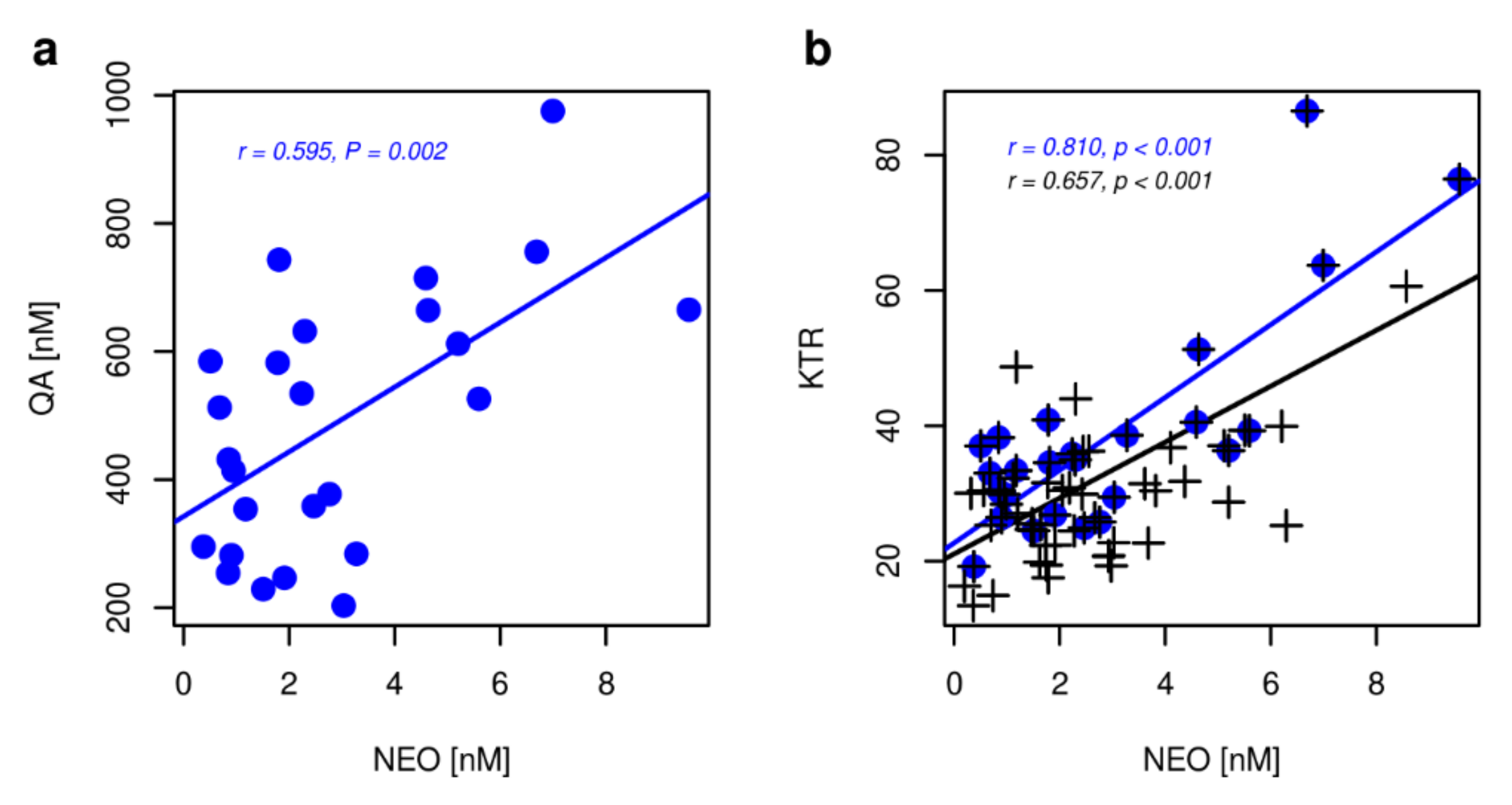
2. Results
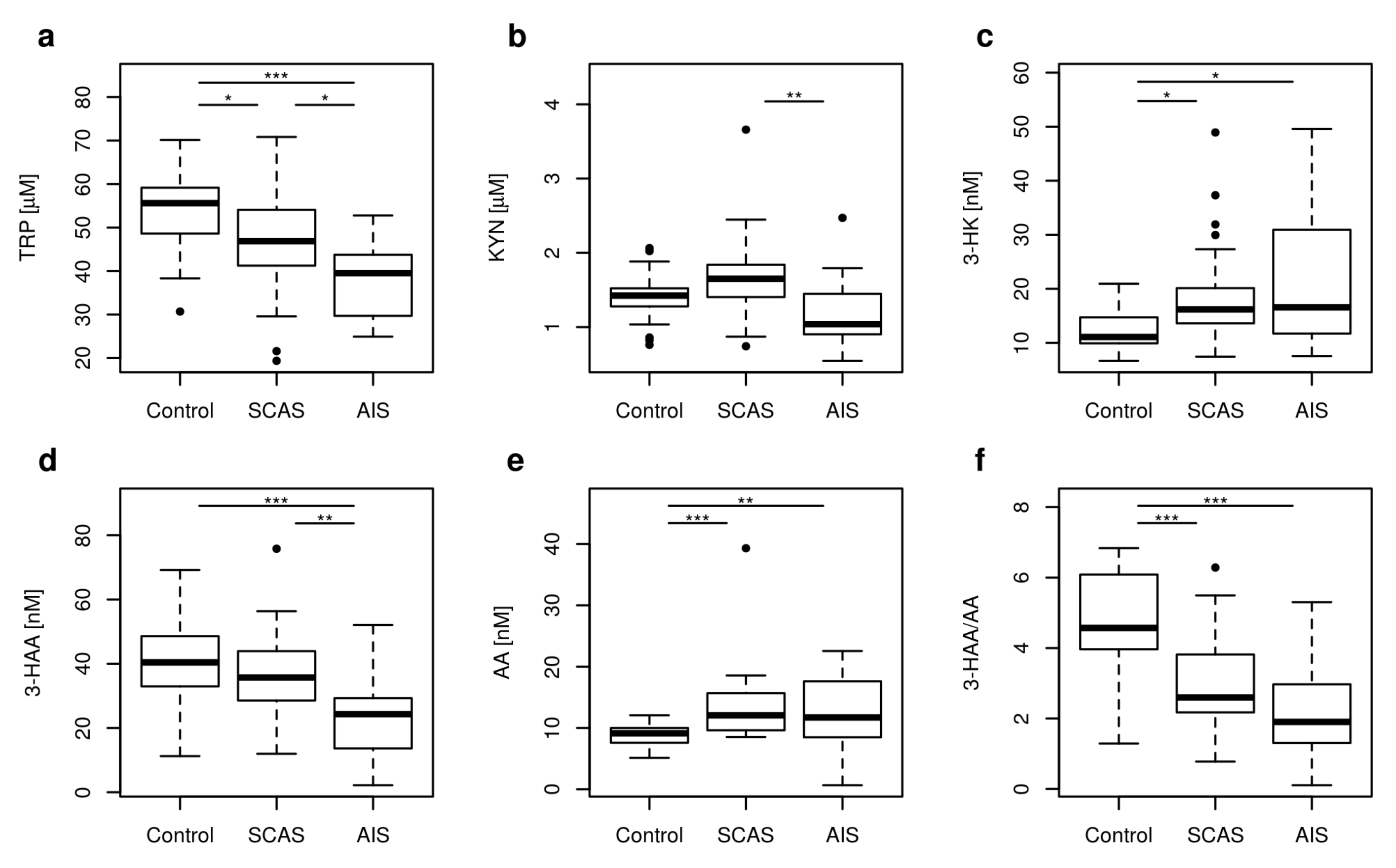
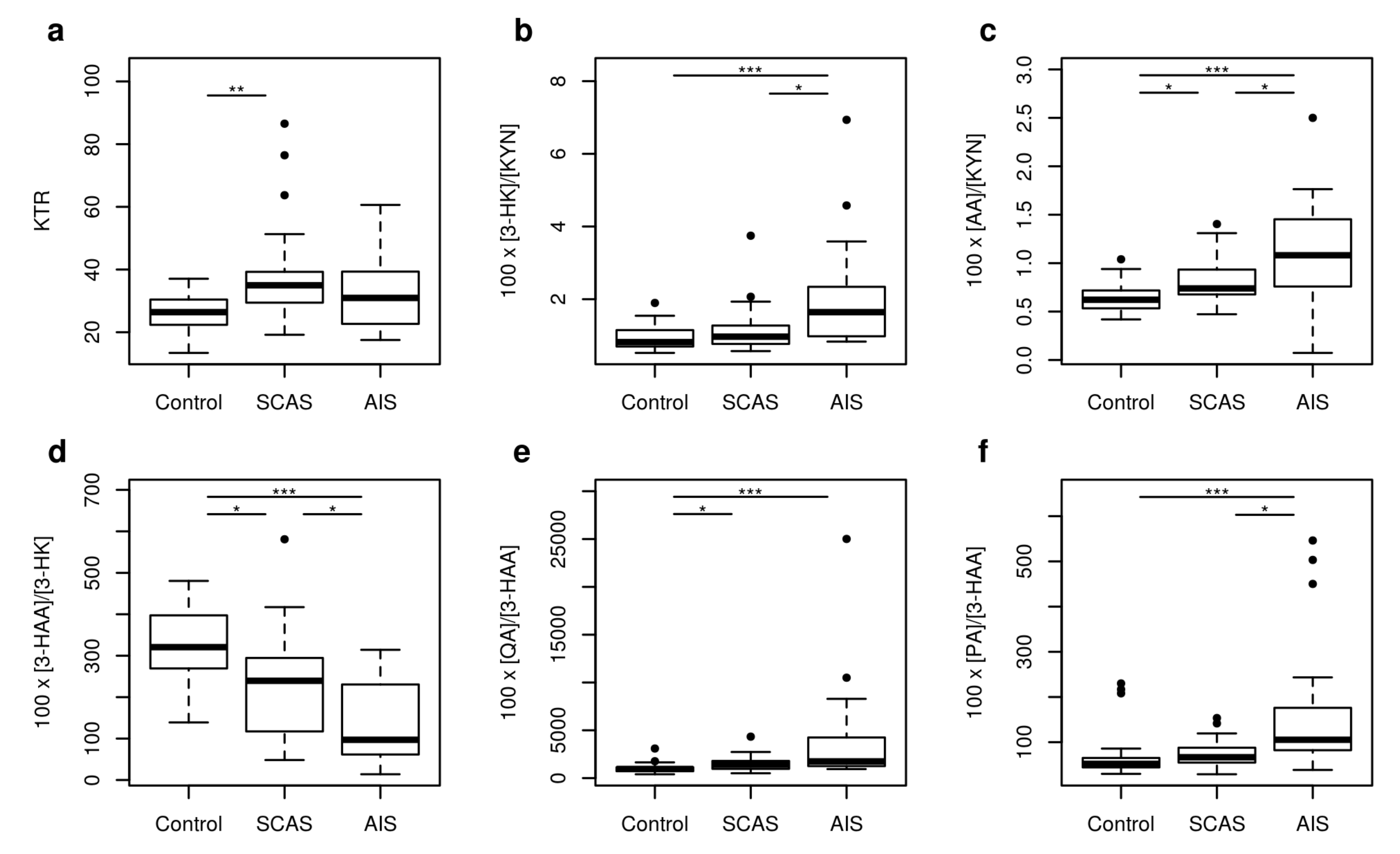
2.1. Metabolites of KP
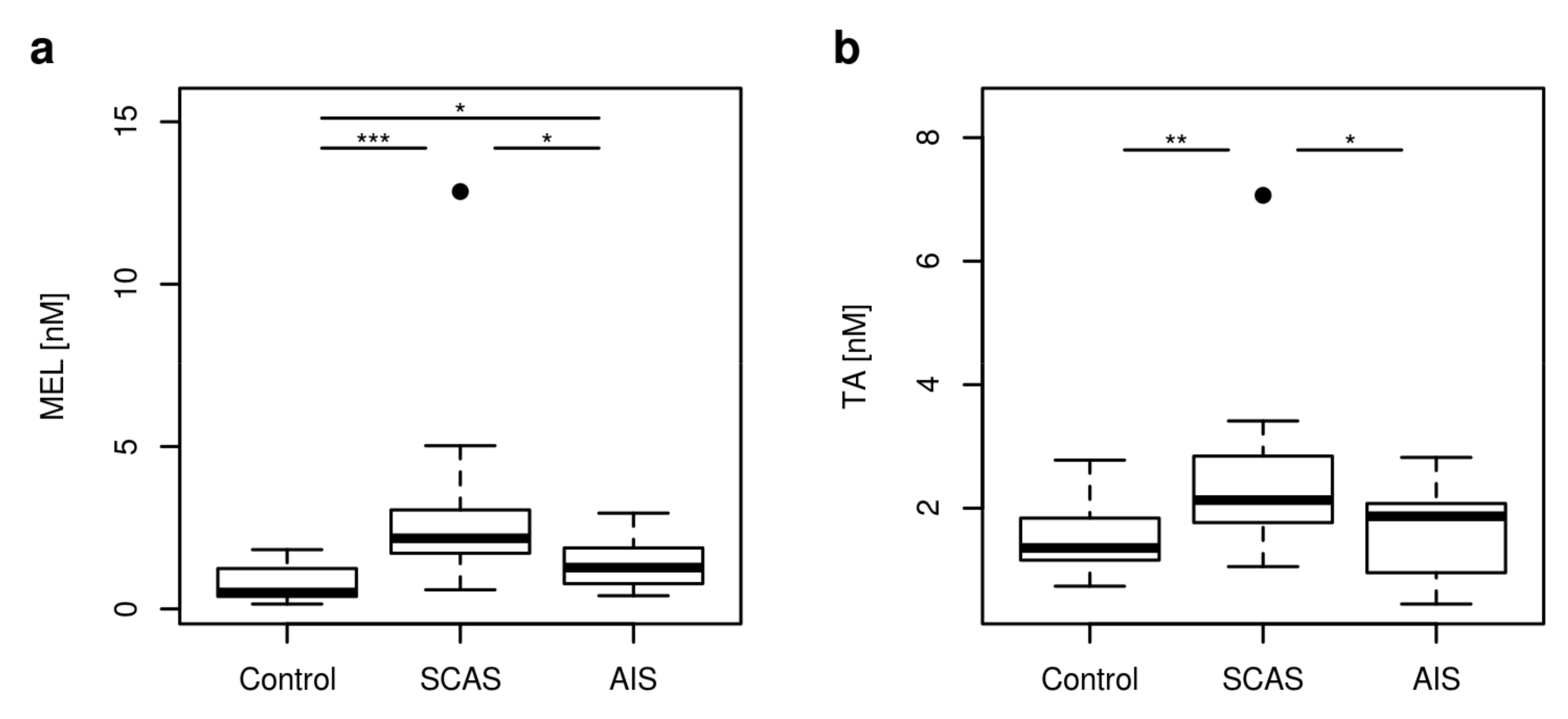
2.2. Non Kynurenines Metabolites
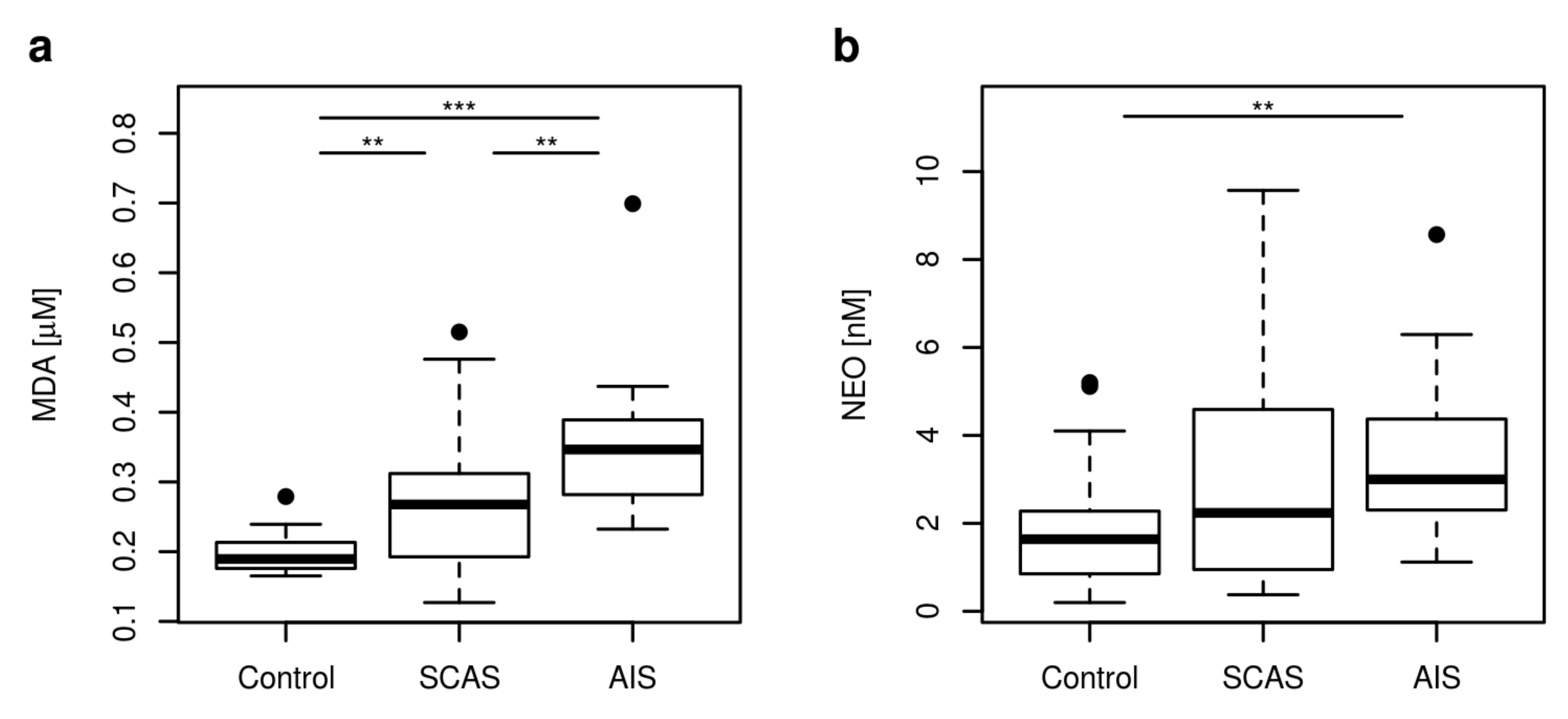
2.3. Markers of Inflammation, OS, and RBF
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Reagents
4.3. Total MDA Analysis
4.4. Tryptophan Metabolites, NEO and RBF Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Analyte | Q1 m/z | Q3 m/z | DP [V] | CE [V] | CXP [V] | RT [min] |
|---|---|---|---|---|---|---|
| TRP | 205.1 | 118.0 | 40 | 28 | 15 | 6.35 ± 0.03 |
| KYN | 209.1 | 192.0 | 30 | 13 | 14 | 5.75 ± 0.02 |
| 3-HK | 225.1 | 110.0 | 41 | 23 | 10 | 5.34 ± 0.03 |
| 3-HAA | 154.0 | 136.0 | 46 | 17 | 12 | 6.76 ± 0.04 |
| KA | 190.1 | 162.0 | 65 | 24 | 11 | 7.90 ± 0.04 |
| AA | 138.1 | 120.1 | 35 | 15 | 11 | 9.63 ± 0.03 |
| QA | 168.0 | 124.0 | 33 | 20 | 12 | 5.36 ± 0.03 |
| PA | 123.9 | 77.9 | 56 | 33 | 14 | 3.45 ± 0.01 |
| 5-HT | 177.1 | 160.0 | 39 | 15 | 11 | 5.43 ± 0.03 |
| 5-HIAA | 192.2 | 146.0 | 31 | 21 | 10 | 7.62 ± 0.04 |
| MEL | 233.1 | 129.9 | 76 | 65 | 8 | 10.11 ± 0.02 |
| TA | 161.1 | 143.9 | 61 | 15 | 8 | 6.45 ± 0.06 |
| NEO | 254.1 | 206.3 | 86 | 29 | 18 | 3.83 ± 0.03 |
| RBF | 154.0 | 136.0 | 90 | 30 | 11 | 7.75 ± 0.04 |
| 6-F-TRP (IS) | 223.0 | 163.8 | 71 | 25 | 10 | 7.01 ± 0.05 |
| Metabolite | Calibration Range [nM] | LOD [nM] | r2 | Slope in Aqueous Solution | Slope in Plasma | %ME |
|---|---|---|---|---|---|---|
| TRP | 100–200,000 | 10.0 | 0.9962 | 0.0001391 | 0.0000307 | 22 |
| KYN | 10–20,000 | 0.8 | 0.9981 | 0.0047248 | 0.0030131 | 64 |
| 3-HK | 1–200 | 0.5 | 0.9933 1 | 0.0021426 | 0.0019954 | 93 |
| 3-HAA | 1–200 | 1.0 | 0.9972 | 0.0003743 | 0.0003837 | 102 |
| KA | 1–200 | 0.7 | 0.9867 | 0.0003448 | 0.0003360 | 97 |
| AA | 1–200 | 0.9 | 0.9982 | 0.0018654 | 0.0018587 | 100 |
| QA | 5–1000 | 80 | 0.9879 | 0.0004685 | 0.0005207 | 111 |
| PA | 1–200 | 0.6 | 0.9938 | 0.0033485 | 0.0026671 | 80 |
| 5-HT | 10–2000 | 1.1 | 0.9987 | 0.0027633 | 0.0034016 | 123 |
| 5-HIAA | 2–400 | 2.8 | 0.9967 | 0.0002519 | 0.0002477 | 98 |
| MEL | 0.1–20 | 0.1 | 0.9858 | 0.0014024 | 0.0009017 | 64 |
| TA | 0.15–30 | 0.2 | 0.9941 | 0.0006594 | 0.0005528 | 84 |
| NEO | 0.5–100 | 0.5 | 0.9897 | 0.0004474 | 0.0002293 | 51 |
| RBF | 0.2–40 | 0.3 | 0.9975 | 0.0002688 | 0.0002780 | 103 |
| Measured | Concentration 1 | |||
|---|---|---|---|---|
| Metabolites | Added | Measured | Recovery, % | RSD, % |
| TRP 2 | 100 | 173.65 | 107.3 | 7.50 |
| 50 | 121.62 | 110.5 | 5.21 | |
| 25 | 95.31 | 115.8 | 7.64 | |
| KYN | 10 | 10.64 | 96.3 | 5.55 |
| 5 | 5.57 | 91.2 | 8.26 | |
| 1.25 | 2.15 | 91.0 | 4.65 | |
| 3-HK | 50 | 46.22 | 84.3 | 5.65 |
| 25 | 27.44 | 93.5 | 9.55 | |
| 12.5 | 14.90 | 86.7 | 23.42 | |
| 3-HAA | 100 | 116.16 | 98.8 | 7.03 |
| 50 | 67.61 | 100.4 | 5.01 | |
| 12.5 | 28.74 | 90.7 | 5.98 | |
| KA | 100 | 132.02 | 97.2 | 14.22 |
| 50 | 89.56 | 109.4 | 9.67 | |
| 12.5 | 45.41 | 84.5 | 33.10 | |
| AA | 50 | 53.62 | 97.8 | 4.81 |
| 25 | 28.76 | 96.2 | 6.85 | |
| 6.25 | 10.11 | 86.3 | 13.75 | |
| QA | 1000 | 1289.54 | 99.4 | 8.53 |
| 500 | 750.88 | 91.0 | 9.37 | |
| 125 | 429.44 | 106.9 | 17.92 | |
| PA | 100 | 105.63 | 94.4 | 7.16 |
| 50 | 57.68 | 92.8 | 6.28 | |
| 12.5 | 23.22 | 95.7 | 13.31 | |
| 5-HT | 1000 | 1212.85 | 97.6 | 5.48 |
| 500 | 716.00 | 95.8 | 4.41 | |
| 125 | 361.49 | 99.7 | 7.92 | |
| 5-HIIA | 200 | 231.06 | 93.0 | 7.81 |
| 100 | 145.42 | 100.3 | 6.16 | |
| 25 | 68.65 | 94.2 | 11.80 | |
| MEL | 10 | 10.13 | 98.5 | 4.94 |
| 5 | 5.26 | 99.6 | 8.37 | |
| 1.25 | 1.27 | 78.4 | 18.90 | |
| TA | 7.5 | 9.23 | 111.6 | 5.96 |
| 3.75 | 4.98 | 109.7 | 8.23 | |
| 1.875 | 2.82 | 104.3 | 13.12 | |
| NEO | 10 | 12.73 | 98.4 | 11.39 |
| 5 | 7.62 | 94.6 | 16.27 | |
| 2.5 | 5.71 | 112.8 | 18.39 | |
| RBF | 20 | 24.19 | 100.9 | 4.30 |
| 10 | 14.36 | 103.5 | 4.94 | |
| 2.5 | 6.65 | 105.5 | 5.86 | |
Appendix B
References
- Wolf, D.; Ley, K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochemistry 2016, 81, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Miteva, K.; Madonna, R.; De Caterina, R.; Van Linthout, S. Innate and adaptive immunity in atherosclerosis. Vascul. Pharmacol. 2018, 107, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Capuron, L.; Geisler, S.; Kurz, K.; Leblhuber, F.; Sperner-Unterweger, B.; Fuchs, D. Activated immune system and inflammation in healthy ageing: Relevance for tryptophan and neopterin metabolism. Curr. Pharm. Des. 2014, 20, 6048–6057. [Google Scholar] [CrossRef] [Green Version]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The interplay between cytokines and the kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef]
- Wu, H.; Gong, J.; Liu, Y. Indoleamine 2, 3-dioxygenase regulation of immune response (Review). Mol. Med. Rep. 2018, 17, 4867–4873. [Google Scholar] [CrossRef] [Green Version]
- Coletti, A.; Greco, F.A.; Dolciami, D.; Camaioni, E.; Sardella, R.; Pallotta, M.T.; Volpi, C.; Orabona, C.; Grohmann, U.; Macchiarulo, A. Advances in indoleamine 2,3-dioxygenase 1 medicinal chemistry. MedChemComm 2017, 8, 1378–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, P.B.; Sathyasaikumar, K.V.; Notarangelo, F.M.; Funakoshi, H.; Nakamura, T.; Schwarcz, R.; Muchowski, P.J. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim. Biophys. Acta 2016, 1860, 2345–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Ueland, P.M.; Ulvik, A.; Eussen, S.J.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Theofylaktopoulou, D.; Meyer, K.; Tell, G.S. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality: The Hordaland Health Study. Am. J. Epidemiol. 2016, 183, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A.; Guillemin, G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: Time for appraisal. Int. J. Tryptophan Res. 2019, 12, 1178646919868978. [Google Scholar] [CrossRef] [Green Version]
- Badawy, A.A.; Dougherty, D.M. Assessment of the human kynurenine pathway: Comparisons and clinical implications of ethnic and gender differences in plasma tryptophan, kynurenine metabolites, and enzyme expressions at baseline and after acute tryptophan loading and depletion. Int. J. Tryptophan Res. 2016, 9, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Theofylaktopoulou, D.; Ulvik, A.; Midttun, O.; Ueland, P.M.; Vollset, S.E.; Nygard, O.; Hustad, S.; Tell, G.S.; Eussen, S.J. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-gamma-mediated immune activation in the community-based Hordaland Health Study. Br. J. Nutr. 2014, 112, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Majewski, M.; Kozlowska, A.; Thoene, M.; Lepiarczyk, E.; Grzegorzewski, W.J. Overview of the role of vitamins and minerals on the kynurenine pathway in health and disease. J. Physiol. Pharmacol. 2016, 67, 3–19. [Google Scholar]
- Jadavji, N.M.; Emmerson, J.T.; MacFarlane, A.J.; Willmore, W.G.; Smith, P.D. B-vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiol. Dis. 2017, 103, 89–100. [Google Scholar] [CrossRef]
- Spence, J.D. Nutrition and risk of stroke. Nutrients 2019, 11, 647. [Google Scholar] [CrossRef] [Green Version]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Ramprasath, T.; Wang, H.; Zou, M.H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol. Life Sci. 2017, 74, 2899–2916. [Google Scholar] [CrossRef] [PubMed]
- Colpo, G.D.; Venna, V.R.; McCullough, L.D.; Teixeira, A.L. Systematic review on the involvement of the kynurenine pathway in stroke: Pre-clinical and clinical evidence. Front. Neurol. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.L.; Kissela, B.; Khoury, J.C.; Alwell, K.; Moomaw, C.J.; Woo, D.; Khatri, P.; Ferioli, S.; Adeoye, O.; Broderick, J.P.; et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013, 40, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhfnawy, A.M.; Volkmann, J.; Schliesser, M.; Fluri, F. Symptomatic vs. asymptomatic 20-40% internal carotid artery stenosis: Does the plaque size matter. Front. Neurol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. 2015, 20, 1116–1143. [Google Scholar]
- Sallée, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014, 6, 934–949. [Google Scholar]
- Shinde, R.; McGaha, T.L. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol. 2018, 39, 1005–1020. [Google Scholar] [CrossRef]
- Cuartero, M.I.; Ballesteros, I.; de la Parra, J.; Harkin, A.L.; Abautret-Daly, A.; Sherwin, E.; Fernández-Salguero, P.; Corbí, A.L.; Lizasoain, I.; Moro, M.A. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 2014, 130, 2040–2051. [Google Scholar] [CrossRef] [Green Version]
- Hertelendy, P.; Toldi, J.; Fülöp, F.; Vécsei, L. Ischemic stroke and kynurenines: Medicinal chemistry aspects. Curr. Med. Chem. 2018, 25, 5945–5957. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- González-Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Egerton, M.; Christofides, J.; Stone, T.W.; Darlington, L.G. Tryptophan loading induces oxidative stress. Free Radic. Res. 2004, 38, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Réus, G.Z.; Becker, I.R.T.; Scaini, G.; Petronilho, F.; Oses, J.P.; Kaddurah-Daouk, R.; Ceretta, L.B.; Zugno, A.I.; Dal-Pizzol, F.; Quevedo, J.; et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 55–63. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A potent immune cell modulator in autoimmune diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, E367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamtaji, O.R.; Mobini, M.; Reiter, R.J.; Azami, A.; Gholami, M.S.; Asemi, Z. Melatonin, a toll-like receptor inhibitor: Current status and future perspectives. J. Cell. Physiol. 2019, 234, 7788–7795. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Brzosko, S.; Mysliwiec, M.; Pawlak, D. Kynurenine, quinolinic acid—The new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis 2009, 204, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Verkerk, R.; Aass, H.C.; Amthor, K.F.; Sandvik, L. Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J. Mol. Neurosci. 2013, 51, 893–902. [Google Scholar] [CrossRef]
- Mo, X.; Pi, L.; Yang, J.; Xiang, Z.; Tang, A. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J. Clin. Neurosci. 2014, 21, 482–486. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Z.; Xiao, C.; Zhang, M.; Li, Z.; Xie, J.; Zhang, Y.; Zhao, X.; Zeng, P.; Mo, L.; et al. Peripheral kynurenine/tryptophan ratio is not a reliable marker of systemic indoleamine 2,3-dioxygenase: A lesson drawn from patients on hemodialysis. Oncotarget 2017, 8, 25261–25269. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.S.; Tsai, T.H.; Liu, C.F.; Lu, C.H.; Chang, W.N.; Chen, S.F.; Huang, C.W.; Huang, C.R.; Tsai, N.W.; Huang, C.C.; et al. Serum level and prognostic value of neopterin in patients after ischemic stroke. Clin. Biochem. 2012, 45, 1596–1601. [Google Scholar] [CrossRef]
- Baran, H.; Schwarcz, R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. J. Neurochem. 1990, 55, 738–744. [Google Scholar] [CrossRef]
- Badawy, A.A. Hypothesis kynurenic and quinolinic acids: The main players of the kynurenine pathway and opponents in inflammatory disease. Med. Hypotheses 2018, 118, 129–138. [Google Scholar] [CrossRef]
- Krause, D.; Suh, H.S.; Tarassishin, L.; Cui, Q.L.; Durafourt, B.A.; Choi, N.; Bauman, A.; Cosenza-Nashat, M.; Antel, J.P.; Zhao, M.L.; et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation. Role of hemeoxygenase-1. Am. J. Pathol. 2011, 179, 1360–1372. [Google Scholar] [CrossRef]
- Leipnitz, G.; Schumacher, C.; Dalcin, K.B.; Scussiato, K.; Solano, A.; Funchal, C.; Dutra-Filho, C.S.; Wyse, A.T.; Wannmacher, C.M.; Latini, A.; et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem. Int. 2007, 50, 83–94. [Google Scholar] [CrossRef]
- Thomas, S.R.; Witting, P.K.; Stocker, R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for alpha-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. J. Biol. Chem. 1996, 271, 32714–32721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-González, A.; Alvarez-Idaboy, J.R.; Galano, A. Dual antioxidant/pro-oxidant behavior of the tryptophan metabolite 3-hydroxyanthranilic acid: A theoretical investigation of reaction mechanisms and kinetics. New J. Chem. 2017, 41, 3829–3845. [Google Scholar] [CrossRef]
- Darlington, L.G.; Mackay, G.M.; Forrest, C.M.; Stoy, N.; George, C.; Stone, T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 2007, 26, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Darlington, L.G.; Forrest, C.M.; Mackay, G.M.; Smith, R.A.; Smith, A.J.; Stoy, N.; Stone, T.W. On the biological importance of the 3-hydroxyanthranilic acid: Anthranilic acid ratio. Int. J. Tryptophan Res. 2010, 3, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Sakotnik, A.; Liebmann, P.M.; Stoschitzky, K.; Lercher, P.; Schauenstein, K.; Klein, W.; Eber, B. Decreased melatonin synthesis in patients with coronary artery disease. Eur. Heart J. 1999, 20, 1314–1317. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Sainz, R.M.; Mayo, J.C.; León, J.; Reiter, R.J. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: A hypothesis. Endocrine 2005, 27, 149–158. [Google Scholar] [CrossRef]
- Young, S.N.; Gauthier, S. Tryptophan availability and the control of 5-hydroxytryptamine and tryptamine synthesis in human CNS. Adv. Exp. Med. Biol. 1981, 133, 221–230. [Google Scholar]
- Sim, A.S.; Salonikas, C.; Naidoo, D.; Wilcken, D.E. Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 785, 337–344. [Google Scholar] [CrossRef]
- Mendonça, R.; Gning, O.; Di Cesaré, C.; Lachat, L.; Bennett, N.C.; Helfenstein, F.; Glauser, G. Sensitive and selective quantification of free and total malondialdehyde in plasma using UHPLC-HRMS. J. Lipid Res. 2017, 58, 1924–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttnar, J.; Cermák, J.; Dyr, J.E. Solid-phase extraction in malondialdehyde analysis. Anal. Biochem. 1997, 249, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Stevens, A.P.; Dettmer, K.; Gottfried, E.; Hoves, S.; Kreutz, M.; Holler, E.; Canelas, A.B.; Kema, I.; Oefner, P.J. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- LC–MS Method Validation. Available online: https://sisu.ut.ee/lcms_method_validation/course-introduction (accessed on 7 April 2020).
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 January 2020).
| Metabolites | Control (n = 25) | SCAS (n = 25) | AIS (n = 18) | ||||
|---|---|---|---|---|---|---|---|
| and Metabolites Ratios | Median | 5–95 Percentile | Median | 5–95 Percentile | Median | 5–95 Percentile | p-Value |
| TRP [μM] | 55.62 | 38.91–68.30 | 46.87 | 23.19–61.30 | 39.51 | 25.46–51.47 | <0.001 |
| KYN [μM] | 1.42 | 0.83–2.00 | 1.65 | 0.91–2.43 | 1.04 | 0.65–1.89 | 0.003 |
| 3-HK [nM] | 11.08 | 8.22–19.28 | 16.18 | 8.09–36.23 | 16.57 | 9.71–46.66 | 0.008 |
| 3-HAA [nM] | 40.42 | 17.95–63.90 | 35.72 | 15.17–55.66 | 24.31 | 3.32–39.17 | <0.001 |
| KA [nM] | 43.44 | 25.48–65.99 | 52.06 | 21.72–82.21 | 42.48 | 21.14–77.63 | 0.379 |
| AA [nM] | 9.13 | 5.96–11.95 | 12.06 | 8.80–18.52 | 11.71 | 6.80–21.01 | <0.001 |
| QA [nM] | 374.60 | 218.07–492.88 | 512.61 | 232.49–753.16 | 438.44 | 252.62–648.12 | 0.065 |
| PA [nM] | 24.36 | 11.84–45.24 | 23.78 | 14.47–34.56 | 23.69 | 9.05–43.45 | 0.984 |
| 5-HT [nM] | 23.71 | 5.85–60.18 | 43.49 | 5.78–134.81 | 19.92 | 6.70–86.72 | 0.263 |
| 5-HIAA [nM] | 44.05 | 25.60–111.94 | 54.64 | 36.08–91.78 | 49.48 | 31.63–77.69 | 0.102 |
| MEL [nM] | 0.51 | 0.26–1.63 | 2.17 | 0.76–4.81 | 1.27 | 0.52–2.60 | <0.001 |
| TA [nM] | 1.36 | 0.77–2.49 | 2.13 | 1.21–3.41 | 1.87 | 0.70–2.64 | 0.001 |
| MDA [μM] | 0.19 | 0.17–0.24 | 0.27 | 0.16–0.46 | 0.35 | 0.24–0.48 | <0.001 |
| NEO [nM] | 1.64 | 0.33–4.91 | 2.24 | 0.54–6.93 | 3.00 | 1.17–6.63 | 0.004 |
| RBF [nM] | 7.34 | 4.01–39.21 | 10.52 | 3.99–41.02 | 12.62 | 5.83–37.92 | 0.126 |
| 3-HAA/AA | 4.57 | 2.26–6.81 | 2.60 | 1.02–5.33 | 1.90 | 0.45–4.48 | <0.001 |
| KTR | 26.41 | 15.18–36.66 | 34.97 | 24.56–73.90 | 30.94 | 19.02–50.48 | 0.003 |
| 100 × 3-HK/KYN | 0.82 | 0.58–1.51 | 0.97 | 0.66–2.04 | 1.64 | 0.84–4.93 | <0.001 |
| 100 × KA/KYN | 3.11 | 2.16–4.66 | 3.17 | 1.49–5.34 | 3.44 | 2.15–8.78 | 0.275 |
| 100 × AA/KYN | 0.62 | 0.44–0.93 | 0.74 | 0.57–1.26 | 1.08 | 0.57–1.87 | <0.001 |
| 100 × 3-HAA/3-HK | 320.65 | 182.70–465.81 | 239.45 | 85.77–403.39 | 97.04 | 21.29–293.86 | <0.001 |
| 100 × QA/3-HAA | 953.89 | 448.87–1738.37 | 1410.83 | 578.12–2677.36 | 1745.90 | 1012.07–12,676.51 | <0.001 |
| 100 × PA/3-HAA | 51.97 | 35.32–215.07 | 66.62 | 37.65–137.12 | 105.34 | 49.73–509.71 | <0.001 |
| Patients’ Characteristics | SCAS | AIS | p-Value |
|---|---|---|---|
| Number of patients | 25 | 18 | - |
| Sex female/male | 8/17 | 11/7 | 0.071 2 |
| Arterial hypertension | 21 | 15 | >0.999 2 |
| Diabetes mellitus | 12 | 3 | 0.052 2 |
| Active smoking | 12 | 3 | 0.052 2 |
| Age 1 | 70 (59.0–78.6) | 71 (46.8–82.6) | >0.999 3 |
| BMI (mean ± SD) | 28 ± 6 | 29 ± 8 | 0.653 3 |
| GF (mean ± SD) | 1.31 ± 0.25 | 1.25 ± 0.36 | 0.522 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajsl, M.; Hlavackova, A.; Broulikova, K.; Sramek, M.; Maly, M.; Dyr, J.E.; Suttnar, J. Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease. Metabolites 2020, 10, 208. https://doi.org/10.3390/metabo10050208
Hajsl M, Hlavackova A, Broulikova K, Sramek M, Maly M, Dyr JE, Suttnar J. Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease. Metabolites. 2020; 10(5):208. https://doi.org/10.3390/metabo10050208
Chicago/Turabian StyleHajsl, Martin, Alzbeta Hlavackova, Karolina Broulikova, Martin Sramek, Martin Maly, Jan E. Dyr, and Jiri Suttnar. 2020. "Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease" Metabolites 10, no. 5: 208. https://doi.org/10.3390/metabo10050208
APA StyleHajsl, M., Hlavackova, A., Broulikova, K., Sramek, M., Maly, M., Dyr, J. E., & Suttnar, J. (2020). Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease. Metabolites, 10(5), 208. https://doi.org/10.3390/metabo10050208







