Oxylipin Response to Acute and Chronic Exercise: A Systematic Review
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Exercise-Related Oxylipin Formation
3.2. Matrix
3.3. Limitations
4. Materials and Methods
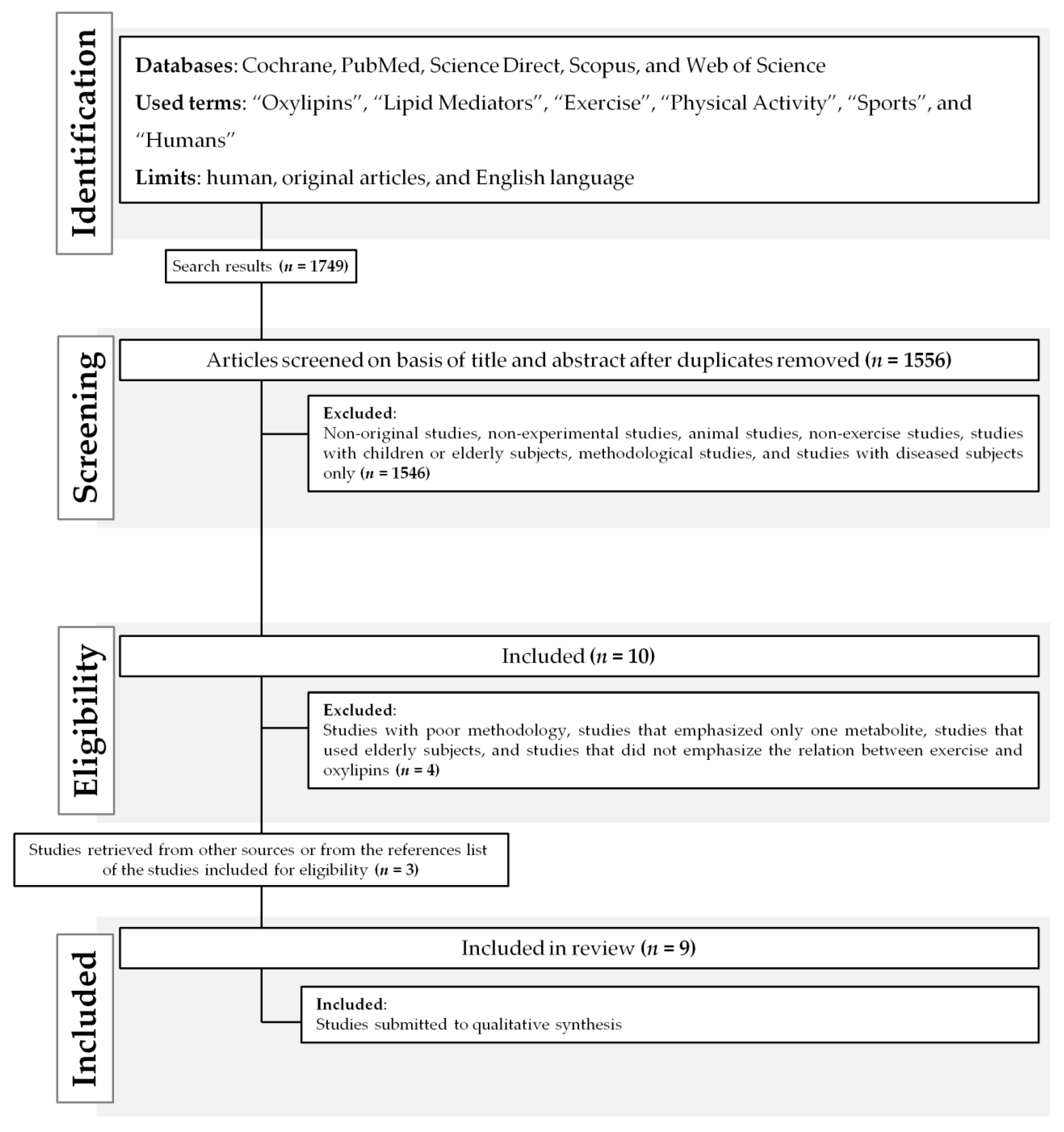
4.1. Search Strategy
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction
4.4. Studies Quality Assessment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Maddipati, K.R.; Cameron-Smith, D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc. Immunol. Rev. 2016, 22, 110–134. [Google Scholar] [PubMed]
- Spector, A.A.; Kim, H.-Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef]
- Willenberg, I.; Ostermann, A.I.; Schebb, N.H. Targeted metabolomics of the arachidonic acid cascade: Current state and challenges of LC-MS analysis of oxylipins. Anal. Bioanal. Chem. 2015, 407, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Massey, K.A.; Nicolaou, A. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 2011, 39, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Walker, R.E. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot. Essent. Fatty Acids 2018, 137, 26–38. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Zimmer, B.; Angioni, C.; Osthues, T.; Toewe, A.; Thomas, D.; Pierre, S.C.; Geisslinger, G.; Scholich, K.; Sisignano, M. The oxidized linoleic acid metabolite 12,13-DiHOME mediates thermal hyperalgesia during inflammatory pain. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2018, 1863, 669–678. [Google Scholar] [CrossRef]
- Nieman, D.C.; Mitmesser, S.H. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients 2017, 9, 513. [Google Scholar] [CrossRef]
- Nieman, D.C.; Lila, M.A.; Gillitt, N.D. Immunometabolism: A multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu. Rev. Food Sci. Technol. 2019, 10, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport Health Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.-Y.; Zhang, Q.; Sakaguchi, C.A.; Stephan, E.H. Carbohydrate intake attenuates post-exercise plasma levels of cytochrome P450-generated oxylipins. PLoS ONE 2019, 14, e0213676. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.; Gillitt, N.; Chen, G.-Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and banana consumption mitigate arachidonic, cytochrome P450 oxylipin generation during recovery from 75-Km cycling: A randomized trial. Front. Nutr. (in press). [CrossRef]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R1281–R1296. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef]
- García-Flores, L.A.; Medina, S.; Gómez, C.; Wheelock, C.E.; Cejuela, R.; Martínez-Sanz, J.M.; Oger, C.; Galano, J.-M.; Durand, T.; Hernández-Sáez, Á.; et al. Aronia-citrus juice (polyphenol-rich juice) intake and elite triathlon training: A lipidomic approach using representative oxylipins in urine. Food Funct. 2018, 9, 463–475. [Google Scholar] [CrossRef]
- Gollasch, B.; Dogan, I.; Rothe, M.; Gollasch, M.; Luft, F.C. Maximal exercise and plasma cytochrome P450 and lipoxygenase mediators: A lipidomics study. Physiol. Rep. 2019, 7, e14165. [Google Scholar] [CrossRef]
- Giordano, R.M.; Newman, J.W.; Pedersen, T.L.; Ramos, M.I.; Stebbins, C.L. Effects of dynamic exercise on plasma arachidonic acid epoxides and diols in human volunteers. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 471–479. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Cejuela-Anta, R.; Villaño, D.; Martínez-Sanz, J.M.; Gil, P.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, A. Assessment of oxidative stress markers and prostaglandins after chronic training of triathletes. Prostaglandins Other Lipid Mediat. 2012, 99, 79–86. [Google Scholar] [CrossRef]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Meaney, M.P.; Dew, D.A.; Pappan, K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R68–R74. [Google Scholar] [CrossRef]
- Shoieb, S.M.; El-Sherbeni, A.A.; El-Kadi, A.O. Subterminal hydroxyeicosatetraenoic acids: Crucial lipid mediators in normal physiology and disease states. Chem. Biol. Interact. 2018, 299, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Dubois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; Van De Putte, L.B.; Lipsky, P.E. Cyclooxygenase in biology and disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Murakami, M.; Yamamoto, K.; Miki, Y.; Murase, R.; Sato, H.; Taketomi, Y. The roles of the secreted phospholipase A2 gene family in immunology. Adv. Immunol. 2016, 132, 91–134. [Google Scholar]
- Sato, H.; Taketomi, Y.; Murakami, M. Metabolic regulation by secreted phospholipase A2. Inflamm. Regen. 2016, 36, 7. [Google Scholar] [CrossRef]
- Ghosh, M.; Tucker, D.E.; Burchett, S.A.; Leslie, C.C. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 2006, 45, 487–510. [Google Scholar] [CrossRef]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef]
- Guijas, C.; Rodríguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 regulation of lipid droplet formation. Biochim. Biophys. Acta (BBA)—Molec. Cell Biol. Lipids 2014, 1841, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.F.; Goodyear, L.J. Exercise, MAPK, and NF-κB signaling in skeletal muscle. J. Appl. Physiol. 2007, 103, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.-T.; Pae, H.-O. Reactive oxygen species in the activation of MAP kinases. Meth. Enzymol. 2013, 528, 27–48. [Google Scholar] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef]
- Demers, L.M.; Harrison, T.S.; Halbert, D.R.; Santen, R.J. Effect of prolonged exercise on plasma prostaglandin levels. Prostaglandins Med. 1981, 6, 413–418. [Google Scholar] [CrossRef]
- Karamouzis, M.; Karamouzis, I.; Vamvakoudis, E.; Ampatzidis, G.; Christoulas, K.; Angelopoulou, N.; Mandroukas, K. The response of muscle interstitial prostaglandin E(2)(PGE(2)), prostacyclin I(2)(PGI(2)) and thromboxane A(2)(TXA(2)) levels during incremental dynamic exercise in humans determined by in vivo microdialysis. Prostaglandins Leukot. Essent. Fatty Acids 2001, 64, 259–263. [Google Scholar] [CrossRef]
- Nowak, J.; Wennmalm, Å. Effect of exercise on human arterial and regional venous plasma concentrations of prostaglandin E. Prostaglandins Med. 1978, 1, 489–497. [Google Scholar] [CrossRef]
- Kaley, G.; Weiner, R. Prostaglandin E1: A potential mediator of the inflammatory response. Ann. N. Y. Acad. Sci. 1971, 180, 338–350. [Google Scholar] [CrossRef]
- Carroll, C.C.; O’Connor, D.T.; Steinmeyer, R.; Del Mundo, J.D.; McMullan, D.R.; Whitt, J.A.; Ramos, J.E.; Gonzales, R.J. The influence of acute resistance exercise on cyclooxygenase-1 and -2 activity and protein levels in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R24–R30. [Google Scholar] [CrossRef] [PubMed]
- Markworth, J.F.; Cameron-Smith, D. Prostaglandin F2α stimulates PI3K/ERK/mTOR signaling and skeletal myotube hypertrophy. Am. J. Physiol. Cell Physiol. 2011, 300, C671–C682. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, J.A.; Hofheinz, K.; Zaiss, M.M.; Krönke, G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2017, 1862, 371–381. [Google Scholar] [CrossRef]
- Savas, Ü.; Wei, S.; Hsu, M.-H.; Falck, J.R.; Guengerich, F.P.; Capdevila, J.H.; Johnson, E.F. 20-Hydroxyeicosatetraenoic Acid (HETE)-dependent Hypertension in Human Cytochrome P450 (CYP) 4A11 transgenic mice: Normalization of blood pressure by sodium restriction, hydrochlorothiazide, or blockade of the type 1 angiotensin II receptor. J. Biol. Chem. 2016, 291, 16904–16919. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of α-linolenic acid, (13-(S)-HPOTrE and 13-(S)-HOTrE), mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Fischer, R.; Konkel, A.; Mehling, H.; Blossey, K.; Gapelyuk, A.; Wessel, N.; von Schacky, C.; Dechend, R.; Muller, D.N.; Rothe, M.; et al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 2014, 55, 1150–1164. [Google Scholar] [CrossRef]
- VanRollins, M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J. Pharmacol. Exp. Ther. 1995, 274, 798–804. [Google Scholar]
- Kim, N.; Ramon, S.; Thatcher, T.H.; Woeller, C.F.; Sime, P.J.; Phipps, R.P. Specialized pro-resolving mediators (SPMs) inhibit human B-cell IgE production. Eur. J. Immunol. 2016, 46, 81–91. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005, 174, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-based studies assessing exercise-induced alterations of the human metabolome: A systematic review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Wolfer, A.M.; Gaudin, M.; Taylor-Robinson, S.D.; Holmes, E.; Nicholson, J.K. Development and validation of a high-throughput ultrahigh-performance liquid chromatography—Mass spectrometry approach for screening of oxylipins and their precursors. Anal. Chem. 2015, 87, 11721–11731. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, S.; Silva, C.; Hernandes, E.; Octaviano, F.; Di Thommazo, A.; Belgamo, A. Improvements in the StArt tool to better support the systematic review process. In Proceedings of the 20th International Conference on Evaluation and Assessment in Software Engineering, Limerick, Ireland, 1–3 June 2016; ACM: New York, NY, USA, 2016. [Google Scholar]
- Sparks, L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia 2017, 60, 2329–2336. [Google Scholar] [CrossRef] [PubMed]

| Investigators, Year Published | Research Design | Methodology | Novelty | Final Score | Classification | ||
|---|---|---|---|---|---|---|---|
| Subjects Number | Studies Characteristics | Analysis Methods | Statistical Support | ||||
| Nieman et al. (2019) [14] | 2 | 2 | 3 | 2 | 2 | 11 | Excellent |
| Nieman et al. (2020) [15] | 0 | 2 | 3 | 2 | 2 | 9 | Excellent |
| Garcia-Flores et al. (2018) [18] | 2 | 2 | 3 | 0 | 1 | 8 | Good |
| Markworth et al. (2013) [16] | 0 | 2 | 3 | 1 | 2 | 8 | Good |
| Vella et al. (2019) [17] | 0 | 1 | 3 | 0 | 2 | 6 | Good |
| Gollach et al. (2019) [19] | 0 | 1 | 3 | 0 | 1 | 5 | Fair |
| Nieman et al.(2014) [22] | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
| Giordano et al. (2011) [20] | 0 | 1 | 1 | 0 | 1 | 3 | Poor |
| Medina et al. (2012) [21] | 0 | 0 | 1 | 0 | 1 | 2 | Poor |
| Investigators, Year Published | Study Population | Research Design | Exercise Intensity and Duration | Enzymatic Pathway | Analytical Platform | Matrix | Key Findings, Exercise Effect |
|---|---|---|---|---|---|---|---|
| Garcia-Flores et al. (2018) [18] | 16 triathletes (10 men 19.0 ± 1.7 years, and 6 women 21.1 ± 3.0 years of age) | Randomized, double-blinded, placebo-controlled, and crossover design. Triathlon training at different conditions: control baseline (15 days), control training (15 days), placebo, and supplement drink crossover (100 days) and washout between these conditions (10 days), and control post-training (15 days). Training was based on objective load scale (ECOs). Urine samples timepoints: pre- and post-each condition (24 h). | Training, high-intensity, long-duration | COX, LOX, and non- enzymatic pathways | UHPLC-MS/MS | Urine | 37 oxylipins detected, with small decreases in F2-IsoPs and PGF1α, and small increases in PGDM, 11-β-PGF2α and PGE1 |
| Medina et al. (2012) [21] | 15 triathletes (10 men 19.0 ± 1.7 years and 5 women 21.8 ± 3.0 years of age) | Intense triathlon training for two weeks (cycling, swimming, running). No control group. Urine samples timepoints: pre-training (24 h) and post-training (24 h). | Training, high-intensity, long-duration | COX and non- enzymatic pathways | UPLC–QqQ-MS/MS | Urine | 13 oxylipins detected, with small decreases in F2-IsoPs, tetranor-PGEM and 11-β-PGF2α, and an increase in 6-keto-PGF1α |
| Giordano et al. (2011) [20] | 14 light to moderately active healthy subjects (6 men and 8 women, 36.9 ± 8.4 years of age) | In three visits, participants randomly performed submaximal bicycle tests for 20 min at 30%, 60%, and 80% of their maximal workload. In an additional visit, a test was performed at 60% of maximal work capacity for 40 min. Blood samples timepoints: pre-exercise, during exercise (20th min), and post-exercise (2 min) in the three visits, and pre-exercise and during exercise (39th min) in the additional visit | Low, moderate, and high-intensity, short-duration | CYP | UPLC-MS/MS | Plasma | 6 oxylipins detected, with small increases after 80% exercise in 8,9-DiHETrE, 11,12-DiHETrE, 14,15-DiHETrE, and after 40 min 60% exercise for 14,15-EpETrE and 14,15-DiHETrE |
| Gollasch et al. (2019) [19] | Six healthy subjects (5 men and 1 woman 38.0 ± 15.0 years of age) | Subjects performed a maximal graded treadmill test. Blood samples timepoints: pre-exercise test (10 min before), during exercise (when heart rate reached 150 bpm), post-exercise (0 min, 10 min) | High-intensity, short-duration | LOX and CYP | HPLC-MS/MS | Plasma | 56 oxylipins detected, with small increases in 5,6-DiHETrE, 12,13-EpOME, 5,6-DiHETE, 17,18-DiHETE. Most of them returned to close pre-exercise values 10 min after the end exercise |
| Markworth et al. (2013) [16] | 16 healthy men (2 groups of 8 each, 23 ± 1.3 years and 23.0 ± 0.5 years of age) | Parallel group design randomized with two groups: placebo and ibuprofen. Exercise included a 10 min warmup; 3 sets of 8 –10 repetitions of squatting with bar; leg press 45° and knee extension at 80% 1 RM. Circuit with 1 min of recovery between sets, 3 min recovery between stations. Blood samples timepoints: after 10 h fasting; pre-exercise (15 min), post-exercise protocol (0 h, 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 24 h) | High-intensity resistance, short-duration | COX, LOX, and CYP | HPLC-MRM-MS/MS | Serum | 87 oxylipins detected with small to moderate increases in 29 oxylipins, especially between 1 and 3 h post-exercise. Most of them returned to close pre-exercise values between 3 and 24 h after the end exercise |
| Nieman et al. (2020) [15] | 59 healthy cyclists (38.6 ± 1.5 years of age) | Parallel group design, randomized, double blind and placebo-controlled intervention. Two-week supplementation period (freeze-dried blueberry powder or placebo) followed by a 75 km cycling time trial (while consuming water only or water with bananas). Four groups (blueberry-water trial / blueberry–banana trial / placebo-banana trial / placebo-water trial). Blood samples timepoints: pre-exercise, post-exercise (0 h, 1.5 h, 3 h, 5 h, 24 h, and 48 h) | High-intensity, long-duration | COX, LOX, and CYP | LC-MRM-MS | Plasma | Large increases in plasma concentrations of 64 of 67 oxylipins detected, with most near pre-exercise levels within 5 h post-exercise |
| Nieman et al. (2019) [14] | 20 healthy cyclists (39.1 ± 2.4 years of age) | Randomized, crossover, counterbalanced approach. Four sessions of 75-km cycling time trial, 2-weekwashout. Four groups (Cavendish banana trial / mini-yellow banana trial / sugar beverage trial / water trial). Blood samples timepoints: pre-exercise, post-exercise (0 h, 0.75 h, 1.5 h, 3 h, 4.5 h, 21 h and 45 h) | High-intensity, long-duration | COX, LOX, and CYP | LC-MRM-MS/UHPLC/MS | Plasma | Large increases in plasma concentrations of 43 of 45 oxylipins detected, with most near pre-exercise levels within 4.5 h post-exercise |
| Nieman et al., (2014) [22] | 19 male cyclists (38.1 ± 1.6 years of age) | Subjects performed a 75 km cycling time trial without any beverage or food containing energy or nutrients. Blood samples timepoints: pre-exercise and post-exercise (0 h, 1.5 h, 21 h) | High-intensity, long-duration | LOX and CYP | UHPLC- MS/MS and GC-MS | Plasma | Large increases in 9-HODE, 13-HODE, 9,10-DiHOME, and 12,13-DiHOME, with most near pre-exercise levels between 1.5 and 21 h post-exercise |
| Vella et al. (2019) [17] | 12 recreationally active men (22.1 ± 0.6 years of age) | Acute bout of maximal concentric and eccentric isokinetic unilateral knee extension exercise, three sets of 12 maximal repetitions, 2 min of rest between sets. Muscle biopsy timepoints: pre-protocol, post-protocol (2 h, 4 h, 24 h) | High-intensity, resistance, short-duration | COX, LOX, and CYP | HPLC-MRM-MS/MS | Muscle Biopsy | 84 oxylipins detected. Modest to small increases in 22 oxylipins at 2 h post-exercise including TXB2, PGE2, PGF2α, 15d-D12,14-PGJ3), 12-oxo-LTB4, 20-COOH-LTB4, 5-HETE, 12-HETE, tetranor 12-HETE, 15-HETE, 12-HEPE, 4HDoHE, 7-HDoHE, 14-HDoHE, 5,6-EpETrE, 11,12-DiHETrE, and 14,15-DiHETrE. Most of them returned to close pre-exercise values 4 h after the end exercise |
| Pathway | Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Giordano et al. (2011) [20] | Medina et al. (2012) [21] | Markworth et al. (2013) [16] | Nieman et al. (2014) [22] | Garcia-Flores et al. (2018) [18] | Nieman et al. (2019) [14] | Gollach et al. (2019) [19] | Vella et al. (2019) [17] | Nieman et al. (2020) [15] | |
| Acute Effect (Short Duration) | Chronic Effect (Long Duration) | Acute Effect (Short Duration) | Acute Effect (Long Duration) | Chronic Effect (long Duration) | Acute Effect (Long Duration) | Acute Effect (Short Duration) | Acute Effect (Short Duration) | Acute Effect (long Duration) | |
| COX | - | ↓ Tetranor- PGEM ↓ 11-β-PGF2α ↑ 6-keto-PGF1α | ↑↑ TXB2 ↑↑ 12-HHTrE ↑ PGD2 ↑ PGE2 ↑ 15-keto-PGE2 ↑ 15-keto- PGF2α ↑ 6-keto-PGF1α ↑ 13,14- dihydro-15-keto-PGE2 ↑ RvE1 | - | ↑ PGDM ↑ 11-β-PGF2α ↑ PGE1 ↓↓ PGF1α |
↑↑ TXB2 ↑↑ 12-HHTrE ↑ PGFM ↑↑ 18-HEPE | - | ↑ TXB2 ↑ 12-HHTrE ↑ PGE2 ↑ PGF2α ↑ 15d- D12,14-PGJ3 | ↑↑TXB2 ↑↑ 12-HHTrE ↑ PGFM ↑↑ PGE2 ↑↑ dh-PGE2 ↑↑ TXB1 ↑↑ TXB3 ↑ PGE3 ↑ 18-HEPE ↑ RvE1 |
| LOX | - | - | ↑ 12-HETE ↑ 5,12- DiHETE ↑ Tetranor- 12-HETE ↑ 15-HETE ↑ 15-oxo-ETE ↑ LTB4 ↑ LXA4 ↑ LXB4 ↑ 9-oxo-ODE ↑ 13-HODE ↑ 13-oxo-ODE ↑ 10(S),17(S)- DiHDoHE ↑ RvD1 | ↑ 9-HODE ↑ 13-HODE | ↑ 5-HETE ↑ 8-HETE ↑↑ 9-HETE ↑↑ 11-HETE ↑↑ 12-HETE ↑↑ Tetranor 12-HETE ↑↑ 15-HETE ↑↑ 9-HODE ↑↑ 9-oxo-ODE ↑↑ 13-HODE ↑ 13-oxo-ODE ↑↑ 5-HETrE ↑↑ 8-HETrE ↑↑ 15-HETrE ↑↑ 4-HdoHE ↑↑ 8-HdoHE ↑↑ 10-HdoHE ↑ 13-HdoHE ↑↑ 14-HdoHE ↑ 16-HdoHE ↑↑ 5-HEPE ↑↑ 12-HEPE ↑ 15-HEPE | - | ↑ 5-HETE ↑ 12-HETE ↑↑ Tetranor 12-HETE ↑ 12-oxo- LTB4 ↑ 20-COOH- LTB4 ↑ 4-HdoHE ↑ 7-HdoHE ↑ 14-HdoHE ↑↑ 12-HEPE | ↑ 5-HETE ↑ 5-oxo-ETE ↑↑ 5,15- DiHETE ↑↑ 8-HETE ↑↑ 9-HETE ↑↑ 11-HETE ↑↑ 12-HETE ↑ 12-oxo-ETE ↑↑ Tetranor 12-HETE ↑ 15-HETE ↑ 15R-LXA4 ↑↑ 9-HODE ↑↑ 9-oxo-ODE ↑↑ 13-HODE ↑↑ 13- oxo-ODE ↑↑ 5-HETrE ↑↑ 8-HETrE ↑↑ 15-HETrE ↑ 4-HdoHE ↑↑ 7-HdoHE ↑↑ 8-HdoHE ↑↑ 10-HdoHE ↑↑ 11-HDoHE | |
| LOX | - | - | ↑↑ 9-HOTrE ↑↑ 13-HOTrE | - | ↑↑ 13-HDoHE ↑↑ 14-HDoHE ↑ 16-HDoHE ↑↑ 17-HDoHE ↑↑ PD1 ↑↑ 5-HEPE ↑ 8-HEPE ↑↑ 9-HEPE ↑ 11-HEPE ↑↑ 12-HEPE ↑↑ 15-HEPE ↑↑ 9-HOTrE ↑↑ 13-HOTrE | ||||
| CYP | ↑ 8,9- DiHETrE ↑ 11,12- DiHETrE ↓ 14,15- EpETrE ↑ 14,15- DiHETrE | - | ↑ 11,12- DiHETrE ↑ 14,15- DiHETrE ↑ 9,10-EpOME ↑ 9,10- DiHOME | ↑ 9,10- DiHOME ↑ 12,13- DiHOME | - | ↑↑ 8,9- DiHETrE ↑↑ 11,12- DiHETrE ↑ 14,15- DiHETrE ↑↑ 17-HETE ↑ 18-HETE ↑ 19-HETE ↑↑ 20-HETE ↑↑ 20- COOH-AA ↑ 9,10- EpOME ↑↑ 9,10- DiHOME ↑↑ 12,13- DiHOME ↑↑ 19,20- DiHDPE ↑↑ 20- HDoHE | ↑ 5,6- DiHETrE ↑ 12,13- EpOME ↑ 5,6- DiHETE ↑ 17,18- DiHETE | ↑ 5,6-EpETrE ↑ 11,12- DiHETrE ↑ 14,15- DiHETrE | ↑↑ 5,6- EpETrE ↑↑ 5,6- DiHETrE ↑↑ 8,9- DiHETrE ↑↑ 11,12- EpETrE ↑↑ 11,12- DiHETrE ↑↑ 14,15- DiHETrE ↑ 16-HETE ↑↑ 17-HETE ↑↑ 18-HETE ↑↑ 19-HETE ↑↑ 20-HETE ↑ 9,10- EpOME ↑ 9,10- DiHOME ↑ 12,13- DiHOME ↑↑ 19,20- DiHDPE ↑↑ 20-COOH- AA ↑↑ 20-HDoHE |
| Non- Enzymatic | - | ↓ 8-iso- PGF2α | - | - | ↓ 15-keto- 15-F2t-iso ↓ 9-epi- 15-F2t-iso ↓ 5-epi-5F2t-iso | ↑↑ 5-iso- PGF2α-VI | - | - | ↑ 8,12-iso- Isoprostane- F2α-VI ↑ 13,14- Dihydro-15-keto-PGF2α |
| PICOS | |
|---|---|
| Population | Healthy adult subjects |
| Intervention | Physical Exercise influence |
| Comparator | Non-exercise/intervention condition |
| Outcome | Exercise-related oxylipins |
| Study Design | Analytical studies |
| Score Setting | |||
|---|---|---|---|
| Section | Maximum Score | Aspects | Score Attribution |
| Research Design | 2 | Number of Participants | Parallel Studies 0—n < 20 2—n > 20 Crossover Studies 0—n < 13 2—n > 13 |
| 2 | Study Characteristics | —Randomized control group —Proper matrix —> 2 timepoints data collection —Duration ≥ 3wk (chronic studies only) 0—None of the previous items 1—At least 2 of the first 3 criteria listed 2—All 3 of the first 3 criteria listed | |
| Methodology | 3 | Analysis Methods | 1—< 10 oxylipins measured using global metabolomics 2—40–10 oxylipins measured using LC-MS/MS targeted oxylipins panel 3—> 40 oxylipins measured using LC-MS/MS targeted oxylipins panel |
| 2 | Statistical Support | 0—simple univariate statistics 1—Univariate statistics + additional analyses to sort and group the data, and to control for confounding factors 2—univariate statistics + PCA, OPLS-DA, PLS-DA, or similar advanced bioinformatics procedures | |
| Novelty | 2 | 0–2—New information in the literature | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signini, É.F.; Nieman, D.C.; Silva, C.D.; Sakaguchi, C.A.; Catai, A.M. Oxylipin Response to Acute and Chronic Exercise: A Systematic Review. Metabolites 2020, 10, 264. https://doi.org/10.3390/metabo10060264
Signini ÉF, Nieman DC, Silva CD, Sakaguchi CA, Catai AM. Oxylipin Response to Acute and Chronic Exercise: A Systematic Review. Metabolites. 2020; 10(6):264. https://doi.org/10.3390/metabo10060264
Chicago/Turabian StyleSignini, Étore F., David C. Nieman, Claudio D. Silva, Camila A. Sakaguchi, and Aparecida M. Catai. 2020. "Oxylipin Response to Acute and Chronic Exercise: A Systematic Review" Metabolites 10, no. 6: 264. https://doi.org/10.3390/metabo10060264
APA StyleSignini, É. F., Nieman, D. C., Silva, C. D., Sakaguchi, C. A., & Catai, A. M. (2020). Oxylipin Response to Acute and Chronic Exercise: A Systematic Review. Metabolites, 10(6), 264. https://doi.org/10.3390/metabo10060264








