L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
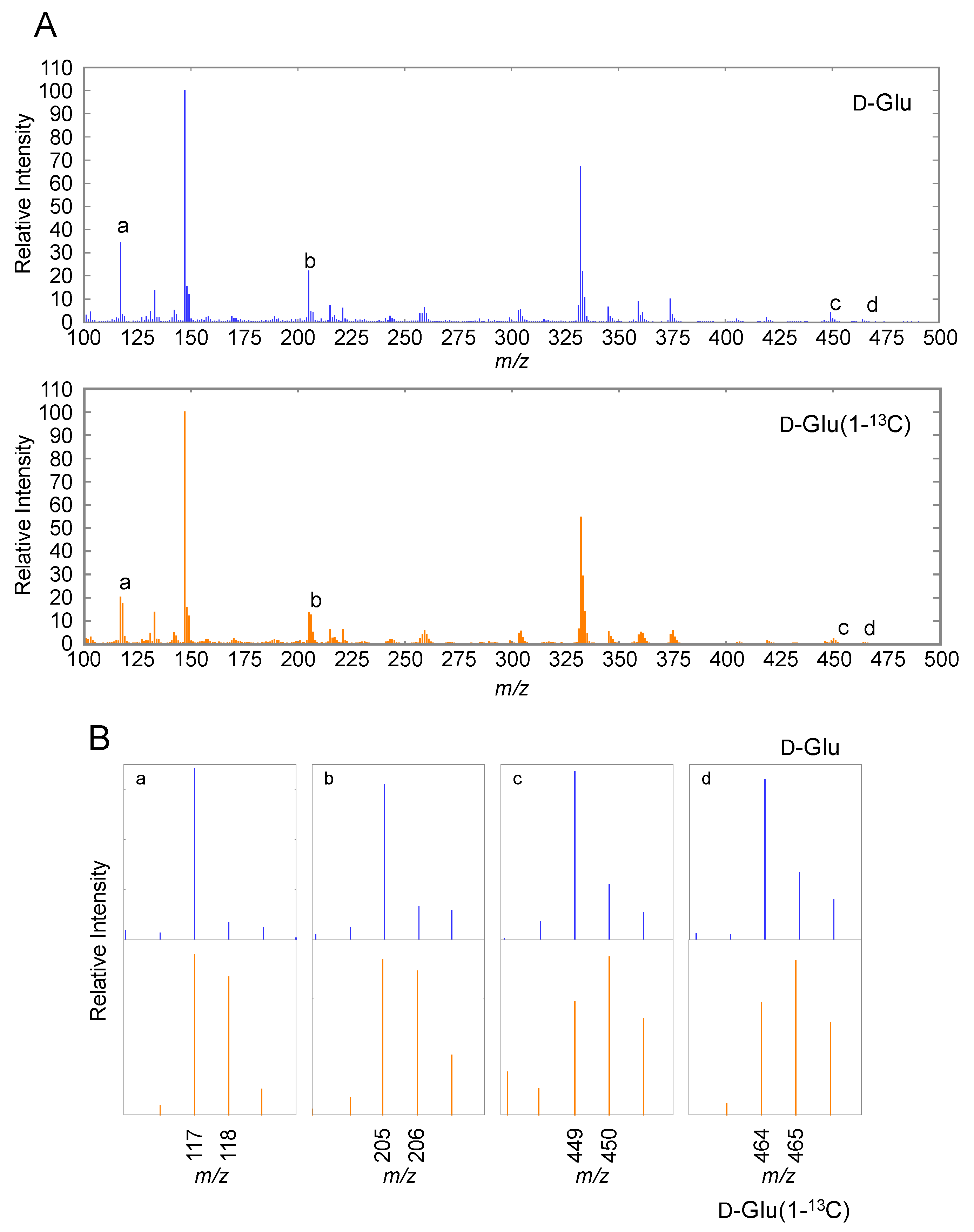
2.1. AsA Biosynthesis in C. elegans Involves Carbon Skeleton Rearrangement
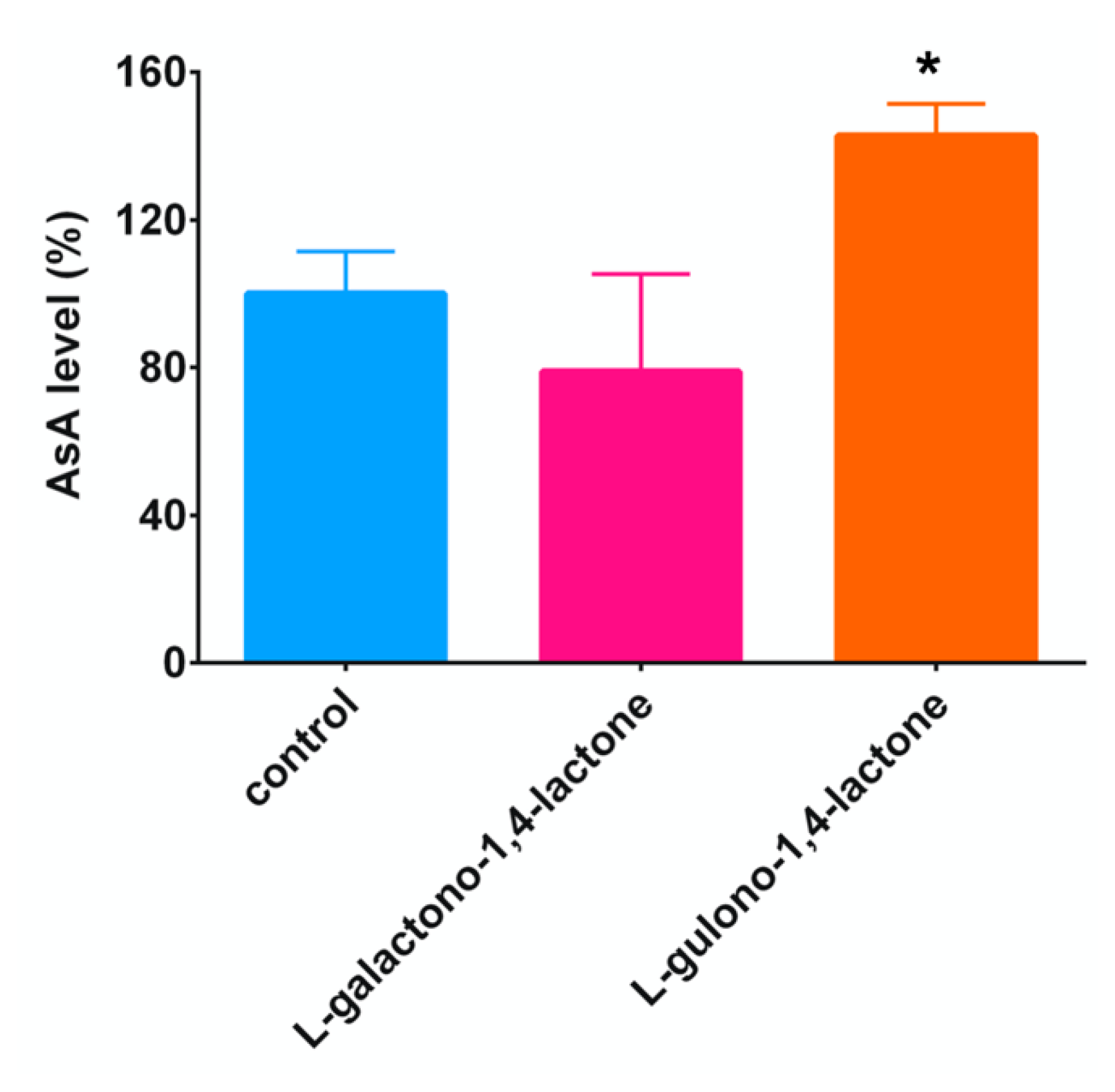
2.2. Addition of L-Gulono-1,4-lactone or L-Galactono-1,4-lactone, AsA Precursors, to C. elegans
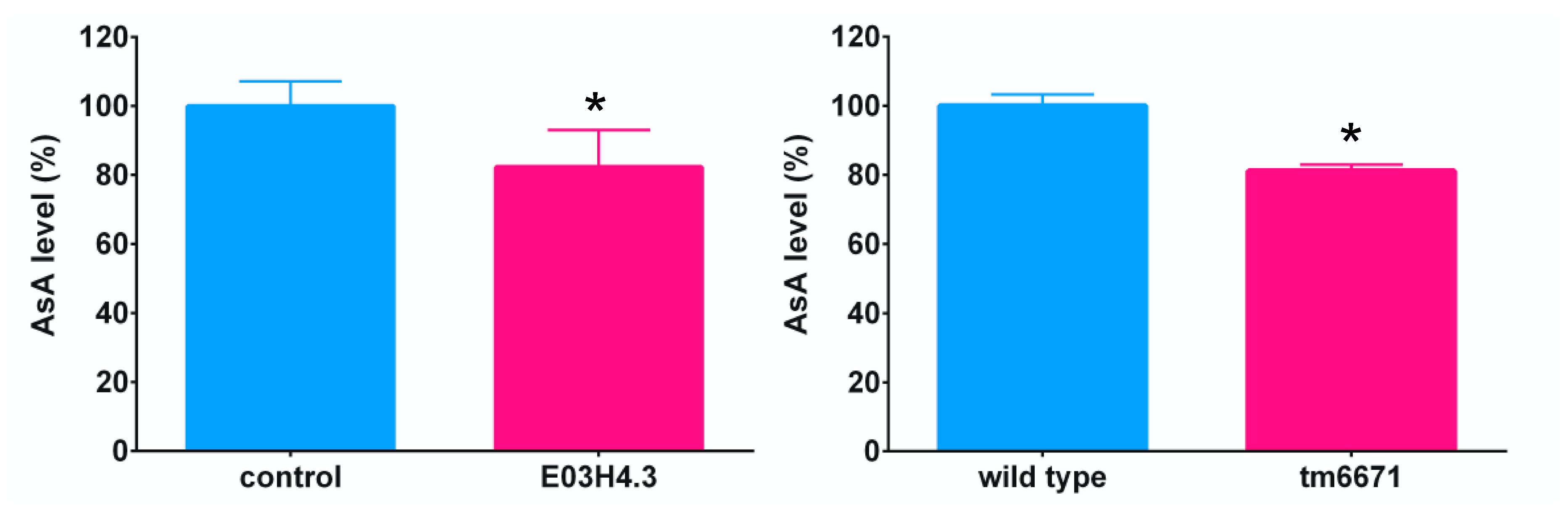
2.3. Identification of the AsA Biosynthetic Pathway in C. elegans Using Knockout or Knockdown Worms
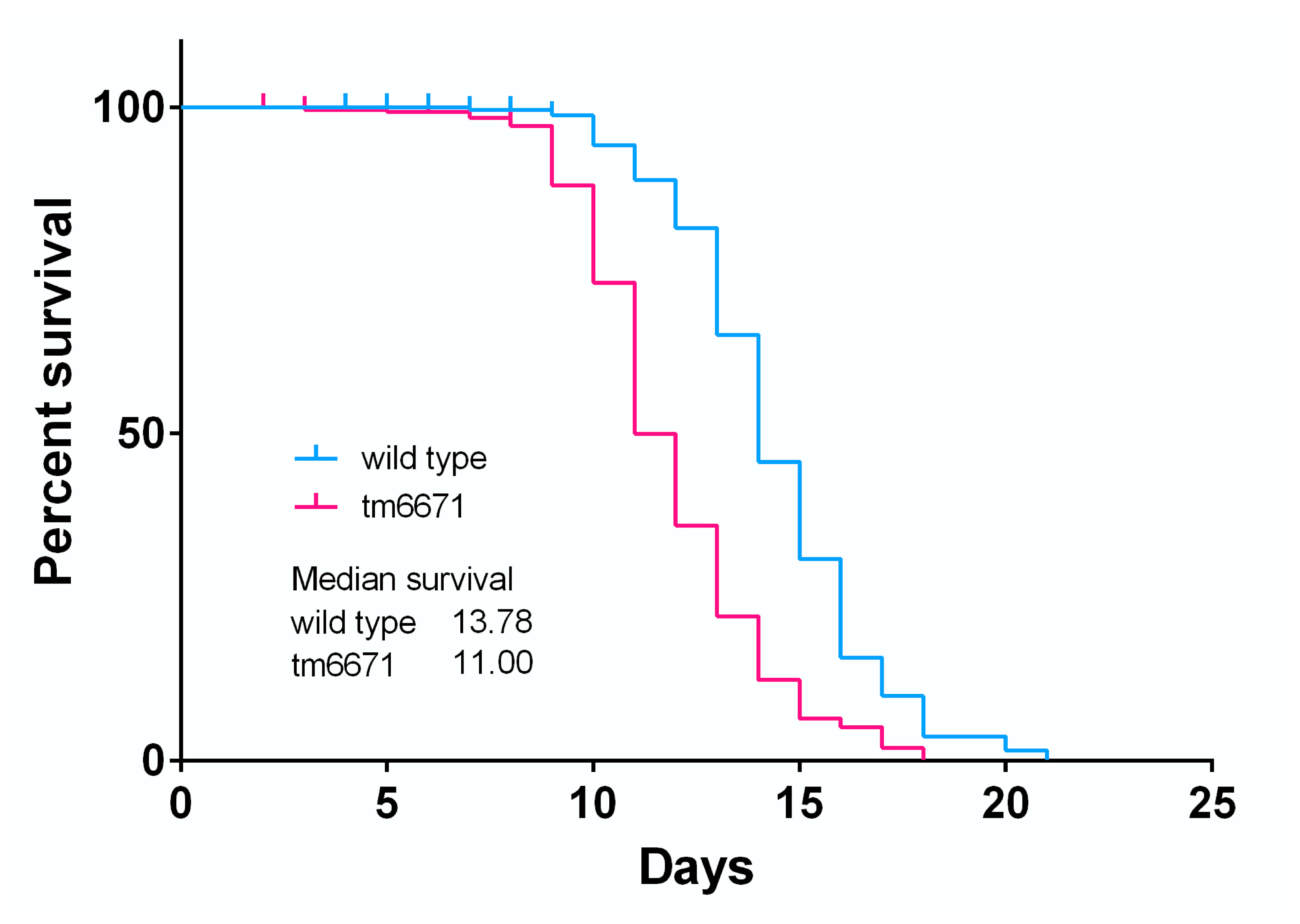
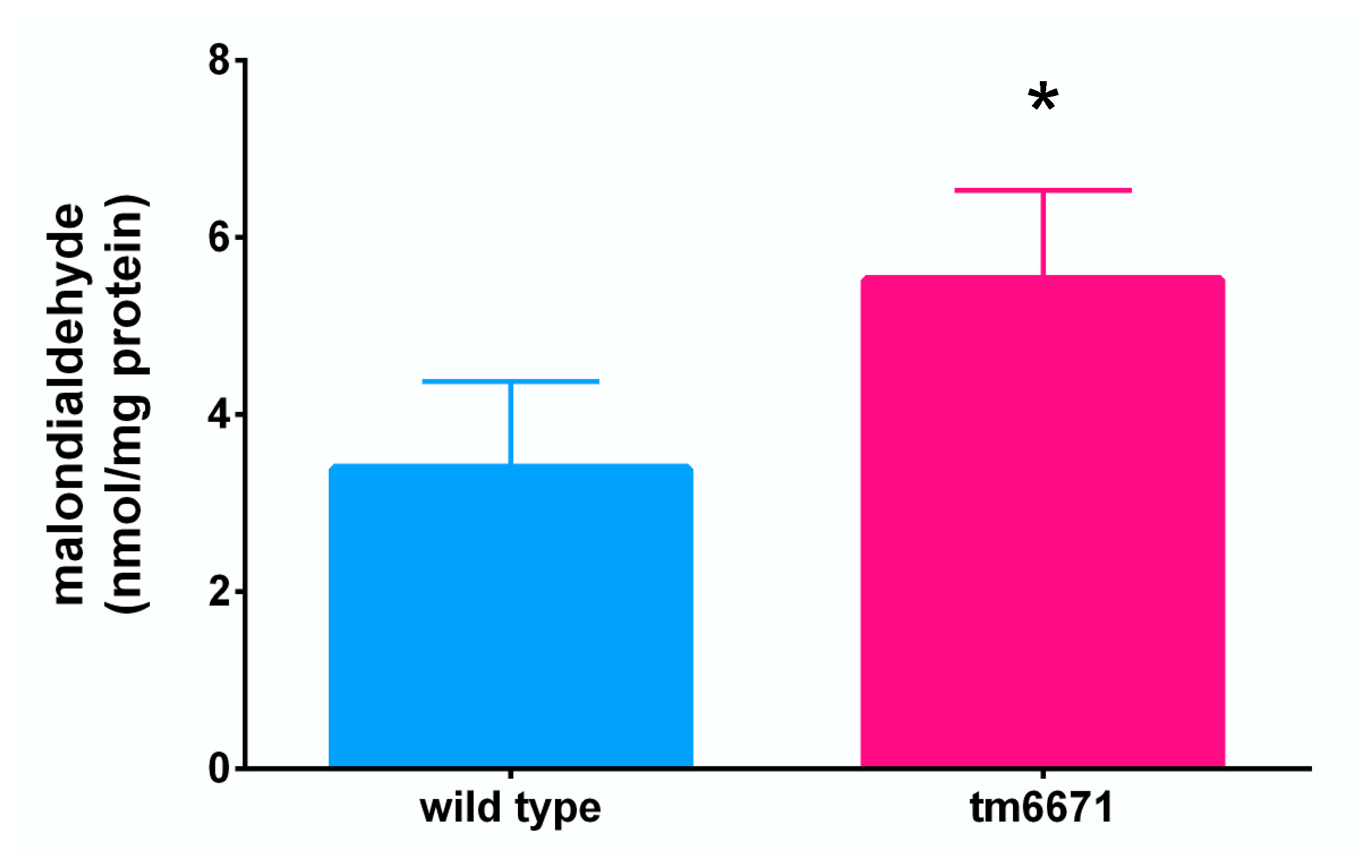
2.4. Reduced AsA Levels Affect C. elegans Lifespan and Lipid Peroxide Levels
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Worm Cultures and Strains
4.3. Preparation of 13C-Labeled Escherichia coli
4.4. Preparation of 13C-Labeled Spinach Leaves
4.5. GC-MS Analysis
4.6. RNAi Vector Construction
4.7. RNAi Experiments
4.8. AsA Measurement
4.9. C. elegans Supplementation with AsA Precursor L-Gulono-1,4-lactone or L-Galactono-1,4-lactone
4.10. qPCR Analysis
4.11. Collagen to Non-Collagen Protein Ratio Measurement
4.12. C. elegans Lifespan Determination
4.13. Malondialdehyde Measurement
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Kivirikko, K.I.; Myllylä, R. Post-translational processing of procollagens. Ann. N. Y. Acad. Sci. 1985, 460, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Kivirikko, K. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar]
- Vogel, W.; Gish, G.D.; Alves, F.; Pawson, T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1997, 1, 13–23. [Google Scholar] [CrossRef]
- Bhadriraju, K.; Chung, K.H.; Spurlin, T.A.; Haynes, R.J.; Elliott, J.T.; Plant, A.L. The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells. Biomaterials 2009, 30, 6687–6694. [Google Scholar] [CrossRef]
- Jurgensen, H.J.; Madsen, D.H.; Ingvarsen, S.; Melander, M.C.; Gardsvoll, H.; Patthy, L.; Engelholm, L.H.; Behrendt, N. A novel functional role of collagen glycosylation: Interaction with the endocytic collagen receptor uparap/ENDO180. J. Biol. Chem. 2011, 286, 32736–32748. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Gu, J.; El Ayadi, A.; Oberhauser, A.F.; Zhou, J.; Sousse, L.E.; Finnerty, C.C.; Herndon, D.N.; Boor, P.J. Effect of N-(2-aminoethyl) ethanolamine on hypertrophic scarring changes in vitro: Finding novel anti-fibrotic therapies. Toxicol. Appl. Pharmacol. 2019, 362, 9–19. [Google Scholar] [CrossRef]
- Hudson, D.M.; Kim, L.S.; Weis, M.; Cohn, D.H.; Eyre, D.R. Peptidyl 3-hydroxyproline binding properties of type I collagen suggest a function in fibril supramolecular assembly. Biochemistry 2012, 51, 2417–2424. [Google Scholar]
- Hulse, J.D.; Ellis, S.R.; Henderson, L.M. Carnitine biosynthesis. β-hydroxylation of trimethyllysine by an α-ketoglutarate-dependent mitochondrial dioxygenase. J. Biol. Chem. 1978, 253, 1654–1659. [Google Scholar]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.; Kaufman, S. 3,4-dihydroxyphenylethylamine ß-hydroxylase. physical properties, copper content, and role of copper in the catalytic activity. J. Biol. Chem. 1965, 240, 4763–4773. [Google Scholar] [PubMed]
- Nishikimi, M.; Yagi, K. Biochemistry and molecular biology of ascorbic acid biosynthesis. Subcell. Biochem. 1996, 25, 17–39. [Google Scholar] [PubMed]
- Kondo, Y.; Inai, Y.; Sato, Y.; Handa, S.; Kubo, S.; Shimokado, K.; Goto, S.; Nishikimi, M.; Maruyama, N.; Ishigami, A. Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc. Natl. Acad. Sci. USA 2006, 103, 5723–5728. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Uchida, K.; Maruyama, N. Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim. Biophys. Acta 1992, 1116, 122–128. [Google Scholar] [CrossRef]
- Mori, T.; Ishigami, A.; Seyama, K.; Onai, R.; Kubo, S.; Shimizu, K.; Maruyama, N.; Fukuchi, Y. Senescence marker protein-30 knockout mouse as a novel murine model of senile lung. Pathol. Int. 2004, 54, 167–173. [Google Scholar] [CrossRef]
- Nishikimi, M.; Tolbert, B.M.; Udenfriend, S. Purification and characterization of L-gulono-γ-lactone oxidase from rat and goat liver. Arch. Biochem. Biophys. 1976, 175, 427–435. [Google Scholar] [CrossRef]
- Nishikimi, M.; Kawai, T.; Yagi, K. Guinea pigs possess a highly mutated gene for L-gulono-γ-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species. J. Biol. Chem. 1992, 267, 21967–21972. [Google Scholar]
- Inai, Y.; Ohta, Y.; Nishikimi, M. The whole structure of the human nonfunctional L-gulono-γ-lactone oxidase gene—The gene responsible for scurvy--and the evolution of repetitive sequences thereon. J. Nutr. Sci Vitaminol. 2003, 49, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Wheeler, G.; Ishikawa, T.; Pornsaksit, V.; Smirnoff, N. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lorence, A.; Chevone, B.; Mendes, P.; Nessler, C.L. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. An Arabidopsis purple acid phosphatase with phytase activity increases foliar ascorbate. Plant Physiol. 2008, 146, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Østergaard, J.; Persiau, G.; Davey, M.W.; Bauw, G.; Van Montagu, M. Isolation of a cDNA coding for L-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. Purification, characterization, cDNA cloning, and expression in yeast. J. Biol. Chem. 1997, 272, 30009–30016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, T.; Karita, S.; Shiratori, G.; Hattori, M.; Nunome, T.; Oba, K.; Hirai, M. L-galactono-γ-lactone dehydrogenase from sweet potato: Purification and cDNA sequence analysis. Plant Cell Physiol. 1998, 39, 1350–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabuta, Y.; Yoshimura, K.; Takeda, T.; Shigeoka, S. Molecular characterization of tobacco mitochondrial L-galactono-γ-lactone dehydrogenase and its expression in Escherichia coli. Plant Cell Physiol. 2000, 41, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Nakano, Y.; Kitaoka, S. The biosynthetic pathway of L-ascorbic acid in Euglena gracilis Z. J. Nutr. Sci. Vitaminol. 1979, 25, 299–307. [Google Scholar] [CrossRef]
- Ishikawa, T.; Nishikawa, H.; Gao, Y.; Sawa, Y.; Shibata, H.; Yabuta, Y.; Maruta, T.; Shigeoka, S. The pathway via D-galacturonate/L-galactonate is significant for ascorbate biosynthesis in Euglena gracilis: Identification and functional characterization of aldonolactonase. J. Biol. Chem. 2008, 283, 31133–31141. [Google Scholar] [CrossRef] [Green Version]
- Winter, A.D.; Page, A.P. Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol. Cell Biol. 2000, 20, 4084–4093. [Google Scholar] [CrossRef] [Green Version]
- Veijola, J.; Koivunen, P.; Annunen, P.; Pihlajaniemi, T.; Kivirikko, K.I. Cloning, baculovirus expression, and characterization of the α subunit of prolyl 4-hydroxylase from the nematode Caenorhabditis elegans. This α subunit forms an active αβ dimer with the human protein disulfide isomerase/β subunit. J. Biol. Chem. 1994, 269, 26746–26753. [Google Scholar] [PubMed]
- Patananan, A.N.; Budenholzer, L.M.; Pedraza, M.E.; Torres, E.R.; Adler, L.N.; Clarke, S.G. The invertebrate Caenorhabditis elegans biosynthesizes ascorbate. Arch. Biochem Biophys. 2015, 569, 32–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WormBase. Available online: https://wormbase.org/#012-34-5 (accessed on 1 May 2020).
- Shi, X.; Tarazona, P.; Brock, T.J.; Browse, J.; Feussner, I.; Watts, J.L. A Caenorhabditis elegans model for ether lipid biosynthesis and function. J. Lipid Res. 2016, 57, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, K.R.; Moerman, D.G. The let-268 locus of Caenorhabditis elegans encodes a procollagen lysyl hydroxylase that is essential for type IV collagen secretion. Dev. Biol. 2000, 227, 690–705. [Google Scholar] [CrossRef] [Green Version]
- Friedman, L.; Higgin, J.J.; Moulder, G.; Barstead, R.; Raines, R.T.; Kimble, J. Prolyl 4-hydroxylase is required for viability and morphogenesis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2000, 97, 4736–4741. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.L.; Harfe, B.D.; Dobbins, C.A.; L’Hernault, S.W. dpy-18 encodes an α-subunit of prolyl-4-hydroxylase in Caenorhabditis elegans. Genetics 2000, 55, 1139–1148. [Google Scholar]
- Myllyharju, J.; Kukkola, L.; Winter, A.D.; Page, A.P. The exoskeleton collagens in Caenorhabditis elegans are modified by prolyl 4-hydroxylases with unique combinations of subunits. J. Biol. Chem. 2002, 277, 29187–29196. [Google Scholar] [CrossRef] [Green Version]
- Riihimaa, P.; Nissi, R.; Page, A.P.; Winter, A.D.; Keskiaho, K.; Kivirikko, K.I.; Myllyharju, J. Egg shell collagen formation in Caenorhabditis elegans involves a novel prolyl 4-hydroxylase expressed in spermatheca and embryos and possessing many unique properties. J. Biol. Chem. 2002, 277, 18238–18243. [Google Scholar] [CrossRef] [Green Version]
- Vanfleteren, J.R. Oxidative stress and ageing in Caenorhabditis elegans. Biochem. J. 1993, 292, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Ishii, N. Life span and oxidative stress in nematode. J. Clin. Biochem. Nutr. 2004, 34, 61–68. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hayashi, S.; Yoshida, T. Participation of a cytochrome b5-like hemoprotein of outer mitochondrial membrane (OM cytochrome b) in NADH-semidehydroascorbic acid reductase activity of rat liver. Biochem. Biophys. Res. Commun. 1981, 101, 591–598. [Google Scholar] [CrossRef]
- May, J.M.; Cobb, C.E.; Mendiratta, S.; Hill, K.E.; Burk, R.F. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J. Biol. Chem. 1998, 273, 23039–23045. [Google Scholar] [CrossRef] [Green Version]
- Bode, A.M.; Cunningham, L.; Rose, R.C. Spontaneous decay of oxidized ascorbic acid (dehydro-L-ascorbic acid) evaluated by high-pressure liquid chromatography. Clin. Chem. 1990, 36, 1807–1809. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Ishikawa, T.; Casini, A.F.; Nishikimi, M. Molecular cloning and functional expression of rat liver glutathione-dependent dehydroascorbate reductase. J. Biol. Chem. 1998, 273, 28708–28712. [Google Scholar] [CrossRef] [Green Version]
- Wells, W.W.; Xu, D.P.; Yang, Y.F.; Rocque, P.A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J. Biol. Chem. 1990, 265, 15361–15364. [Google Scholar]
- Whitbread, A.K.; Masoumi, A.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005, 401, 78–99. [Google Scholar]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Del Bello, B.; Maellaro, E.; Sugherini, L.; Santucci, A.; Comporti, M.; Casini, A.F. Purification of NADPH-dependent dehydroascorbate reductase from rat liver and its identification with 3α-hydroxysteroid dehydrogenase. Biochem. J. 1994, 304, 385–390. [Google Scholar] [CrossRef]
- May, J.M.; Mendiratta, S.; Hill, K.E.; Burk, R.F. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 1997, 272, 22607–22610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Sieburth, D.; Ch’ng, Q.; Dybbs, M.; Tavazoie, M.; Kennedy, S.; Wang, D.; Dupuy, D.; Rual, J.F.; Hill, D.E.; Vidal, M.; et al. Systematic analysis of genes required for synapse structure and function. Nature 2005, 436, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, Y.; Honda, Y.; Honda, S.; Iwasaki, T.; Qadota, H.; Benian, G.M.; Kawano, T. Diapause is associated with a change in the polarity of secretion of insulin-like peptides. Nat. Commun. 2016, 7, 10573. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Misaki, T.; Yabuta, Y.; Ishikawa, T.; Kawano, T.; Watanabe, F. Vitamin B12 deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 2017, 11, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Flavel, M.R.; Mechler, A.; Shahmiri, M.; Mathews, E.R.; Franks, A.E.; Chen, W.; Zanker, D.; Xian, B.; Gao, S.; Luo, J.; et al. Growth of Caenorhabditis elegans in defined media is dependent on presence of particulate matter. G3 2018, 8, 567–575. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yabuta, Y.; Nagata, R.; Aoki, Y.; Kariya, A.; Wada, K.; Yanagimoto, A.; Hara, H.; Bito, T.; Okamoto, N.; Yoshida, S.; et al. L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans. Metabolites 2020, 10, 334. https://doi.org/10.3390/metabo10080334
Yabuta Y, Nagata R, Aoki Y, Kariya A, Wada K, Yanagimoto A, Hara H, Bito T, Okamoto N, Yoshida S, et al. L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans. Metabolites. 2020; 10(8):334. https://doi.org/10.3390/metabo10080334
Chicago/Turabian StyleYabuta, Yukinori, Ryuta Nagata, Yuka Aoki, Ayumi Kariya, Kousuke Wada, Ayako Yanagimoto, Hiroka Hara, Tomohiro Bito, Naho Okamoto, Shinichi Yoshida, and et al. 2020. "L-Ascorbate Biosynthesis Involves Carbon Skeleton Rearrangement in the Nematode Caenorhabditis elegans" Metabolites 10, no. 8: 334. https://doi.org/10.3390/metabo10080334




