Metabolic Characterization and Consequences of Mitochondrial Pyruvate Carrier Deficiency in Drosophila melanogaster
Abstract
:1. Introduction
2. Results
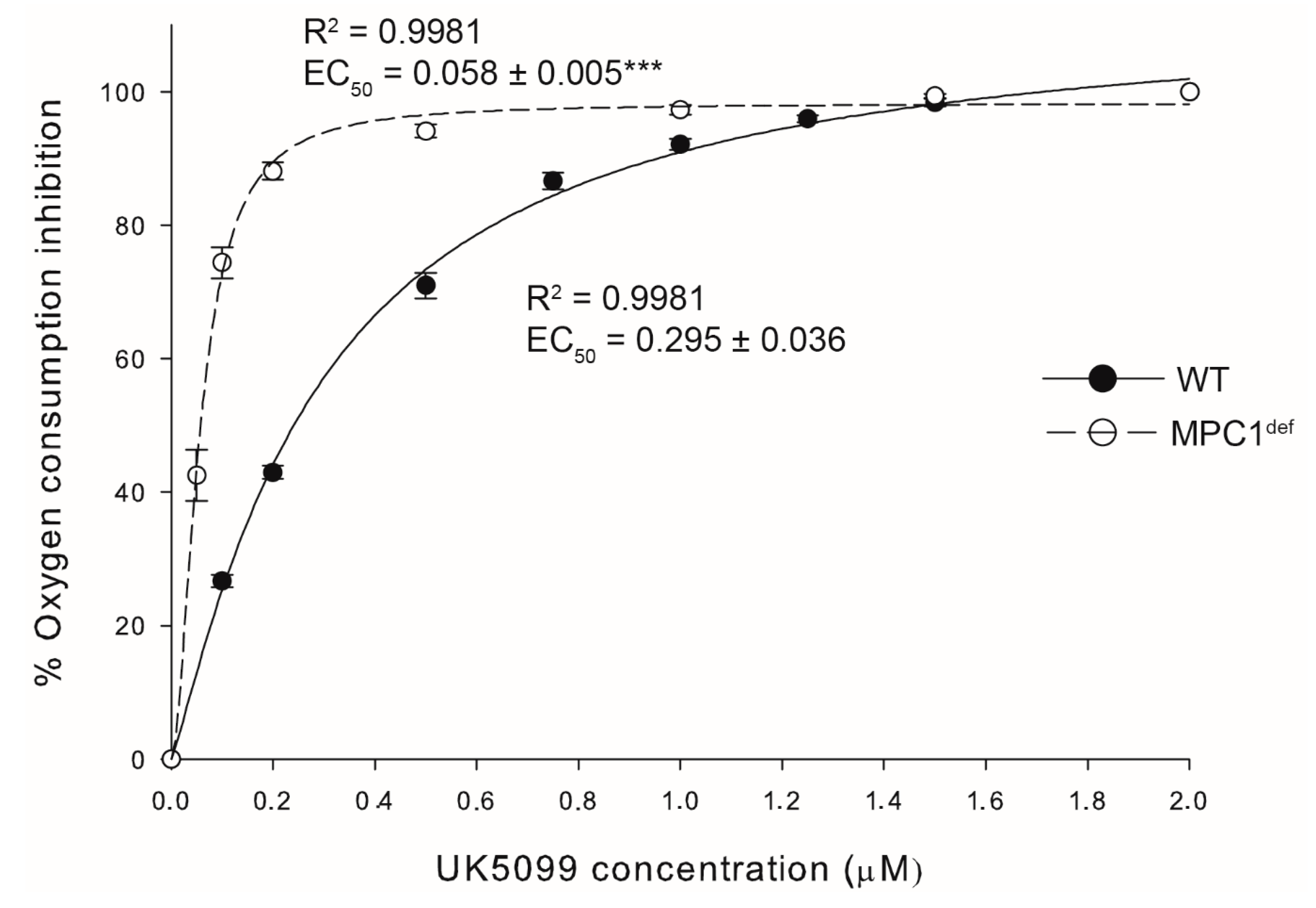
2.1. MPC1 Expression and Mitochondrial Pyruvate Carrier Inhibition Kinetics
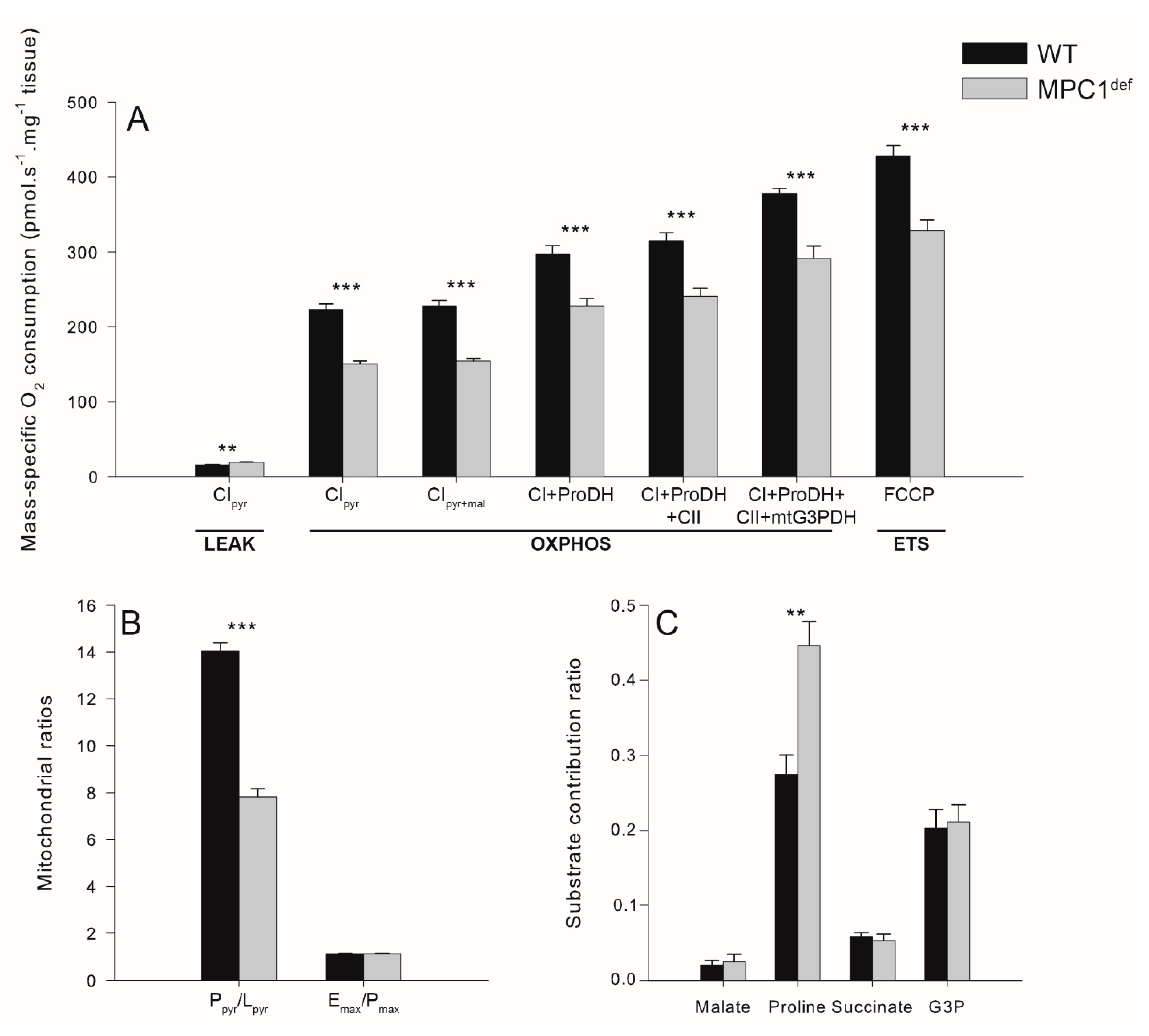
2.2. Mitochondrial Oxygen Consumption and Mitochondrial Ratios
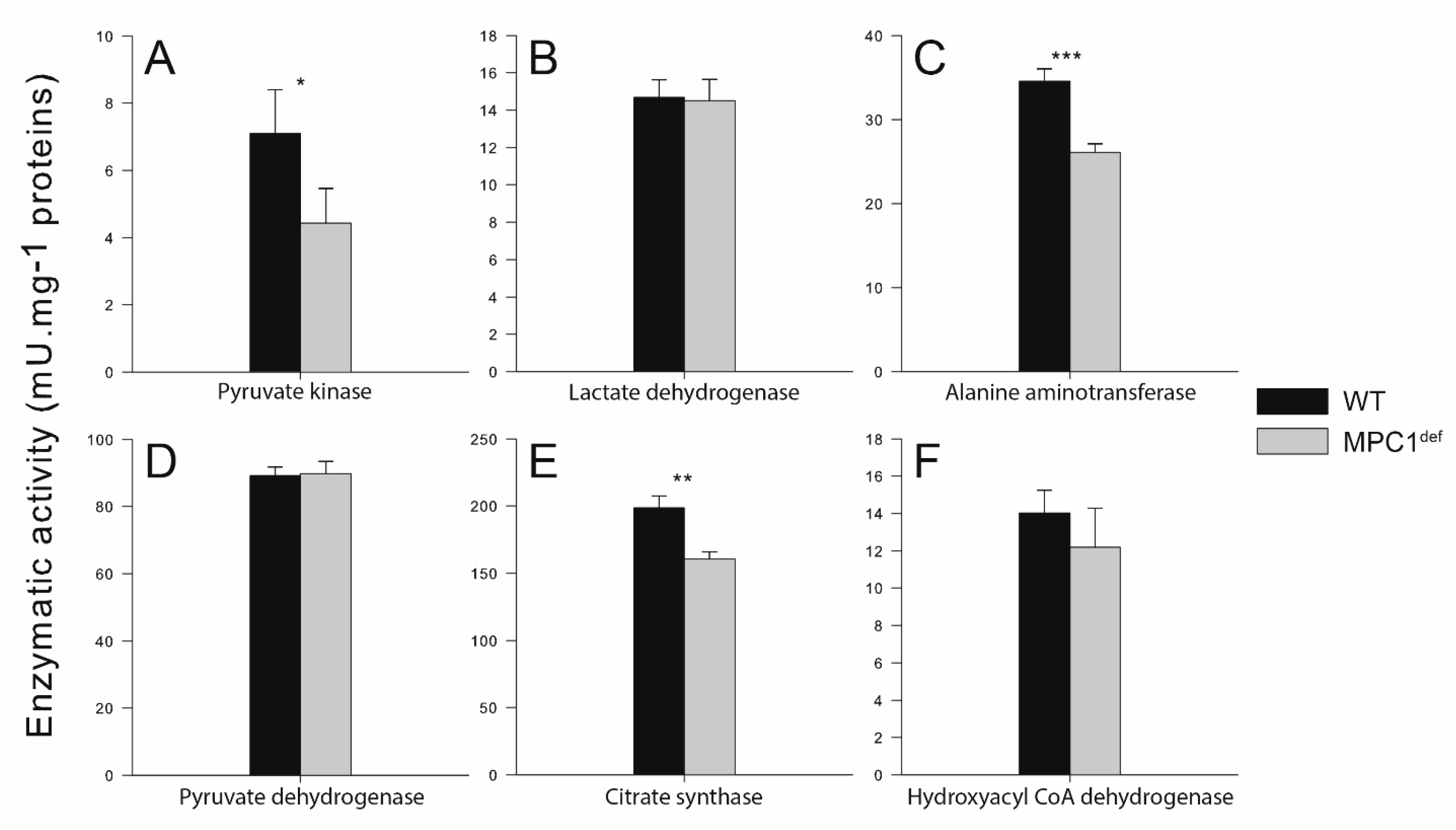
2.3. Enzymatic Activities
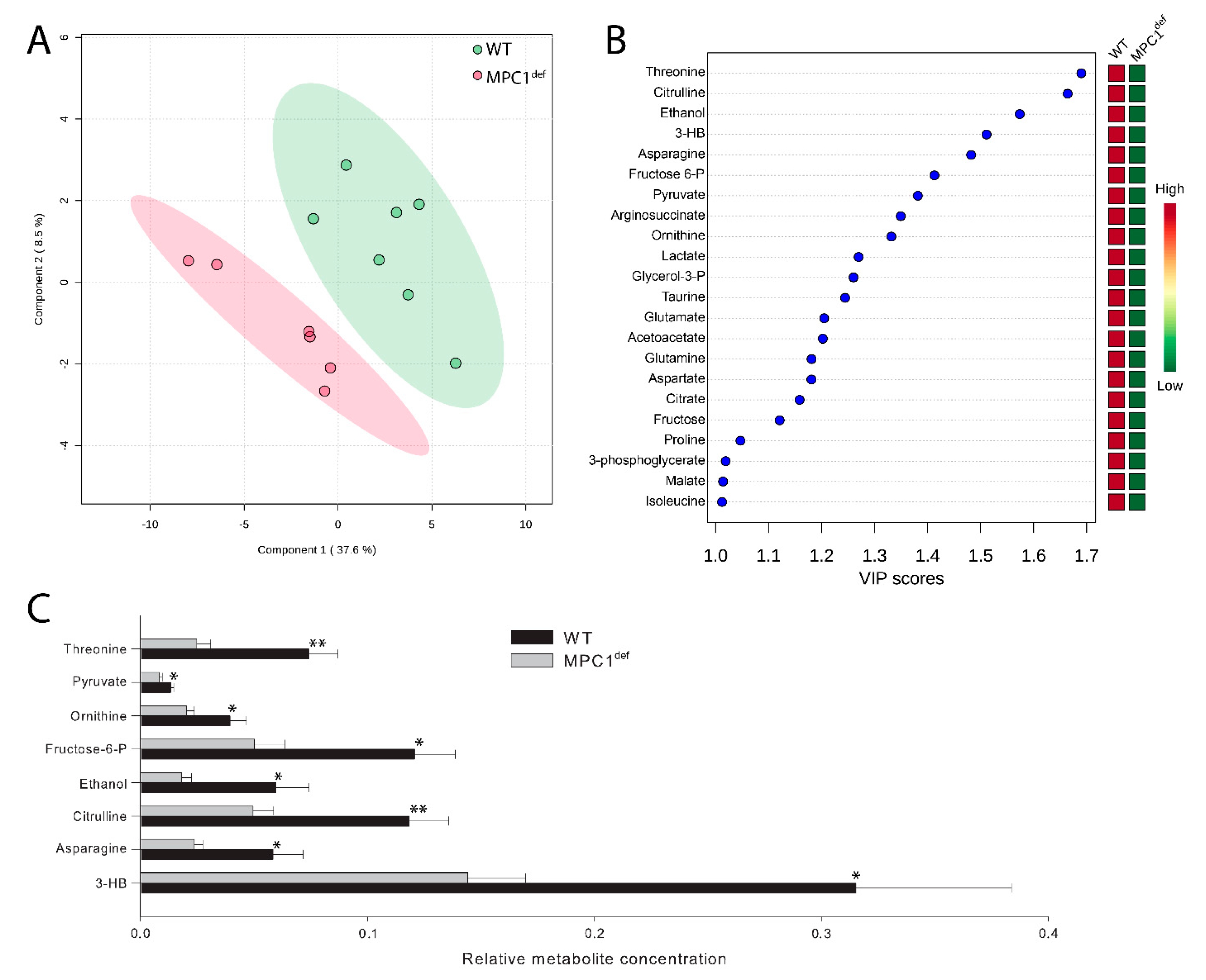
2.4. Relative Metabolites Abundance
2.5. Climbing Assay
3. Discussion
4. Materials and Methods
4.1. Drosophila
4.2. MPC1 Expression
4.3. Mitochondrial Pyruvate Carrier Inhibition
4.4. Mitochondrial Oxygen Consumption
4.5. Enzymatic Activities
4.6. Relative Metabolites Abundance
4.7. Climbing Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Horst, D.J.; van Hoof, D.; van Marrewijk, W.J.A.; Rodenburg, K.W. Alternative lipid mobilization: The insect shuttle system. Mol. Cell. Biochem. 2002, 239, 113–119. [Google Scholar] [CrossRef]
- Beenakkers, A.M.T.; van der Horst, D.J.; van Marrewijk, W.J.A. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Candy, D.J.; Becker, A.; Wegener, G. Coordination and integration of metabolism in insect flight. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 497–512. [Google Scholar] [CrossRef]
- Sacktor, B. Biochemistry of Insect Flight. In Insect Biochemistry and Function; Springer US: Boston, MA, USA, 1975; pp. 1–88. [Google Scholar]
- Bricker, D.K.; Taylor, E.B.; Schell, J.C.; Orsak, T.; Boutron, A.; Chen, Y.C.; Cox, J.E.; Cardon, C.M.; van Vranken, J.G.; Dephoure, N.; et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012, 337, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Pichaud, N.; Ballard, J.W.O.; Tanguay, R.M.; Blier, P.U. Thermal sensitivity of mitochondrial functions in permeabilized muscle fibers from two populations of Drosophila simulans with divergent mitotypes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R48–R59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teulier, L.; Weber, J.M.; Crevier, J.; Darveau, C.A. Proline as a fuel for insect flight: Enhancing carbohydrate oxidation in hymenopterans. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160333. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.B.R.C.; Gaviraghi, A.; Oliveira, M.F.; Veuthey, J.; Zamboni, N.; Westermann, B. Mitochondrial physiology in the major arbovirus vector Aedes aegypti: Substrate preferences and sexual differences define respiratory capacity and superoxide production. PLoS ONE 2015, 10, e0120600. [Google Scholar] [CrossRef] [Green Version]
- Bursell, E. Aspects of the metabolism of amino acids in the tsetse fly, Glossina (Diptera). J. Insect Physiol. 1963, 9, 439–452. [Google Scholar] [CrossRef]
- Weeda, E.; de Kort, C.A.D.; Beenakkers, A.M.T. Oxidation of proline and pyruvate by flight muscle mitochondria of the Colorado beetle, Leptinotarsa decemlineata say. Insect Biochem. 1980, 10, 305–311. [Google Scholar] [CrossRef]
- Gäde, G.; Auerswald, L. Beetles’ choice-Proline for energy output: Control by AKHs. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 117–129. [Google Scholar] [CrossRef]
- Bursell, E. The Role of Proline in Energy Metabolism. In Energy Metabolism in Insects; Springer: New York, NY, USA, 1981; pp. 135–154. [Google Scholar]
- Olembo, N.K.; Pearson, D.J. Changes in the contents of intermediates of proline and carbohydrate metabolism in flight muscle of the tsetse fly Glossina morsit ans and the fleshfly Sarcophaga tibialis. Insect Biochem. 1982, 12, 657–662. [Google Scholar] [CrossRef]
- Vrbacký, M.; Drahota, Z.; Mráček, T.; Vojtíšková, A.; Ješina, P.; Stopka, P.; Houštěk, J. Respiratory chain components involved in the glycerophosphate dehydrogenase-dependent ROS production by brown adipose tissue mitochondria. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, R.P.J.; Champigny, C.M.; Simard, C.J.; St-Coeur, P.D.; Pichaud, N. Dynamic mitochondrial responses to a high-fat diet in Drosophila melanogaster. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- McDonald, A.E.; Pichaud, N.; Darveau, C.A. Alternative fuels contributing to mitochondrial electron transport: Importance of non-classical pathways in the diversity of animal metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 185–194. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Hodges, W.T.; Bricker, D.K.; Wisidagama, D.R.; Compan, V.; Remedi, M.S.; Thummel, C.S.; Finck, B.N. An ancestral role for the mitochondrial pyruvate carrier in glucose-stimulated insulin secretion. Mol. Metab. 2016, 5, 602–614. [Google Scholar] [CrossRef]
- Halestrap, A.P. The mitochondrial pyruvate carrier. Kinetics and specificity for substrates and inhibitors. Biochem. J. 1975, 148, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009, 41, 1837–1845. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Prot. Bioinf. 2019, 68, e86. [Google Scholar] [CrossRef]
- Vanderperre, B.; Bender, T.; Kunji, E.R.S.; Martinou, J.C. Mitochondrial pyruvate import and its effects on homeostasis. Curr. Opin. Cell Biol. 2015, 33, 35–41. [Google Scholar] [CrossRef]
- Vacanti, N.M.; Divakaruni, A.S.; Green, C.R.; Parker, S.J.; Henry, R.R.; Ciaraldi, T.P.; Murphy, A.N.; Metallo, C.M. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 2014, 56, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Schell, J.C.; Olson, K.A.; Jiang, L.; Hawkins, A.J.; vanVranken, J.G.; Xie, J.; Egnatchik, R.A.; Earl, E.G.; DeBerardinis, R.J.; Rutter, J. A role for the mitochondrial pyruvate carrier as a repressor of the warburg effect and colon cancer cell growth. Mol. Cell 2014, 56, 400–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigueira, P.A.; McCommis, K.S.; Schweitzer, G.G.; Remedi, M.S.; Chambers, K.T.; Fu, X.; McDonald, W.G.; Cole, S.L.; Colca, J.R.; Kletzien, R.F.; et al. Mitochondrial pyruvate carrier 2 hypomorphism in mice leads to defects in glucose-stimulated insulin secretion. Cell Rep. 2014, 7, 2042–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Model. Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmbeck, M.A.; Donner, J.R.; Villa-Cuesta, E.; Rand, D.M. A Drosophila model for mito-nuclear diseases generated by an incompatible interaction between tRNA and tRNA synthetase. Dis. Model. Mech. 2015, 8, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.B.; Werkhoven, S.; Harrison, J.F. Metabolomics of anoxia tolerance in Drosophila melanogaster: Evidence against substrate limitation and for roles of protective metabolites and paralytic hypometabolism. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R442–R450. [Google Scholar] [CrossRef] [PubMed]
- Feala, J.D.; Coquin, L.; McCulloch, A.D.; Paternostro, G. Flexibility in energy metabolism supports hypoxia tolerance in Drosophila flight muscle: Metabolomic and computational systems analysis. Mol. Syst. Biol. 2007, 3, 99. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Datta, S.; Johnson, G.A.; Li, P.; Satterfield, M.C.; Spencer, T.E. Proline metabolism in the conceptus: Implications for fetal growth and development. Amino Acids 2008, 35, 691–702. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Servet, C.; Ghelis, T.; Richard, L.; Zilberstein, A.; Savoure, A. Proline dehydrogenase: A key enzyme in controlling cellular homeostasis. Front. Biosci. 2012, 17, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Pant, R.; Kumar, S. Is a urea cycle present in insects? Biochem. J. 1978, 174, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Pichaud, N.; Chatelain, E.H.; Ballard, J.W.O.; Tanguay, R.; Morrow, G.; Blier, P.U. Thermal sensitivity of mitochondrial metabolism in two distinct mitotypes of Drosophila simulans: Evaluation of mitochondrial plasticity. J. Exp. Biol. 2010, 213, 1665–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estabrook, R.W.; Sacktor, B. α-Glycerophosphate oxidase of flight muscle mitochondria. J. Biol. Chem. 1958, 233, 1014–1019. [Google Scholar] [PubMed]
- Pichaud, N.; Bérubé, R.; Côté, G.; Belzile, C.; Dufresne, F.; Morrow, G.; Tanguay, R.M.; Rand, D.M.; Blier, P.U. Age dependent dysfunction of mitochondrial and ros metabolism induced by mitonuclear mismatch. Front. Genet. 2019, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P. Dietary protein: Carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: A test using a chemically defined diet. J. Insect Physiol. 2015, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, L.M.; Johnson, S.; Paight, C.; Bozinovic, G.; Markow, T.A. Dietary protein and sugar differentially affect development and metabolic pools in ecologically diverse Drosophila. J. Nutr. 2011, 141, 1127–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichaud, N.; Messmer, M.; Correa, C.C.; Ballard, J.W.O. Diet influences the intake target and mitochondrial functions of Drosophila melanogaster males. Mitochondrion 2013, 13, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Thibault, S.T.; Singer, M.A.; Miyazaki, W.Y.; Milash, B.; Dompe, N.A.; Singh, C.M.; Buchholz, R.; Demsky, M.; Fawcett, R.; Francis-Lang, H.L.; et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 2004, 36, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Bellen, H.J.; Levis, R.W.; He, Y.; Carlson, J.W.; Evans-Holm, M.; Bae, E.; Kim, J.; Metaxakis, A.; Savakis, C.; Schulze, K.L.; et al. The Drosophila gene disruption project: Progress using transposons with distinctive site specificities. Genetics 2011, 188, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Bier, E.; Vaessin, H.; Shepherd, S.; Lee, K.; McCall, K.; Barbel, S.; Ackerman, L.; Carretto, R.; Uemura, T.; Grell, E.; et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989, 3, 1273–1287. [Google Scholar] [CrossRef] [Green Version]
- Simard, C.J.; Pelletier, G.; Boudreau, L.H.; Hebert-Chatelain, E.; Pichaud, N. Measurement of mitochondrial oxygen consumption in permeabilized fibers of Drosophila using minimal amounts of tissue. J. Vis. Exp. 2018, e57376. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Veksler, V.; Gellerich, F.N.; Saks, V.; Margreiter, R.; Kunz, W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Prot. 2008, 3, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Iftikar, F.I.; MacDonald, J.R.; Baker, D.W.; Renshaw, G.M.C.; Hickey, A.J.R. Could thermal sensitivity of mitochondria determine species distribution in a changing climate? J. Exp. Biol. 2014, 217, 2348–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekström, A.; Sandblom, E.; Blier, P.U.; Dupont Cyr, B.-A.; Brijs, J.; Pichaud, N.; Ekström, A.; Sandblom, E.; Blier, P.U.; Dupont Cyr, B.-A.; et al. Thermal sensitivity and phenotypic plasticity of cardiac mitochondrial metabolism in European perch, Perca fluviatilis. J. Exp. Biol. 2017, 220, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmeyer, H.U.; Schelbe, P.; Wahiefeld, A.W. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin. Chem. 1978, 24, 58. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C.; et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simard, C.; Lebel, A.; Allain, E.P.; Touaibia, M.; Hebert-Chatelain, E.; Pichaud, N. Metabolic Characterization and Consequences of Mitochondrial Pyruvate Carrier Deficiency in Drosophila melanogaster. Metabolites 2020, 10, 363. https://doi.org/10.3390/metabo10090363
Simard C, Lebel A, Allain EP, Touaibia M, Hebert-Chatelain E, Pichaud N. Metabolic Characterization and Consequences of Mitochondrial Pyruvate Carrier Deficiency in Drosophila melanogaster. Metabolites. 2020; 10(9):363. https://doi.org/10.3390/metabo10090363
Chicago/Turabian StyleSimard, Chloé, Andréa Lebel, Eric Pierre Allain, Mohamed Touaibia, Etienne Hebert-Chatelain, and Nicolas Pichaud. 2020. "Metabolic Characterization and Consequences of Mitochondrial Pyruvate Carrier Deficiency in Drosophila melanogaster" Metabolites 10, no. 9: 363. https://doi.org/10.3390/metabo10090363
APA StyleSimard, C., Lebel, A., Allain, E. P., Touaibia, M., Hebert-Chatelain, E., & Pichaud, N. (2020). Metabolic Characterization and Consequences of Mitochondrial Pyruvate Carrier Deficiency in Drosophila melanogaster. Metabolites, 10(9), 363. https://doi.org/10.3390/metabo10090363




