Targeting the Pentose Phosphate Pathway for SARS-CoV-2 Therapy
Abstract
:1. Introduction
2. Results
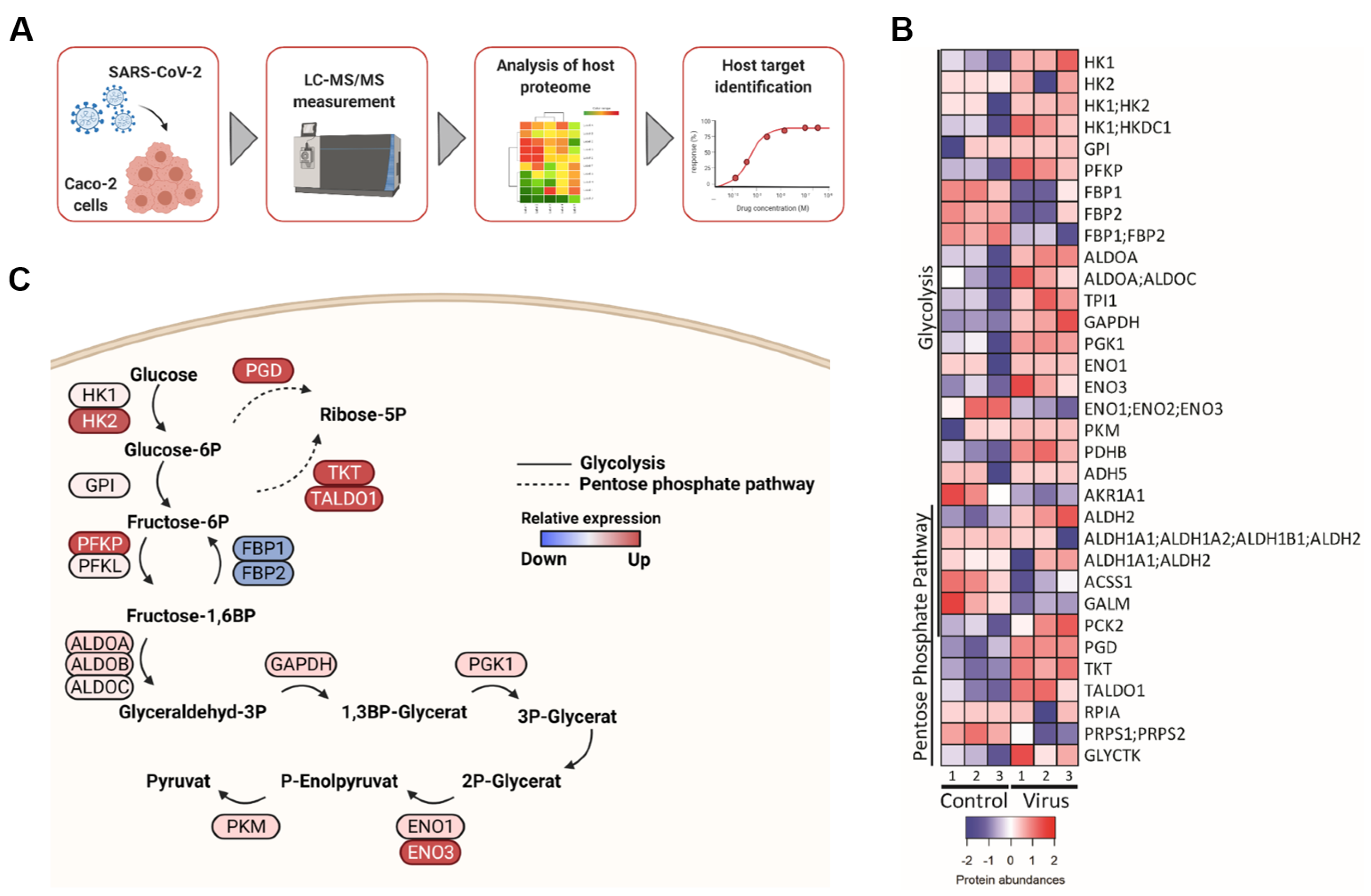
2.1. SARS-CoV-2 Infection Affects Key Enzymes of the Glycolysis Pathway and Non-Oxidative Pentose Phosphate Pathway (PPP)
2.2. Increased Glycolytic Activity in SARS-CoV-2-Infected Cells
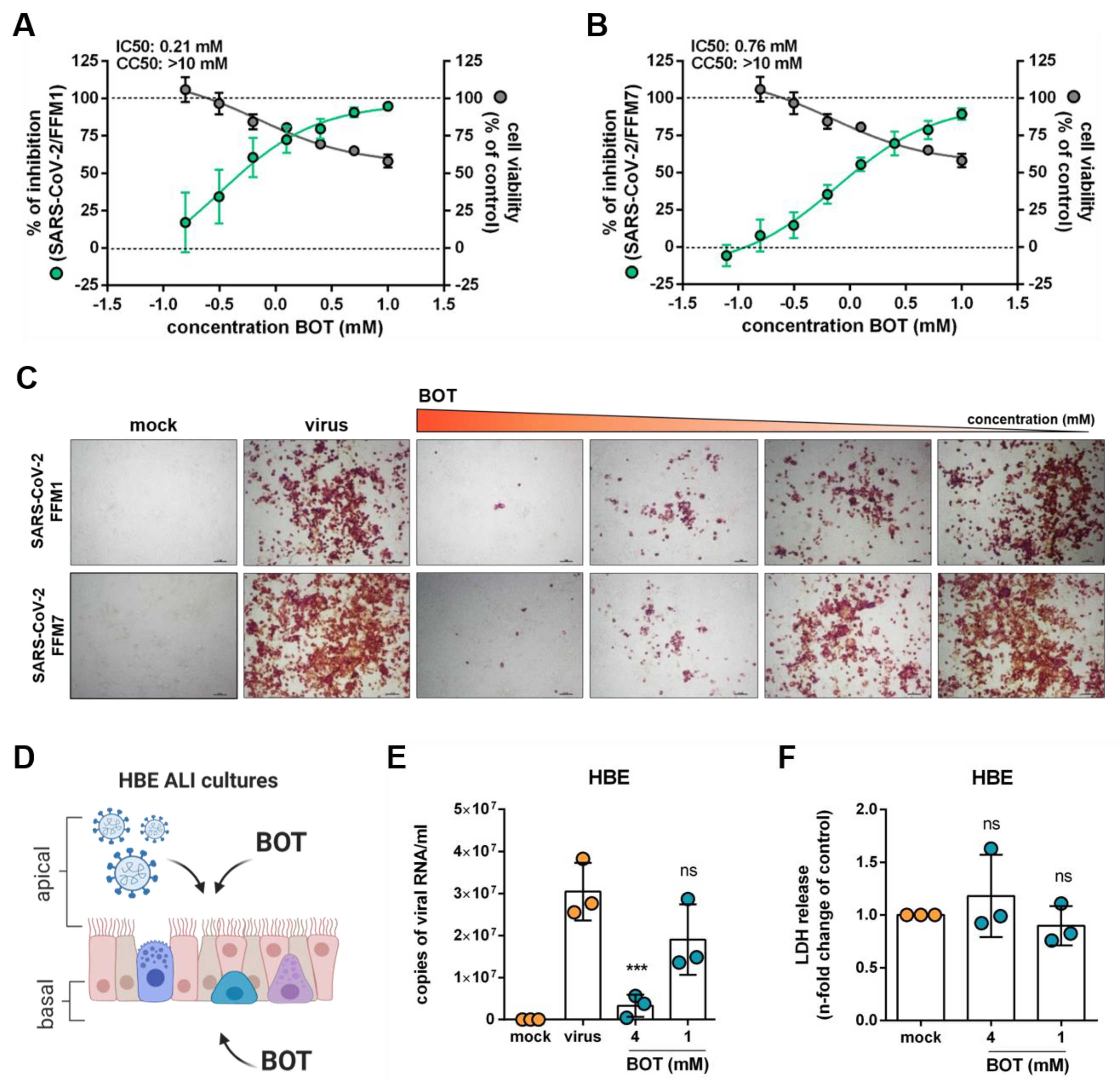
2.3. Inhibition of the Non-Oxidative Pentose Phosphate Pathway (PPP) Interferes with SARS-CoV-2 Replication
2.4. Benfooxythiamine (BOT) Increases the Anti-SARS-CoV-2 Effects of 2-Deoxy-d-Glucose (2DG)
3. Discussion
4. Materials and Methods
4.1. Proteomics Data
4.2. Measurement of ATP Rate
4.3. Measurement of Mitochondrial Respiration and the Glycolytic Function
4.4. Cell Culture and Virus Production
4.5. Antiviral and Cytotoxicity Assay
4.6. Immunostaining
4.7. qRT-PCR of Viral Genome in Supernatants
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hokello, J.; Sharma, A.L.; Shukla, G.C.; Tyagi, M. A narrative review on the basic and clinical aspects of the novel SARS-CoV-2, the etiologic agent of COVID-19. Ann. Transl. Med. 2020, 8, 1686. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Pawlik, A. Therapy of Type 2 Diabetes in Patients with SARS-CoV-2 Infection. Int. J. Mol. Sci. 2021, 22, 7605. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Norouzi, S.; Ruggiero, A.; Khan, M.; Myers, S.; Kavanagh, K.; Vemuri, R. Type-2 Diabetes as a Risk Factor for Severe COVID-19 Infection. Microorganisms 2021, 9, 1211. [Google Scholar] [CrossRef]
- Shin, C.-H.; Kim, K.-H.; Jeeva, S.; Kang, S.-M. Towards Goals to Refine Prophylactic and Therapeutic Strategies Against COVID-19 Linked to Aging and Metabolic Syndrome. Cells 2021, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef] [PubMed]
- Bojkova, D.; Bechtel, M.; McLaughlin, K.-M.; McGreig, J.E.; Klann, K.; Bellinghausen, C.; Rohde, G.; Jonigk, D.; Braubach, P.; Ciesek, S.; et al. Aprotinin Inhibits SARS-CoV-2 Replication. Cells 2020, 9, 2377. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Clinical Trials Registry–India. Available online: http://ctri.nic.in/Clinicaltrials/advancesearchmain.php (accessed on 18 August 2021).
- Halder, S.; Mehta, A.K. 2-Deoxy-d-glucose: Is this the final cure for COVID-19: Or yet another mirage? Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4448–4450. [Google Scholar] [CrossRef]
- DG-Nika. Available online: https://dg-nika.net (accessed on 18 August 2021).
- Novel Coronavirus COVID-19. Available online: https://www.moleculin.com/covid-19/ (accessed on 20 August 2021).
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Alam, M.T.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2014, 90, 927–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, D.; Shah, R.; Karpe, A.; Hillyer, K.; McAuley, A.; Au, G.; Marsh, G.; Vasan, S. Metabolic Profiling from an Asymptomatic Ferret Model of SARS-CoV-2 Infection. Metabolites 2021, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, A.; Łotowski, Z.; Siemieniuk, M.; Ratkiewicz, A. Thiamine and selected thiamine antivitamins—Biological activity and methods of synthesis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coy, J.F. EDIM-TKTL1/Apo10 Blood Test: An Innate Immune System Based Liquid Biopsy for the Early Detection, Characterization and Targeted Treatment of Cancer. Int. J. Mol. Sci. 2017, 18, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yao, C.-F.; Xu, F.-J.; Qu, Y.-Y.; Li, J.-T.; Lin, Y.; Cao, Z.-L.; Lin, P.-C.; Xu, W.; Zhao, S.-M.; et al. APC/CCDH1 synchronizes ribose-5-phosphate levels and DNA synthesis to cell cycle progression. Nat. Commun. 2019, 10, 2502. [Google Scholar] [CrossRef]
- Pácal, L.; Kuricová, K.; Kaňková, K. Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J. Diabetes 2014, 5, 288–295. [Google Scholar] [CrossRef]
- Ziegler, D.; Schleicher, E.; Strom, A.; Knebel, B.; Fleming, T.; Nawroth, P.; Häring, H.-U.; Papanas, N.; Szendrödi, J.; Müssig, K.; et al. Association of transketolase polymorphisms with measures of polyneuropathy in patients with recently diagnosed diabetes. Diabetes/Metab. Res. Rev. 2017, 33, e2811. [Google Scholar] [CrossRef]
- Chalásová, K.; Pácal, L.; Pleskačová, A.; Knopfová, L.; Řehořová, J.; Tomandlová, M.; Tomandl, J.; Kaňková, K. Transketolase Activity but not Thiamine Membrane Transport Change in Response to Hyperglycaemia and Kidney Dysfunction. Exp. Clin. Endocrinol. Diabetes 2018, 126, 255–262. [Google Scholar] [CrossRef]
- Peiró, C.; Romacho, T.; Azcutia, V.; Villalobos, L.; Fernández, E.; Bolaños, J.P.; Moncada, S.; Sánchez-Ferrer, C.F. Inflammation, glucose, and vascular cell damage: The role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a systemic vascular hemopathy: Insight for mechanistic and clinical aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef]
- Deng, Y.; Lei, L.; Chen, Y.; Zhang, W. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1634–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Tseng, C.-W.; Kuo, W.-H.; Chan, S.-H.; Chan, H.-L.; Chang, K.-J.; Wang, L.-H. Transketolase Regulates the Metabolic Switch to Control Breast Cancer Cell Metastasis via the α-Ketoglutarate Signaling Pathway. Cancer Res. 2018, 78, 2799–2812. [Google Scholar] [CrossRef] [Green Version]
- Rebold, N.; Holger, D.; Alosaimy, S.; Morrisette, T.; Rybak, M. COVID-19: Before the Fall, An Evidence-Based Narrative Review of Treatment Options. Infect. Dis. Ther. 2021, 10, 93–113. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.-H.; Michailidis, E.; et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Vostok, J.; Johnson, H.; Burns, M.; Gharpure, R.; Sami, S.; Sabo, R.T.; Hall, N.; Foreman, A.; Schubert, P.L.; et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings—Barnstable County, Massachusetts, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1059–1062. [Google Scholar] [CrossRef]
- Sabino, E.C.; Buss, L.F.; Carvalho, M.P.S.; Prete, C.A., Jr.; Crispim, M.A.E.; Fraiji, N.A.; Pereira, R.H.M.; Parag, K.V.; da Silva Peixoto, P.; Kraemer, M.U.G.; et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet 2021, 397, 452–455. [Google Scholar] [CrossRef]
- Vignier, N.; Bérot, V.; Bonnave, N.; Peugny, S.; Ballet, M.; Jacoud, E.; Michaud, C.; Gaillet, M.; Djossou, F.; Blanchet, D.; et al. Breakthrough Infections of SARS-CoV-2 Gamma Variant in Fully Vaccinated Gold Miners, French Guiana, 2021. Emerg. Infect. Dis. 2021, 27, 2673–2676. [Google Scholar] [CrossRef] [PubMed]
- Pum, A.; Ennemoser, M.; Adage, T.; Kungl, A.J. Cytokines and Chemokines in SARS-CoV-2 Infections—Therapeutic Strategies Targeting Cytokine Storm. Biomolecules 2021, 11, 91. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-Analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Hadid, T.; Kafri, Z.; Al-Katib, A. Coagulation and anticoagulation in COVID-19. Blood Rev. 2020, 47, 100761. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Garn, H.; Unger, S.D.; Renz, H. Antisense molecules: A new class of drugs. J. Allergy Clin. Immunol. 2016, 137, 1334–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potaczek, D.P.; Unger, S.D.; Zhang, N.; Taka, S.; Michel, S.; Akdağ, N.; Lan, F.; Helfer, M.; Hudemann, C.; Eickmann, M.; et al. Development and characterization of DNAzyme candidates demonstrating significant efficiency against human rhinoviruses. J. Allergy Clin. Immunol. 2019, 143, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.-M.; Tu, M.-J. Deliver the promise: RNAs as a new class of molecular entities for therapy and vaccination. Pharmacol. Ther. 2021, 107967, in press. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, A.-B.; Saiz, J.-C. Potential for Protein Kinase Pharmacological Regulation in Flaviviridae Infections. Int. J. Mol. Sci. 2020, 21, 9524. [Google Scholar] [CrossRef]
- Lingappa, J.; Lingappa, V.; Reed, J. Addressing Antiretroviral Drug Resistance with Host-Targeting Drugs—First Steps towards Developing a Host-Targeting HIV-1 Assembly Inhibitor. Viruses 2021, 13, 451. [Google Scholar] [CrossRef]
- Riyapa, D.; Rinchai, D.; Muangsombut, V.; Wuttinontananchai, C.; Toufiq, M.; Chaussabel, D.; Ato, M.; Blackwell, J.M.; Korbsrisate, S. Transketolase and vitamin B1 influence on ROS-dependent neutrophil extracellular traps (NETs) formation. PLoS ONE 2019, 14, e0221016. [Google Scholar] [CrossRef] [Green Version]
- Mahrooz, A.; Muscogiuri, G.; Buzzetti, R.; Maddaloni, E. The complex combination of COVID-19 and diabetes: Pleiotropic changes in glucose metabolism. Endocrine 2021, 72, 317–325. [Google Scholar] [CrossRef]
- Ayres, J.S. A metabolic handbook for the COVID-19 pandemic. Nat. Metab. 2020, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, G.; Huyck, H.L.; Misra, R.S.; Bhattacharya, S.; Wang, Q.; Mereness, J.; Lillis, J.; Myers, J.R.; Ashton, J.; Bushnell, T.; et al. Dissociation, cellular isolation, and initial molecular characterization of neonatal and pediatric human lung tissues. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 315, L576–L583. [Google Scholar] [CrossRef] [PubMed]
- Toptan, T.; Hoehl, S.; Westhaus, S.; Bojkova, D.; Berger, A.; Rotter, B.; Hoffmeier, K.; Cinatl, J., Jr.; Ciesek, S.; Widera, M. Optimized qRT-PCR Approach for the Detection of Intra- and Extra-Cellular SARS-CoV-2 RNAs. Int. J. Mol. Sci. 2020, 21, 4396. [Google Scholar] [CrossRef] [PubMed]
- Onafuye, H.; Pieper, S.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Langer, K.; Michaelis, M. Doxorubicin-loaded human serum albumin nanoparticles overcome transporter-mediated drug resistance in drug-adapted cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1707–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojkova, D.; Costa, R.; Reus, P.; Bechtel, M.; Jaboreck, M.-C.; Olmer, R.; Martin, U.; Ciesek, S.; Michaelis, M.; Cinatl, J., Jr. Targeting the Pentose Phosphate Pathway for SARS-CoV-2 Therapy. Metabolites 2021, 11, 699. https://doi.org/10.3390/metabo11100699
Bojkova D, Costa R, Reus P, Bechtel M, Jaboreck M-C, Olmer R, Martin U, Ciesek S, Michaelis M, Cinatl J Jr. Targeting the Pentose Phosphate Pathway for SARS-CoV-2 Therapy. Metabolites. 2021; 11(10):699. https://doi.org/10.3390/metabo11100699
Chicago/Turabian StyleBojkova, Denisa, Rui Costa, Philipp Reus, Marco Bechtel, Mark-Christian Jaboreck, Ruth Olmer, Ulrich Martin, Sandra Ciesek, Martin Michaelis, and Jindrich Cinatl, Jr. 2021. "Targeting the Pentose Phosphate Pathway for SARS-CoV-2 Therapy" Metabolites 11, no. 10: 699. https://doi.org/10.3390/metabo11100699
APA StyleBojkova, D., Costa, R., Reus, P., Bechtel, M., Jaboreck, M.-C., Olmer, R., Martin, U., Ciesek, S., Michaelis, M., & Cinatl, J., Jr. (2021). Targeting the Pentose Phosphate Pathway for SARS-CoV-2 Therapy. Metabolites, 11(10), 699. https://doi.org/10.3390/metabo11100699





