Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
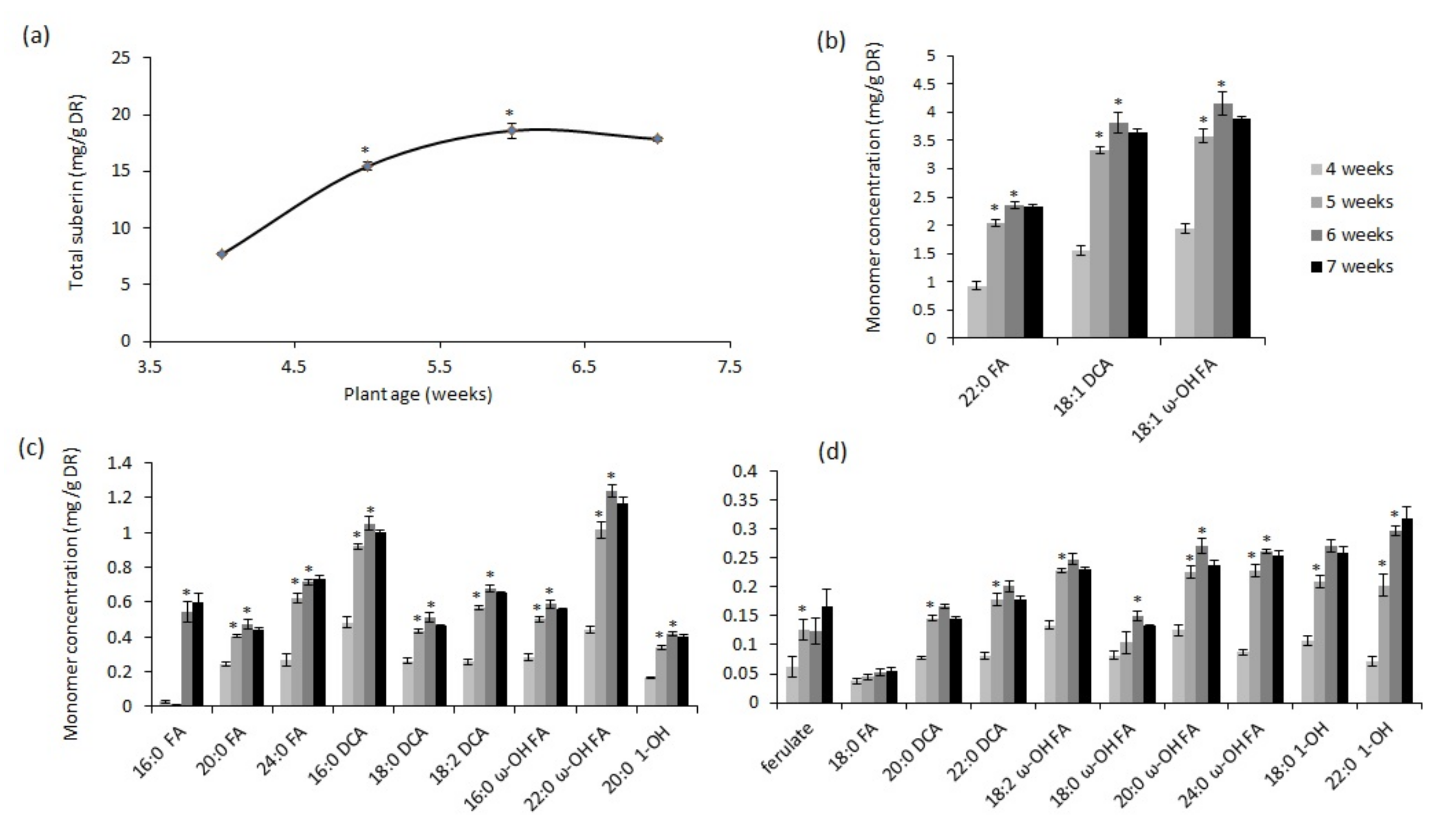
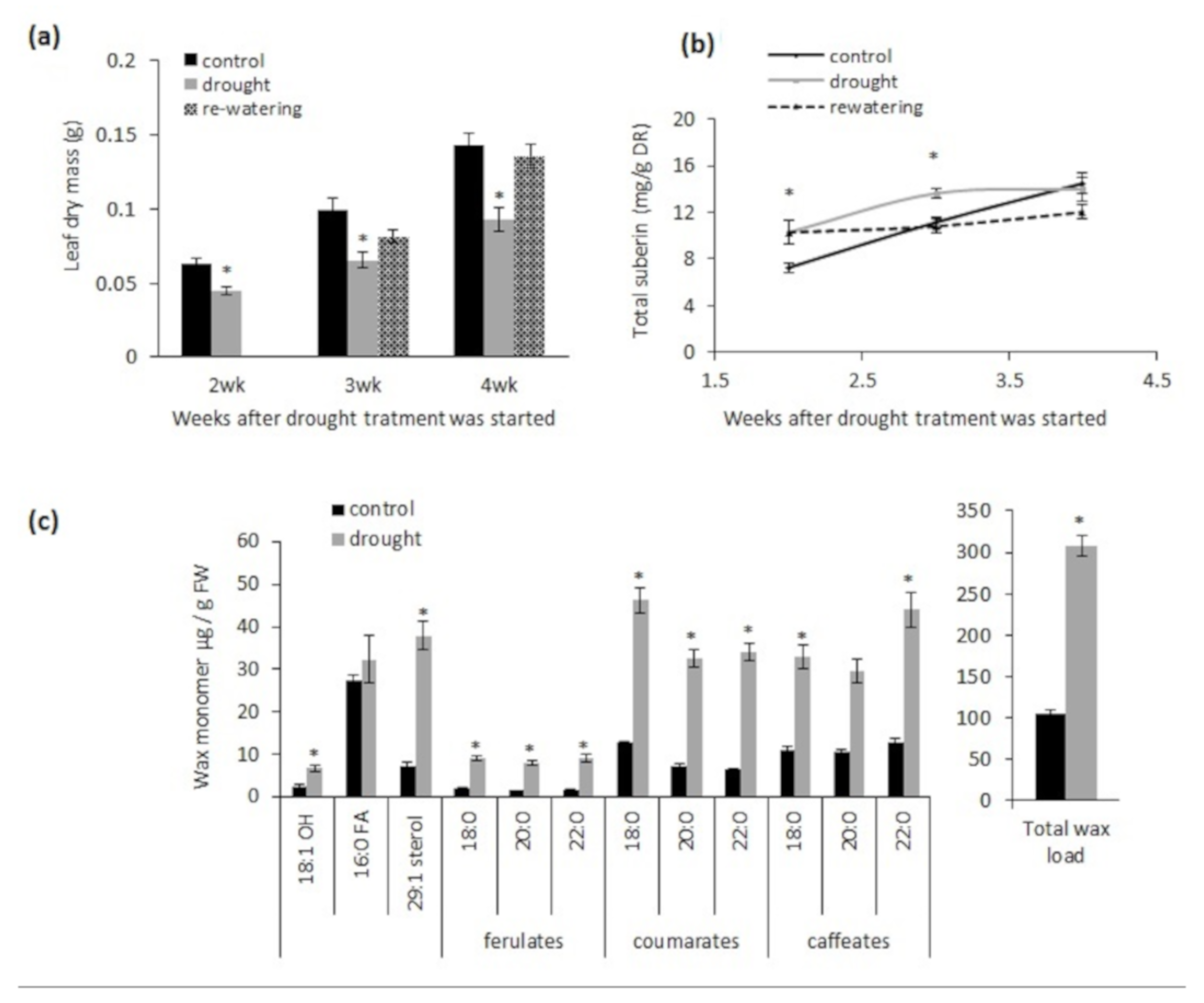
2.1. Chronic Drought Stress Induces Suberin and Suberin-Associated Waxes in Wild-Type Plants
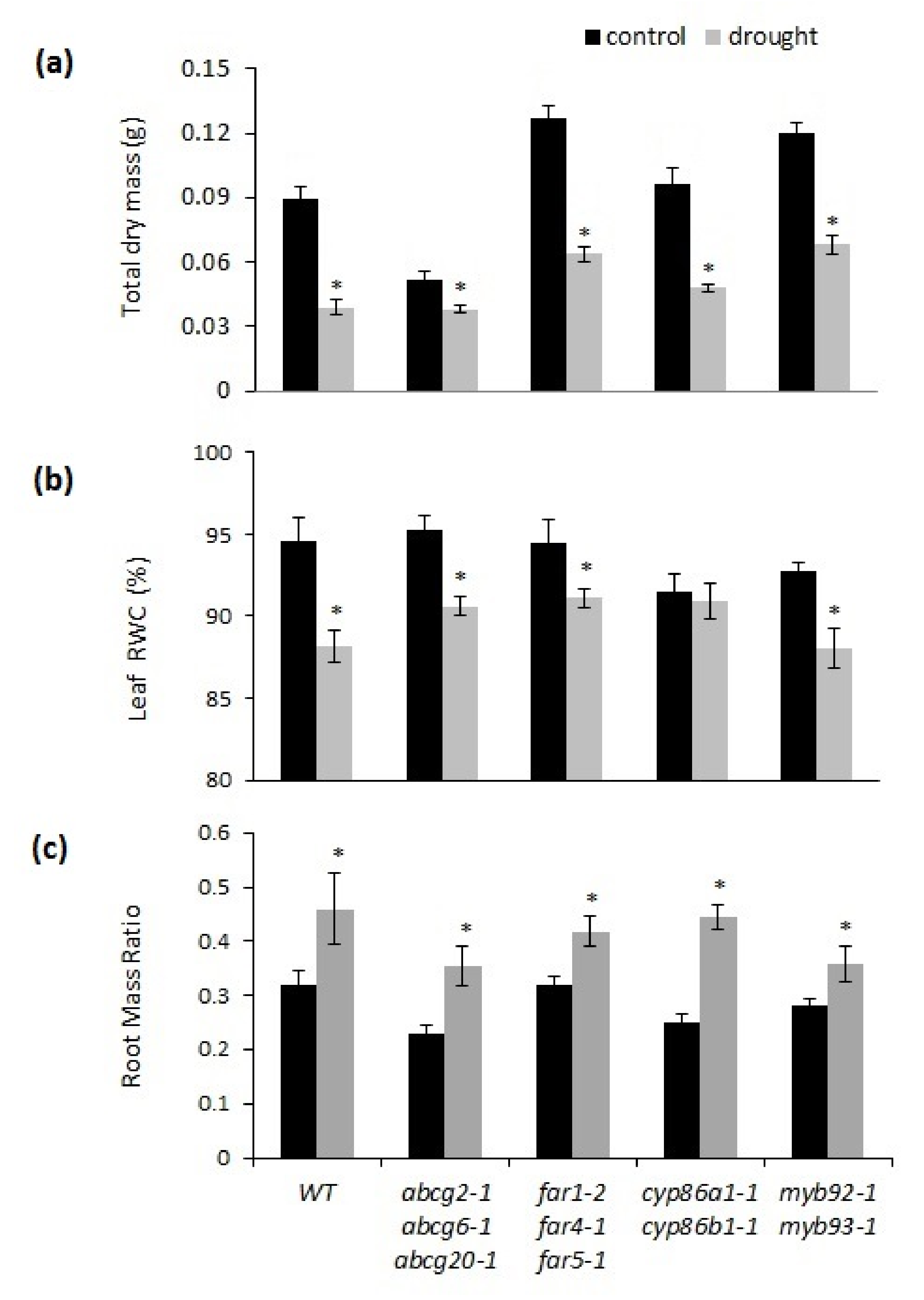
2.2. Chronic Drought Stress Has a Similar Effect on Suberin-Deficient Mutants versus Wild-Type Plants
2.3. Cyp86a1-1 cyp86b1-1 Has Reduced Total Suberin Content and Ultrastructural Defects
2.4. Chronic Drought Stress Increases Root Suberin Content but Does Not Alter Its Lamellae Structure
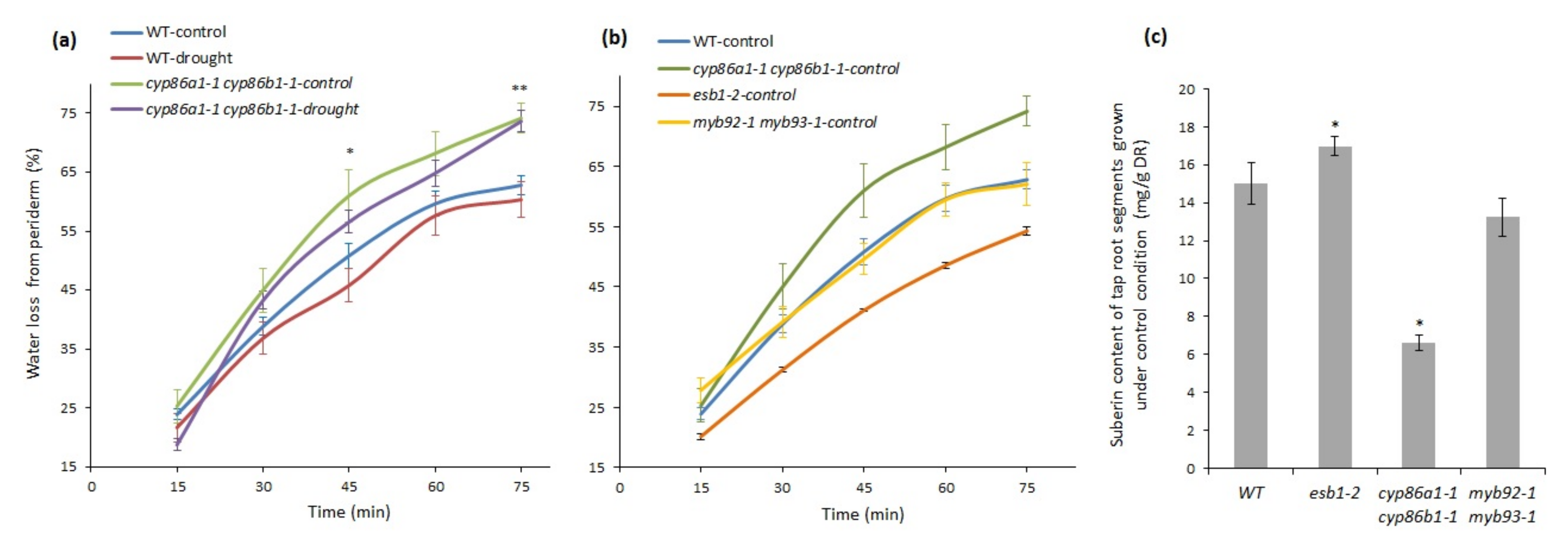
2.5. Suberin Deficiency and Aberrant Lamellae Structure Correlates with Increased Water Loss in Root Periderm
2.6. Salt Stress Induced Suberin Biosynthesis in Roots of Wild-Type Plants
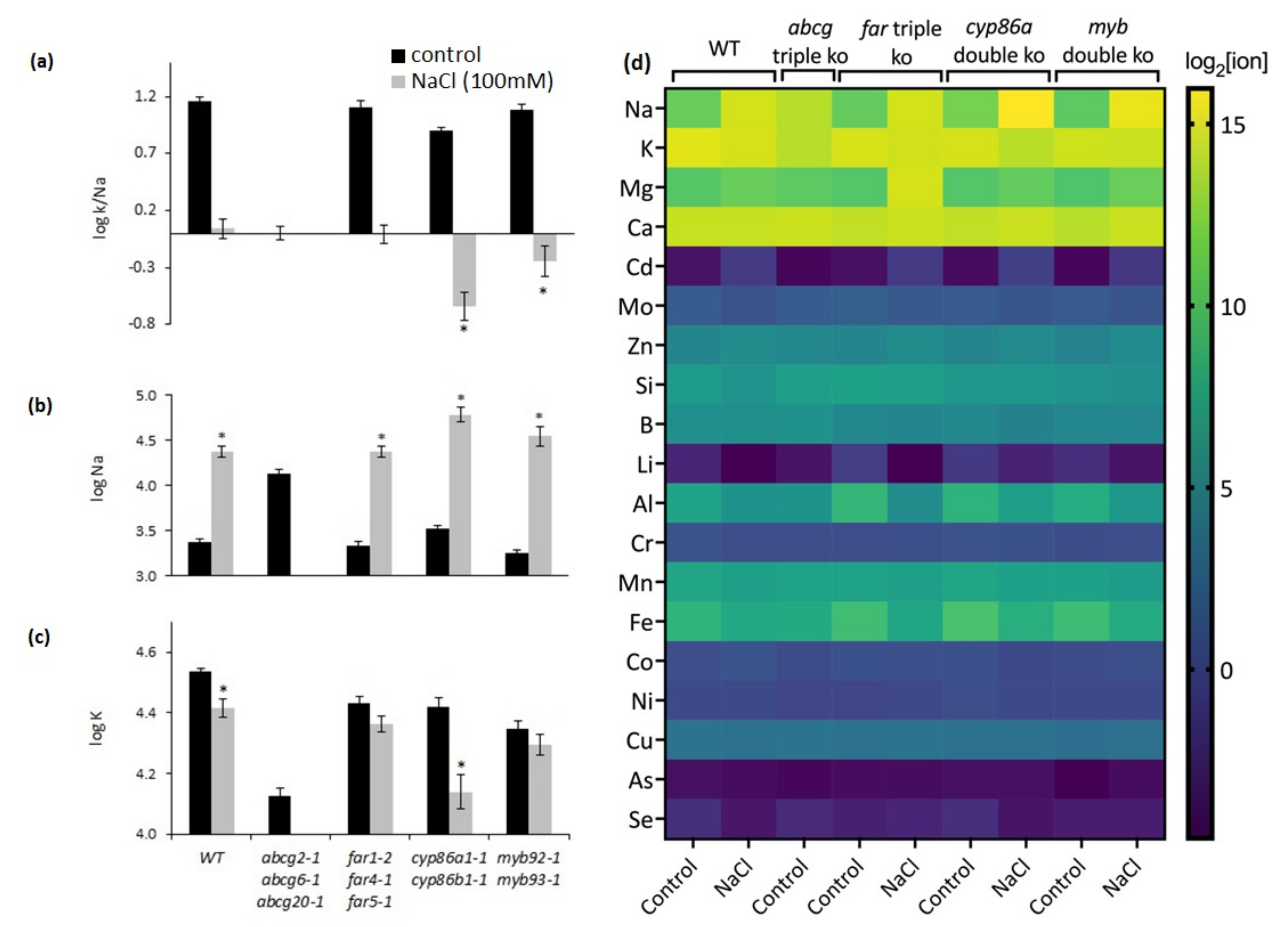
2.7. NaCl Treatment Causes Ion Imbalances in Mutants with Altered Suberin Composition and Structure
3. Discussion
3.1. Wild-Type Roots Show Plasticity in Suberin Production in Response to Different Water Levels
3.2. Root Suberin Modulates Water Loss through the Periderm
3.3. Mutants Defective in Suberin Composition and Lamellae Structure Show Higher Sensitivity to NaCl Treatment
4. Materials and Methods
4.1. Plant Growth Conditions and Drought and Salt Assays
4.2. Plant Trait Measurements
4.3. Root Wax Extraction and Analysis
4.4. Root Suberin Analysis
4.5. Transmission Electron Microscopy (TEM)
4.6. Measurement of Root Periderm Segments for Water Loss
4.7. Tissue Elemental Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cominelli, E.; Conti, L.; Tonelli, C.; Galbiati, M. Challenges and perspectives to improve crop drought and salinity tolerance. New Biotechnol. 2013, 30, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [Green Version]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, S.J.; Delude, C.; Domergue, F.; Rowland, O. Suberin: Biosynthesis, regulation, and polymer assembly of a protective extracellular barrier. Plant Cell Rep. 2015, 34, 573–586. [Google Scholar] [CrossRef]
- Bernards, M. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Briesen, I.; Wojciechowski, T.; Faust, A.; Yephremov, A.; Nawrath, C.; Schreiber, L. Apoplastic polyesters in Arabidopsis surface tissues—A typical suberin and a particular cutin. Phytochemistry 2005, 66, 2643–2658. [Google Scholar] [CrossRef] [Green Version]
- Kosma, D.K.; Molina, I.; Ohlrogge, J.B.; Pollard, M. Identification of an Arabidopsis fatty alcohol:caffeoyl-coenzyme A acyltransferase required for the synthesis of alkyl hydroxycinnamates in root waxes. Plant Physiol. 2012, 160, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Delude, C.; Fouillen, L.; Bhar, P.; Cardinal, M.J.; Pascal, S.; Santos, P.; Kosma, D.K.; Joubès, J.; Rowland, O.; Domergue, F. Primary fatty alcohols are major components of suberized root tissues of Arabidopsis in the form of alkyl hydroxycinnamates. Plant Physiol. 2016, 171, 1934–1950. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, S.J.; Kosma, D.K.; Pulsifer, I.P.; Scandola, S.; Pascal, S.; Joubes, J.; Dittrich-Domergue, F.; Lessire, R.; Rowland, O.; Domergue, F. Suberin-associated fatty alcohols in Arabidopsis thaliana: Distribution in roots and contributions to seed coat barrier properties. Plant Physiol. 2013, 163, 1118–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höfer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Wunderling, A.; Ripper, D.; Barra-Jimenez, A.; Mahn, S.; Sajak, K.; Targem, M.B.; Ragni, L. A molecular framework to study periderm formation in Arabidopsis. New Phytol. 2018, 219, 216–229. [Google Scholar] [CrossRef]
- Campilho, A.; Nieminen, K.; Ragni, L. The development of the periderm: The final frontier between a plant and its environment. Curr. Opin. Plant Biol. 2020, 53, 10–14. [Google Scholar] [CrossRef]
- North, G.B.; Nobel, P.S. Changes in root hydraulic conductivity for two tropical epiphytic cacti as soil-moisture varies. Am. J. Bot. 1994, 81, 46–53. [Google Scholar] [CrossRef]
- Kreszies, T.; Shellakkutti, N.; Osthoff, A.; Yu, P.; Baldauf, J.A.; Zeisler-Diehl, V.V.; Ranathunge, K.; Hochholdinger, F.; Schreiber, L. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: Analysis of chemical, transcriptomic and physiological responses. New Phytol. 2019, 221, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, P.; Ranathunge, K.; Franke, R.; Prakash, H.S.; Schreiber, L.; Mathew, M.K. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 2009, 230, 119–134. [Google Scholar] [CrossRef]
- Kotula, L.; Ranathunge, K.; Schreiber, L.; Steudle, E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J. Exp. Bot. 2009, 60, 2155–2167. [Google Scholar] [CrossRef]
- Ranathunge, K.; Schreiber, L.; Bi, Y.M.; Rothstein, S.J. Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 2016, 243, 231–249. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ranathunge, K.; Nayak, S.; Schreiber, L.; Mathew, M.K. Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 4215–4228. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Jyothi-Prakash, P.A.; Qin, L.; He, J.; Lin, Q.; Loh, C.S.; Kumar, P.P. Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ. 2014, 37, 1656–1671. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishiuchi, S.; Kulichikhin, K.; Nakazono, M. Does suberin accumulation in plant roots contribute to waterlogging tolerance? Front. Plant Sci. 2013, 4, 178. [Google Scholar] [CrossRef] [Green Version]
- Enstone, D.E.; Peterson, C.A. Suberin lamella development in maize seedling roots grown in aerated and stagnant conditions. Plant Cell Environ. 2005, 28, 444–455. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Hartmann, K. Effects of NO3 deficiency and NaCl stress on suberin deposition in rhizo-and hypodermal (RHCW) and endodermal cell walls (ECW) of castor bean (Ricinus communis L.) roots. Plant Soil 2005, 269, 333–339. [Google Scholar] [CrossRef]
- Barberon, M.; Vermeer, J.E.M.; De Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E.; et al. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 2016, 164, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Franke, R.; Hofer, R.; Briesen, I.; Emsermann, M.; Efremova, N.; Yephremov, A.; Schreiber, L. The DAISY gene from Arabidopsis encodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds. Plant J. 2009, 57, 80–95. [Google Scholar] [CrossRef]
- Ranathunge, K.; Schreiber, L. Water and solute permeability of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J. Exp. Bot. 2011, 62, 1961–1974. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.M.; Gao, L.; Cui, Y.N.; Yang, H.L.; de Silva, N.D.G.; Ma, Q.; Bao, A.K.; Flowers, T.J.; Rowland, O.; et al. Aliphatic suberin confers salt tolerance to Arabidopsis by limiting Na+ influx, K+ efflux and water backflow. Plant Soil 2020, 448, 603–620. [Google Scholar] [CrossRef]
- Baxter, I.; Hosmani, P.S.; Rus, A.; Lahner, B.; Borevitz, J.O.; Muthukumar, B.; Mickelbart, M.V.; Schreiber, L.; Franke, R.B.; Salt, D.E. Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet. 2009, 5, e100492. [Google Scholar] [CrossRef] [Green Version]
- Ursache, R.; De Jesus Vieira Teixeira, C.; Tendon, V.D.; Gully, K.; De Bellis, D.; Schmid-Siegert, E.; Andersen, T.G.; Shekhar, V.; Calderon, S.; Pradervand, S.; et al. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 2021, 7, 353–364. [Google Scholar] [CrossRef]
- Shukla, V.; Han, J.-P.; Cléard, F.; Lefebvre-Legendre, L.; Gully, K.; Flis, P.; Berhin, A.; Andersen, T.G.; Salt, D.E.; Nawrath, C.; et al. Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101730118. [Google Scholar] [CrossRef]
- Zhang, L.; Merlin, I.; Pascal, S.; Bert, P.F.; Domergue, F.; Gambetta, G.A. Drought activates MYB41 orthologs and induces suberization of grapevine fine roots. Plant Direct 2020, 4, e00278. [Google Scholar] [CrossRef]
- Yadav, V.; Molina, I.; Ranathunge, K.; Castillo, I.Q.; Rothstein, S.J.; Reed, J.W. ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 2014, 26, 3569–3588. [Google Scholar] [CrossRef] [Green Version]
- Compagnon, V.; Diehl, P.; Benveniste, I.; Meyer, D.; Schaller, H.; Schreiber, K.; Franke, R.; Pinot, F. CYP86B1 is required for very long chain ω-hydroxyacid and α,ω dicarboxylic acid synthesis in root and seed suberin polyester. Plant Physiol. 2009, 150, 1831–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legay, S.; Guerriero, G.; André, C.; Guignard, C.; Cocco, E.; Charton, S.; Boutry, M.; Rowland, O.; Hausman, J.F. MdMyb93 is a regulator of suberin deposition in russeted apple fruit skins. New Phytol. 2016, 212, 977–991. [Google Scholar] [CrossRef]
- To, A.; Joubès, J.; Thueux, J.; Kazaz, S.; Lepiniec, L.; Baud, S. AtMYB92 enhances fatty acid synthesis and suberin deposition in leaves of Nicotiana benthamiana. Plant J. 2020, 103, 660–676. [Google Scholar] [CrossRef]
- Blum, A. Genomics for drought resistance-getting down to earth. Funct. Plant Biol. 2014, 41, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Hosmani, P.S.; Kamiya, T.; Danku, J.; Naseer, S.; Geldner, N.; Guerinot, M.L.; Salt, D.E. Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc. Natl. Acad. Sci. USA 2013, 110, 14498–14503. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, J.I.; Kwak, J.M.; Allen, G.J. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 2001, 410, 327–330. [Google Scholar] [CrossRef]
- Soliday, C.L.; Kolattukudy, P.E.; David, R.W. Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.). Planta 1979, 146, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Lens, F.; Tixier, A.; Cochard, H.; Sperry, J.S.; Jansen, S.; Herbette, S. Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr. Opin. Plant Biol. 2013, 16, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Kreszies, T.; Schreiber, L.; Ranathunge, K. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J. Plant Physiol. 2018, 227, 75–83. [Google Scholar] [CrossRef]
- Kosma, D.K.; Murmu, J.; Razeq, F.M.; Santos, P.; Bourgault, R.; Molina, I.; Rowland, O. AtMYB41 activates ectopic suberin synthesis and assembly in multiple plant species and cell types. Plant J. 2014, 80, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Andersen, T.G.; Molina, D.; Kilian, J.; Franke, R.B.; Ragni, L.; Geldner, N. Tissue-autonomous phenylpropanoid production is essential for establishment of root barriers. Curr. Biol. 2021, 31, 965–977. [Google Scholar] [CrossRef]
- Li, B.; Kamiya, T.; Kalmbach, L.; Yamagami, M.; Yamagichi, K.; Shigenobu, S.; Sawa, S.; Danku, J.M.C.; Salt, D.E.; Geldner, N.; et al. Role of LOTR1 in nutrient transport through organization of spatial distribution of root endodermal barriers. Curr. Biol. 2017, 27, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Calvo-Polanco, M.; Reyt, G.; Barberon, M.; Champeyroux, C.; Santoni, V.; Maurel, C.; Franke, R.B.; Ljung, K.; Novak, O.; et al. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci. Rep. 2019, 9, 4227. [Google Scholar] [CrossRef] [Green Version]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H.; Zhou, M.X.; Mendham, N.; Newman, I.A.; Zhang, G.P.; Shabala, S. Potassium and sodium relations in salinized barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007, 34, 150–162. [Google Scholar] [CrossRef]
- Nassery, H. Salt-induced loss of potassium from plant roots. New Phytol. 1979, 83, 23–27. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britto, D.T.; Ebrahimi-Ardebili, S.; Hamam, A.M.; Coskun, D.; Kronzucker, H.J. 42K analysis of sodium-induced potassium efflux in barley: Mechanism and relevance to salt tolerance. New Phytol. 2010, 186, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Ryser, P.; Bernardi, J.; Merla, A. Determination of leaf fresh mass after storage between moist paper towels: Constraints and reliability of the method. J. Exp. Bot. 2008, 59, 2461–2467. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C. Techniques and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Kosma, D.K.; Rice, A.; Pollard, M. Analysis of aliphatic waxes associated with root periderm or exodermis from eleven plant species. Phytochemistry 2015, 117, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Molina, I.; Bonaventure, G.; Ohlrogge, J.; Pollard, M. The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 2006, 67, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Lévesque-Lemay, M.; Chabot, D.; Hubbard, K.; Chan, J.K.; Miller, S.; Robert, L.S. Tapetal oleosins play an essential role in tapetosome formation and protein relocation to the pollen coat. New Phytol. 2016, 209, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Russin, W.; Trivett, C. Vacuum-microwave combination for processing plant tissues for electron microscopy. In Microwave Techniques and Protocols; Giberson, R.T., Demaree, R.S., Eds.; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Molina, I.; Li-Beisson, Y.; Beisson, F.; Ohlrogge, J.B.; Pollard, M. Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol. 2009, 151, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.; Lv, T.; Shen, H. Regulation of drought tolerance by the F-Box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Silva, N.D.G.; Murmu, J.; Chabot, D.; Hubbard, K.; Ryser, P.; Molina, I.; Rowland, O. Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana. Metabolites 2021, 11, 735. https://doi.org/10.3390/metabo11110735
de Silva NDG, Murmu J, Chabot D, Hubbard K, Ryser P, Molina I, Rowland O. Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana. Metabolites. 2021; 11(11):735. https://doi.org/10.3390/metabo11110735
Chicago/Turabian Stylede Silva, Nayana D. G., Jhadeswar Murmu, Denise Chabot, Keith Hubbard, Peter Ryser, Isabel Molina, and Owen Rowland. 2021. "Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana" Metabolites 11, no. 11: 735. https://doi.org/10.3390/metabo11110735
APA Stylede Silva, N. D. G., Murmu, J., Chabot, D., Hubbard, K., Ryser, P., Molina, I., & Rowland, O. (2021). Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana. Metabolites, 11(11), 735. https://doi.org/10.3390/metabo11110735





