Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of the SPE Protocol
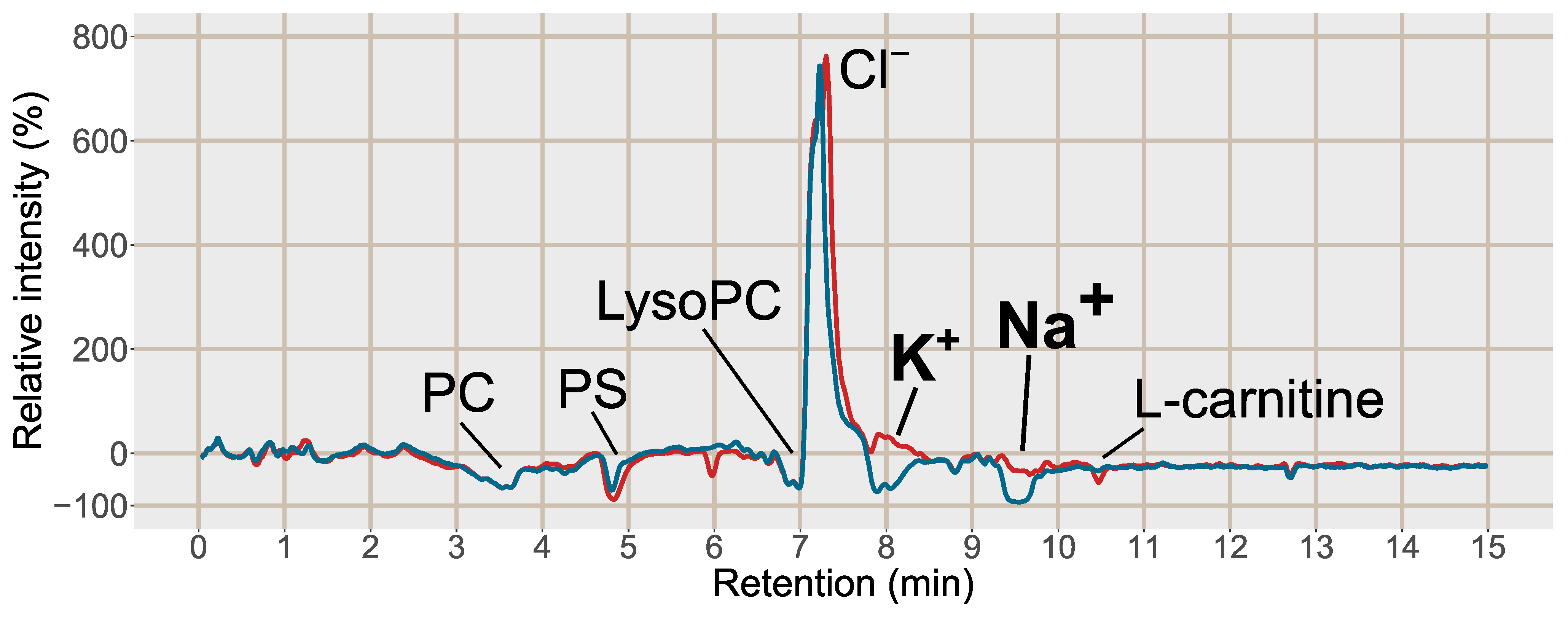
2.2. Evaluation of the Final SPE Protocol in Plasma Samples
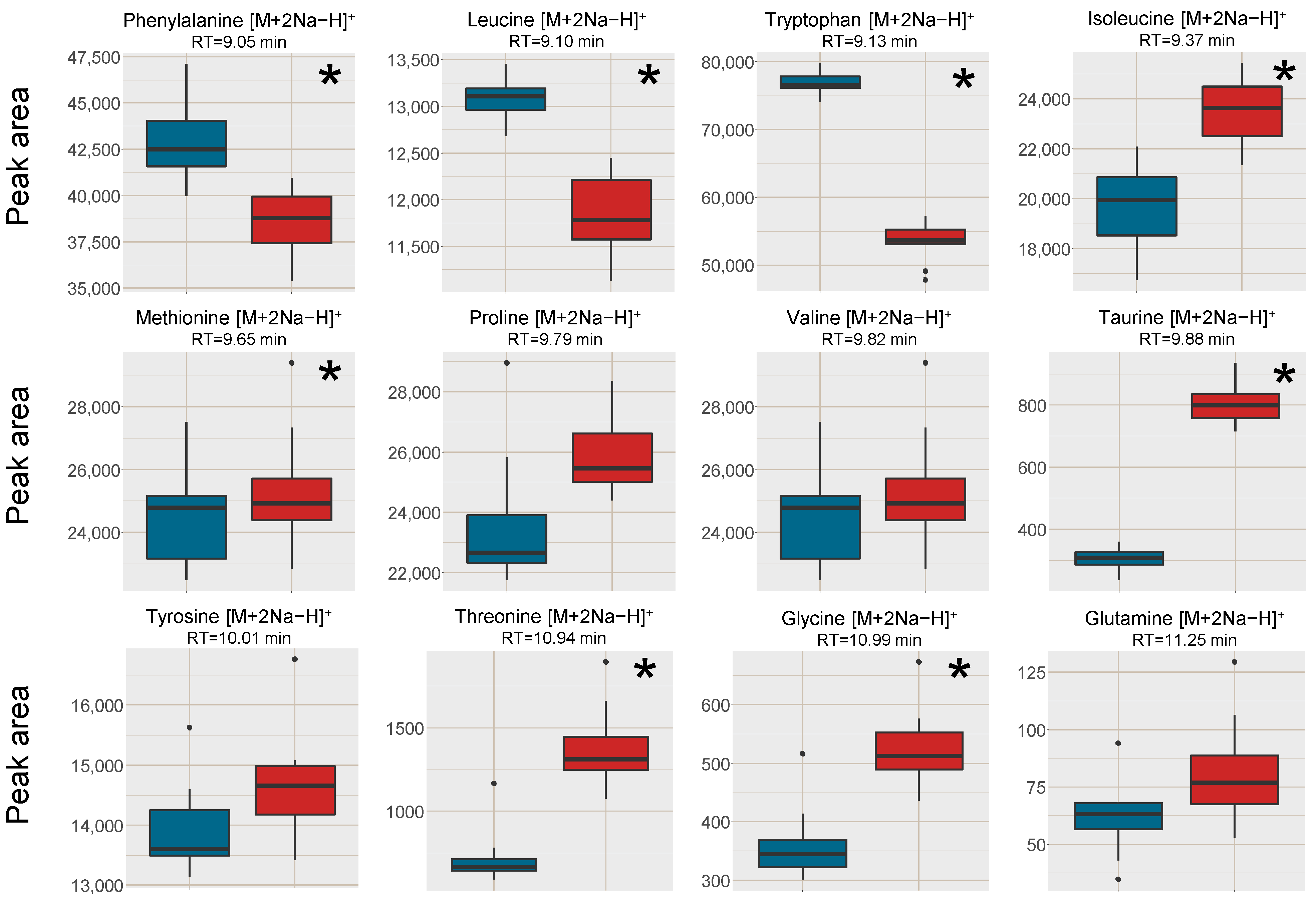
2.2.1. Improved Sensitivity of Hydrophilic Analytes by Reduction in K+ and Na+ Concentrations through SPE
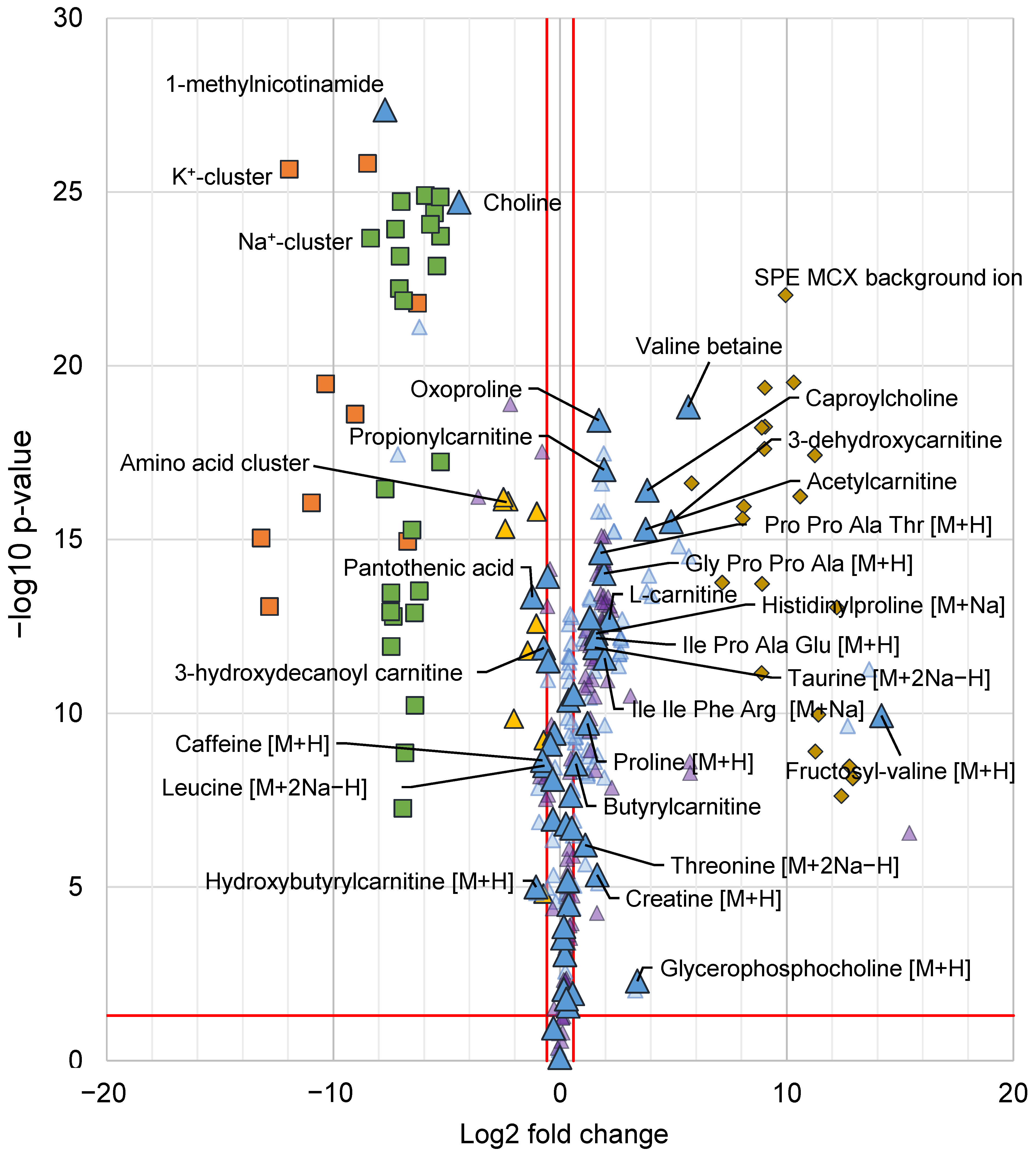
2.2.2. Decreased Adduct and Cluster Formation Due to Reduced Na+ and K+ Concentrations
2.2.3. Multivariate Data Analysis
2.2.4. Linear Response of Spiked Analytes Were Improved
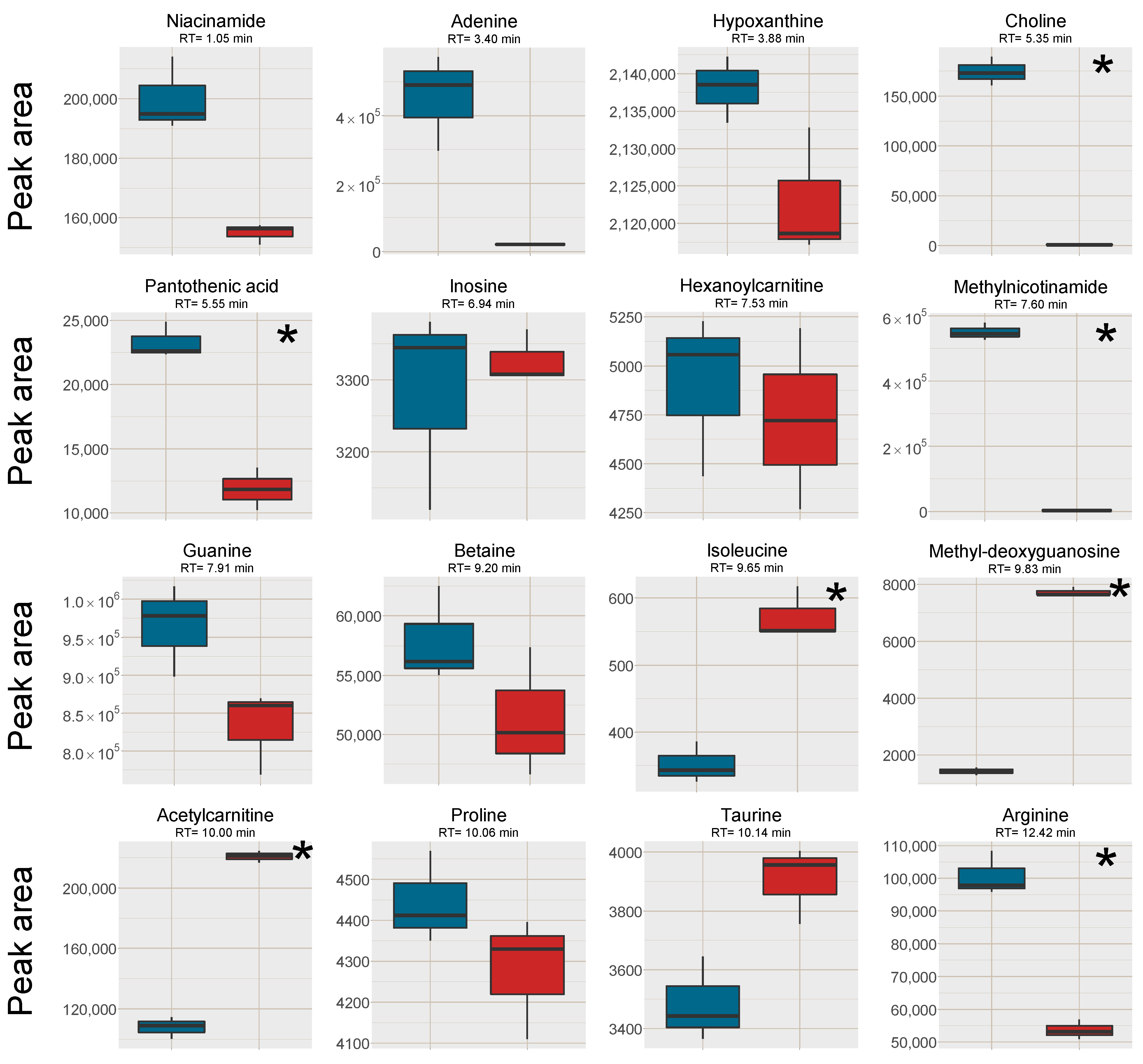
2.3. Cell Samples
3. Material and Methods
3.1. Chemicals
3.2. SPE on Standard Solutios
3.3. Final SPE Method and Preparation of Plasma Samples
3.4. Post-Column Infusion Experiments
3.5. Determination of Linearity
3.6. Preparation of Cell Samples
3.7. HILIC-QTOF-ESI-MS Analysis
3.8. Data Analysis and Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| SCX-2 | MCX | StrataX-C | WCX Acidic | WCX Basic | Standard Solution | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rel. int. (%) 1 | CV (%) | Rel. int. (%) 1 | CV (%) | Rel. int. (%) 1 | CV (%) | Rel. int. (%) 1 | CV (%) | Rel. int. (%) 1 | CV (%) | CV (%) | |
| Butyrylcarnitine | 88.9 | 4.56 | 101 | 3.22 | 59.9 | 14.0 | 119 | 19.0 | 157 | 34.52 | 16.7 |
| Hypoxanthine | 94.2 | 6.77 | 102 | 4.86 | 101 | 28.5 | 50.9 | 15.3 | 185 | 40.56 | 51.0 |
| Choline | 0.070 | 43.9 | 0.800 | 44.2 | 0.080 | 19.1 | 44.6 | 11.1 | 123 | 49.35 | 34.0 |
| Thiamine [F] 2 | 0.210 | 4.28 | 1.10 | 12.9 | 0.040 | 31.2 | 40.3 | 3.43 | 2.98 | 21.15 | 11.4 |
| Adenine | 95.3 | 13.7 | 106 | 5.42 | 90.7 | 18.7 | 70.8 | 6.76 | 164 | 66.83 | 40.7 |
| Betaine | 120 | 8.65 | 132 | 5.39 | 65.7 | 19.2 | 39.1 | 4.73 | 219 | 10.11 | 55.9 |
| Phenylalanine [F] 2 | 165 | 13.5 | 176 | 22.3 | 87.8 | 29.7 | 45.0 | 31.7 | 444 | 17.48 | 44.3 |
| Tryptophan [F] 2 | 119 | 13.6 | 161 | 4.68 | 118 | 27.3 | 45.3 | 10.1 | 353 | 34.54 | 49.4 |
| Acetylcarnitine | 114 | 6.92 | 192 | 4.98 | 151 | 41.2 | 137 | 8.42 | 297 | 40.62 | 12.0 |
| Proline | 336 | 4.14 | 537 | 4.65 | 296 | 27.5 | 62.0 | 12.4 | 1278 | 57.03 | 17.3 |
| Taurine | 329 | 20.0 | 383 | 32.3 | 311 | 34.1 | 18.7 | 24.9 | 1467 | 51.0 | 43.5 |
| Carnitine | 105 | 22.4 | 119 | 7.90 | 103 | 33.6 | 58.2 | 9.47 | 144 | 35.5 | 34.5 |
| Creatine | 92.5 | 15.3 | 119 | 14.3 | 39.0 | 44.1 | 9.62 | 14.2 | 269 | 54.9 | 106 |
| Arginine | 0.180 | 14.1 | 11.6 | 12.3 | 0.820 | 61.9 | 0.540 | 4.81 | 184 | 70.3 | 206 |
| NADH | 13.1 | 5.82 | 53.6 | 3.3 | 185 | 14.5 | 0.350 | 5.28 | 636 | 26.5 | 216 |
| Sodium, Na+ | 2.53 | 14.0 | 4.29 | 11.0 | 1.81 | 50.2 | 18.0 | 7.58 | 17.5 | 59.8 | 76.8 |
| SPE MCX No pH Control | SPE MCX pH Control | WCX Basic No pH Control | WCX Basic pH Control | PPT | |||||
|---|---|---|---|---|---|---|---|---|---|
| Rel. int. (%) 1 | CV % | Rel. int. (%) 1 | CV % | Rel. int. (%) 1 | CV % | Rel. int. (%) 1 | CV % | CV % | |
| Caffeine 0.89 min | 75.06 | 60.24 | 83.65 | 26.55 | 75.79 | 39.91 | 83.98 | 29.66 | 5.58 |
| Theophylline 1.21 min | 62.51 | 5.47 | 71.66 | 14.79 | 91.01 | 5.60 | 96.13 | 4.82 | 4.47 |
| Hypoxanthine 4.39 min | 113.06 | 3.30 | 72.36 | 15.81 | 112.73 | 2.85 | 117.88 | 10.31 | 6.13 |
| Creatinine 4.69 min | 118.92 | 5.50 | 71.64 | 23.31 | 119.93 | 5.19 | 130.80 | 14.93 | 8.21 |
| Palmitoylcarnitine 5.09 min | 96.37 | 9.51 | 73.63 | 17.11 | 95.10 | 0.92 | 155.56 | 17.19 | 2.81 |
| Choline 6.01 min | 1.74 | 3.17 | 5.59 | 28.18 | 11.43 | 5.48 | |||
| Pantothenic acid 2 7.35 min | 95.41 | 3.35 | 67.88 | 3.15 | 97.59 | 5.04 | 64.48 | 11.43 | 2.12 |
| Hexanoylcarnitine 7.8 min | 95.89 | 42.41 | 92.61 | 28.38 | 102.74 | 7.84 | 131.01 | 19.49 | 4.69 |
| 1-Methylnicotinamide 8.02 min | 0.00 | 4.15 | 0.00 | 22.91 | 0.00 | 6.68 | 12.48 | 4.92 | 7.58 |
| Butyrylcarnitine 9.08 min | 273.41 | 1.17 | 77.49 | 20.43 | 278.09 | 9.84 | 83.05 | 13.50 | 12.63 |
| Oxoproline 9.13 min | 311.67 | 15.80 | 110.16 | 38.51 | 357.32 | 21.98 | 77.07 | 22.53 | 11.54 |
| Homostachydrine 9.41 min | 100.71 | 38.70 | 65.39 | 7.51 | 115.96 | 96.24 | 52.53 | 87.20 | 21.20 |
| Propionylcarnitine 9.78 min | 210.52 | 7.09 | 94.03 | 18.04 | 243.53 | 4.66 | 71.86 | 3.49 | 16.65 |
| Betaine 9.83 min | 108.60 | 6.86 | 79.86 | 14.09 | 112.69 | 13.49 | 71.19 | 2.09 | 16.92 |
| Methylnicotinic acid 10.16 min | 181.86 | 4.16 | 80.55 | 14.61 | 112.79 | 12.95 | 46.43 | 8.46 | 9.85 |
| Acetylcarnitine 10.47 min | 604.31 | 3.36 | 277.47 | 14.83 | 784.74 | 5.22 | 139.35 | 10.22 | 11.28 |
| Acetylcholine 10.78 min | 2279.60 | 5.52 | 247.01 | 15.20 | 1183.32 | 8.53 | 142.26 | 20.24 | 12.07 |
| Carnitine 10.89 min | 279.17 | 1.59 | 122.97 | 9.92 | 281.05 | 10.11 | 73.42 | 2.48 | 3.67 |
| Creatine 11.07 min | 181.63 | 10.00 | 79.86 | 12.13 | 178.08 | 8.59 | 42.96 | 15.11 | 3.10 |
| Arginine 12.5 min | 52.15 | 2.95 | 28.85 | 9.38 | 39.26 | 16.09 | 22.32 | 20.15 | 3.68 |
References
- Schug, K.; McNair, H.M. Adduct formation in electrospray ionization mass spectrometry: II. Benzoic acid derivatives. J. Chromatogr. A 2003, 985, 531–539. [Google Scholar] [CrossRef]
- Ekdahl, A.; Johansson, M.C.; Ahnoff, M. Tracing and separating plasma components causing matrix effects in hydrophilic interaction chromatography–electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 923–924, 83–91. [Google Scholar] [CrossRef]
- Haglind, A.; Hedeland, M.; Arvidsson, T.; Pettersson, C.E. Major signal suppression from metal ion clusters in SFC/ESI-MS—Cause and effects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1084, 96–105. [Google Scholar] [CrossRef]
- Svan, A.; Hedeland, M.; Arvidsson, T.; Pettersson, C.E. The differences in matrix effect between supercritical fluid chromatography and reversed phase liquid chromatography coupled to ESI/MS. Anal. Chim. Acta 2018, 1000, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Elmsjö, A.; Haglöf, J.; Engskog, M.K.R.; Erngren, I.; Nestor, M.; Arvidsson, T.; Pettersson, C. Method selectivity evaluation using the co-feature ratio in LC/MS metabolomics: Comparison of HILIC stationary phase performance for the analysis of plasma, urine and cell extracts. J. Chromatogr. A 2018, 1568, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mortier, K.A.; Zhang, G.F.; Van Peteghem, C.H.; Lambert, W.E. Adduct formation in quantitative bioanalysis: Effect of ionization conditions on paclitaxel. J. Am. Soc. Mass Spectrom. 2004, 15, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Erngren, I.; Haglöf, J.; Engskog, M.K.R.; Nestor, M.; Hedeland, M.; Arvidsson, T.; Pettersson, C. Adduct formation in electrospray ionisation-mass spectrometry with hydrophilic interaction liquid chromatography is strongly affected by the inorganic ion concentration of the samples. J. Chromatogr. A 2019, 1600, 174–185. [Google Scholar] [CrossRef]
- Risley, D.S.; Pack, B.W. Simultaneous determination of positive and negative counterions using a hydrophilic interaction chromatography method. LCGC North Am. 2006, 24, 82–90. [Google Scholar]
- Huang, Z.; Richards, M.A.; Zha, Y.; Francis, R.; Lozano, R.; Ruan, J. Determination of inorganic pharmaceutical counterions using hydrophilic interaction chromatography coupled with a Corona® CAD detector. J. Pharm. Biomed. Anal. 2009, 50, 809–814. [Google Scholar] [CrossRef]
- McMillan, A.; Renaud, J.B.; Gloor, G.B.; Reid, G.; Sumarah, M.W. Post-acquisition filtering of salt cluster artefacts for LC-MS based human metabolomic studies. J. Cheminform. 2016, 8, 1–5. [Google Scholar] [CrossRef]
- Johnson, W.M.; Kido Soule, M.C.; Kujawinski, E.B. Extraction efficiency and quantification of dissolved metabolites in targeted marine metabolomics. Limnol. Oceanogr. Methods 2017, 15, 417–428. [Google Scholar] [CrossRef]
- Drouin, N.; Rudaz, S.; Schappler, J. Sample preparation for polar metabolites in bioanalysis. Analyst 2018, 143, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Fujita, N.; Horie, R.; Saito, K.; Tawaraya, K. Solid-phase extraction for metabolomic analysis of high-salinity samples by capillary electrophoresis-mass spectrometry. J. Sep. Sci. 2011, 34, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Whiley, L.; Chekmeneva, E.; Berry, D.J.; Jiménez, B.; Yuen, A.H.Y.; Salam, A.; Hussain, H.; Witt, M.; Takats, Z.; Nicholson, J.; et al. Systematic Isolation and Structure Elucidation of Urinary Metabolites Optimized for the Analytical-Scale Molecular Profiling Laboratory. Anal. Chem. 2019, 91, 8873–8882. [Google Scholar] [CrossRef]
- Vardal, L.; Gjelstad, A.; Huang, C.; Oiestad, E.L.; Pedersen-Bjergaard, S. Efficient discrimination and removal of phospholipids during electromembrane extraction from human plasma samples. Bioanalysis 2017, 9, 631–641. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Huang, C.; Gjelstad, A. Electromembrane extraction–Recent trends and where to go. J. Pharm. Anal. 2017, 7, 141–147. [Google Scholar] [CrossRef]
- Strieglerová, L.; Kubáň, P.; Boček, P. Electromembrane extraction of amino acids from body fluids followed by capillary electrophoresis with capacitively coupled contactless conductivity detection. J. Chromatogr. A 2011, 1218, 6248–6255. [Google Scholar] [CrossRef]
- Schnellbaecher, A.; Binder, D.; Bellmaine, S.; Zimmer, A. Vitamins in cell culture media: Stability and stabilization strategies. Biotechnol. Bioeng. 2019, 116, 1537–1555. [Google Scholar] [CrossRef]
- Bonfiglio, R.; King, R.C.; Olah, T.V.; Merkle, K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 1999, 13, 1175–1185. [Google Scholar] [CrossRef]
- Michopoulos, F.; Gika, H.; Palachanis, D.; Theodoridis, G.; Wilson, I.D. Solid phase extraction methodology for UPLC-MS based metabolic profiling of urine samples. Electrophoresis 2015, 36, 2170–2178. [Google Scholar] [CrossRef]
- Viant, M.R. Revealing the Metabolome of Animal Tissues Using 1H Nuclear Magnetic Resonance Spectroscopy. Methods Mol. Biol. 2007, 358, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Huang, W.; Collette, T.W.; Ekman, D.R.; Tan, C. A direct cell quenching method for cell-culture based metabolomics. Metabolomics 2009, 5, 199–208. [Google Scholar] [CrossRef]
- Brenner, J.C.; Graham, M.P.; Kumar, B.; Saunders, L.M.; Kupfer, R.; Lyons, R.H.; Bradford, C.R.; Carey, T.E. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck 2009, 5, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, E.L.; Erngren, I.; Engskog, M.; Haglöf, J.; Arvidsson, T.; Hedeland, M.; Petterson, C.; Laurell, G.; Nestor, M. Exploring radiation response in two head and neck squamous carcinoma cell lines through metabolic profiling. Front. Oncol. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Engskog, M.K.R.; Haglöf, J.; Arvidsson, T.; Pettersson, C. LC–MS based global metabolite profiling: The necessity of high data quality. Metabolomics 2016, 12, 114. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. LC/MS preprocessing and analysis with xcms. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- Benton, H.P.; Want, E.J.; Ebbels, T.M.D. Correction of mass calibration gaps in liquid chromatography–mass spectrometry metabolomics data. Bioinformatics 2010, 26, 2488–2489. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Böttcher, C.; Neumann, S. Highly sensitive feature detection for high resolution LC/MS. BMC Bioinf. 2008, 9, 504. [Google Scholar] [CrossRef] [PubMed]



| Analyte | Concentration Range of Calibration Curve | Expected Concentration in Blood Plasma | R2 | No. of Standards within ±15% of Nominal Concentration | Concentration Range of “Passed” Standards | |||
|---|---|---|---|---|---|---|---|---|
| PPT | MCX | PPT | MCX | PPT | MCX | |||
| Guanine [M+H]+ | 18 nM–1.9 µM | 0.4 µM | 0.98750 | 0.99475 | 4 | 7 | (0.18–1.9 µM) * | 30 nM–1.9 µM |
| Inosine [F]+ | 79 nM–5.4 µM | 0.2–0.3 µM | 0.98940 | 0.99510 | 7 | 5 | 0.12–5.4 µM | 0.28–5.4 µM |
| Tryptophan [F]+ | 1.1 µM–110 µM | 50–60 µM | 0.99707 | 0. 99396 | 7 | 7 | 2.2–110 µM | 2.2–110 µM |
| Valine [M+H]+ | 77 µM–309 µM | 200–250 µM | 0.60632 | 0.85888 | 2 | 6 | (206–309 µM) * | 103–309 µM |
| Tryptophan [M+2Na−H]+ | 1.1 µM–110 µM | 50–60 µM | 0.98341 | 0.98519 | 4 | 5 | (11–110 µM) * | 11–110 µM |
| Valine [M+2Na−H]+ | 77 µM–309 µM | 200–250 µM | 0.96423 | 0.95860 | 8 | 8 | 77–309 µM | 77–309 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erngren, I.; Nestor, M.; Pettersson, C.; Hedeland, M. Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples. Metabolites 2021, 11, 170. https://doi.org/10.3390/metabo11030170
Erngren I, Nestor M, Pettersson C, Hedeland M. Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples. Metabolites. 2021; 11(3):170. https://doi.org/10.3390/metabo11030170
Chicago/Turabian StyleErngren, Ida, Marika Nestor, Curt Pettersson, and Mikael Hedeland. 2021. "Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples" Metabolites 11, no. 3: 170. https://doi.org/10.3390/metabo11030170
APA StyleErngren, I., Nestor, M., Pettersson, C., & Hedeland, M. (2021). Improved Sensitivity in Hydrophilic Interaction Liquid Chromatography-Electrospray-Mass Spectrometry after Removal of Sodium and Potassium Ions from Biological Samples. Metabolites, 11(3), 170. https://doi.org/10.3390/metabo11030170







