Rapid Evaporative Ionization Mass Spectrometry: A Review on Its Application to the Red Meat Industry with an Australian Context
Abstract
1. Introduction
2. What Is REIMS?
2.1. Compounds Amenable to REIMS Analysis
2.2. Data Analysis/Chemometric Approaches
2.3. Comparison of REIMS to Other Ambient Mass Ionisation-Spectroscopy (AMS) Techniques
3. Provenance
3.1. Meat Products
3.2. Fish
3.3. Botanicals
4. Quality
4.1. Flavour and Sensory Characteristics
4.2. Breed
4.3. Long Aged Shelf-Life
4.4. Nutrition and Essential Fatty Acids (Vitamin E and ω-3)
4.5. Oxidation and Antioxidants
4.6. Tenderness
4.7. Meat Colour, pH and Water Holding Capacity
5. Safety
5.1. REIMS Applications in Bacterial and Yeast Speciation

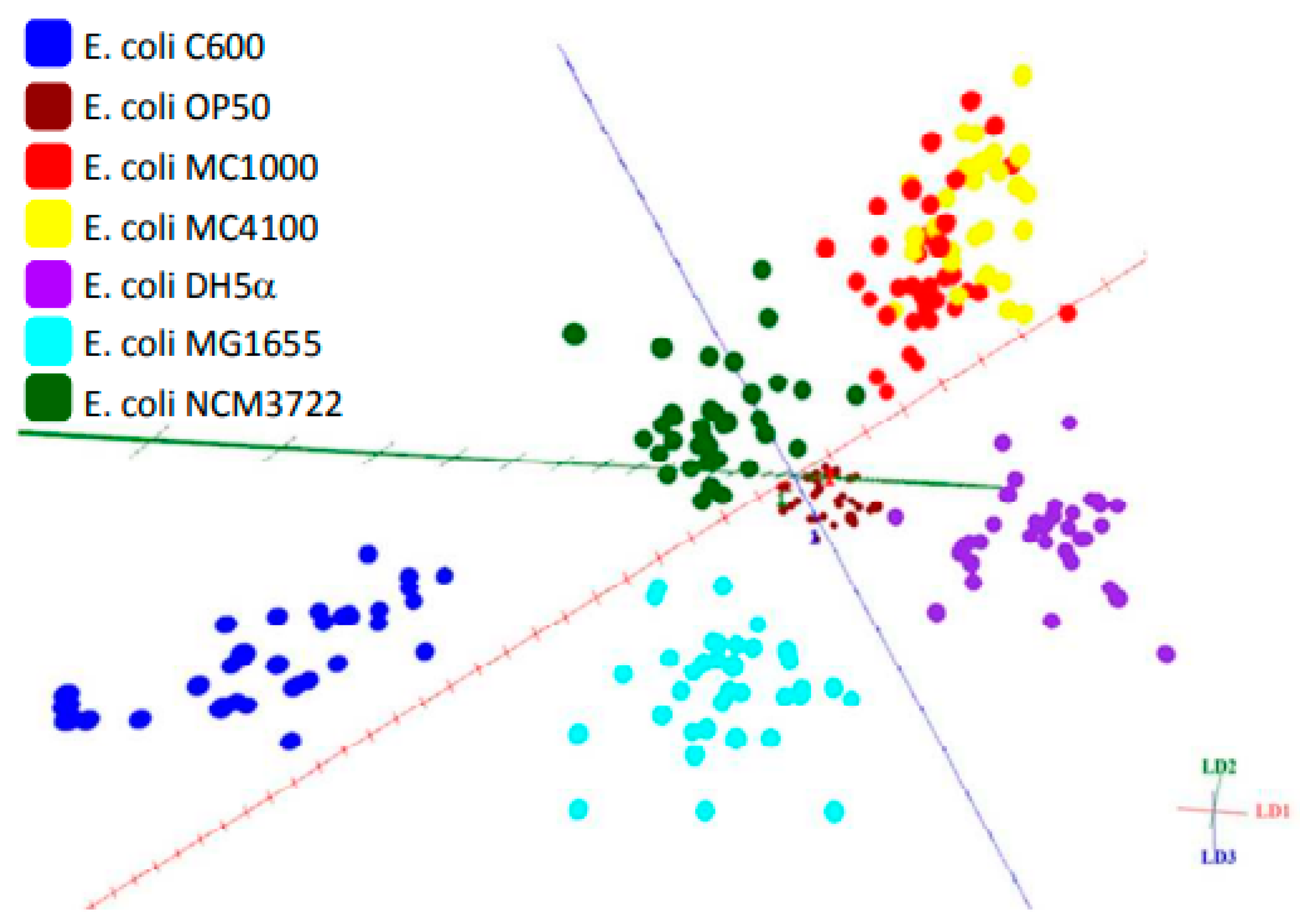
5.2. Subspecies Differentiation
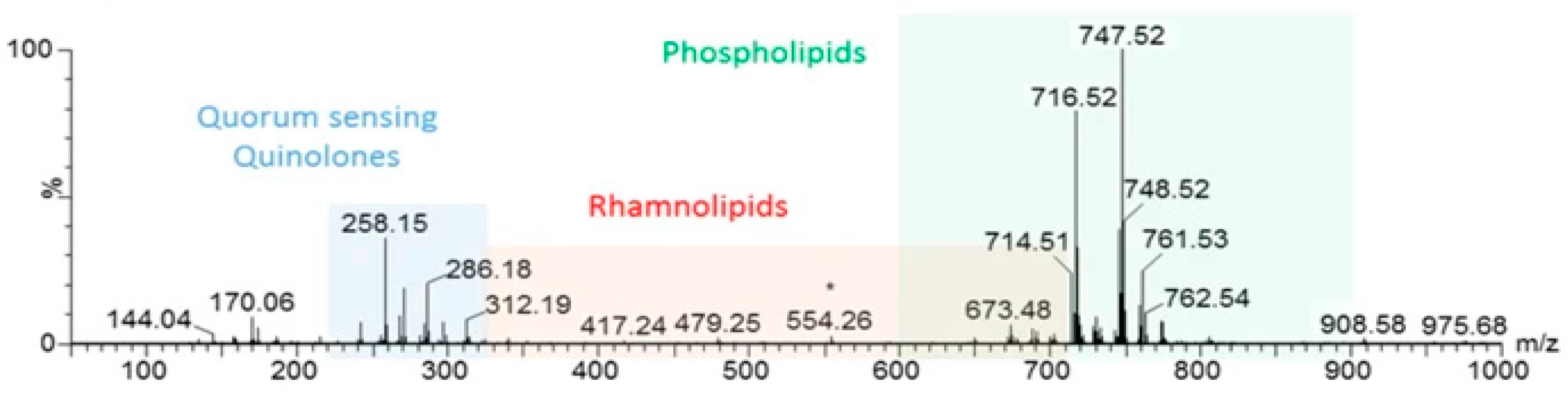
5.3. Quorum Sensing Molecules—Virulence and Antimicrobial Resistance
5.4. Chemical Residues
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLeod, R. Counting the Cost: Lost Australian Food and Wine Export Sales due to Fraud. 2017. Available online: https://www.fial.com.au/blogs/post/Lost-Australian-Food-and-Wine-Export-Sales-Due-to-Fraud (accessed on 2 March 2020).
- Meat & Livestock Australia. Livestock Data Link. Available online: https://www.mla.com.au/research-and-development/livestock-data-link/ (accessed on 2 March 2020).
- Meat & Livestock Australia. 2019 State of the Industry Report—The Australian Red Meat and Livestock Industry. Available online: https://www.mla.com.au/globalassets/mla-corporate/prices--markets/documents/trends--analysis/soti-report/mla-state-of-industry-report-2019.pdf (accessed on 2 March 2020).
- Balog, J.; Szaniszlo, T.; Schaefer, K.C.; Denes, J.; Lopata, A.; Godorhazy, L.; Szalay, D.; Balogh, L.; Sasi-Szabo, L.; Toth, M.; et al. Identification of biological tissues by rapid evaporative ionization mass spectrometry. Anal. Chem. 2010, 82, 7343–7350. [Google Scholar] [CrossRef]
- Harris, G.A.; Galhena, A.S.; Fernández, F.M. Ambient Sampling/Ionization Mass Spectrometry: Applications and Current Trends. Anal. Chem. 2011, 83, 4508–4538. [Google Scholar] [CrossRef] [PubMed]
- REIMS Research System with iKnife Sampling Device. Available online: https://www.waters.com/waters/en_AU/REIMS-Research-System-with-iKnife-Sampling-Device/nav.htm?cid=134846529&locale=en_AU (accessed on 17 January 2020).
- Balog, J.; Perenyi, D.; Guallar-Hoyas, C.; Egri, A.; Pringle, S.D.; Stead, S.; Chevallier, O.P.; Elliott, C.T.; Takats, Z. Identification of the Species of Origin for Meat Products by Rapid Evaporative Ionization Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 4793–4800. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Chevallier, O.P.; Cooper, K.M.; Haughey, S.A.; Balog, J.; Takats, Z.; Elliott, C.T.; Cavin, C. Rapid detection and specific identification of offals within minced beef samples utilising ambient mass spectrometry. Sci. Rep. 2019, 9, 6295. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K.-C.; Dénes, J.; Albrecht, K.; Szaniszló, T.; Balog, J.; Skoumal, R.; Katona, M.; Tóth, M.; Balogh, L.; Takáts, Z. In Vivo, In Situ Tissue Analysis Using Rapid Evaporative Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2009, 48, 8240–8242. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Simon, D.; Karancsi, T.; Balog, J.; Pringle, S.D.; Takats, Z. Matrix Assisted Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2019, 91, 9784–9791. [Google Scholar] [CrossRef] [PubMed]
- Paxton, T. Rapid evaporative ionization mass spectrometry. In Ambient Ionization Mass Spectrometry in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–270. [Google Scholar]
- Strittmatter, N.; Rebec, M.; Jones, E.A.; Golf, O.; Abdolrasouli, A.; Balog, J.; Behrends, V.; Veselkov, K.A.; Takats, Z. Characterization and Identification of Clinically Relevant Microorganisms Using Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2014, 86, 6555–6562. [Google Scholar] [CrossRef] [PubMed]
- Bolt, F.; Cameron, S.J.S.; Karancsi, T.; Simon, D.; Schaffer, R.; Rickards, T.; Hardiman, K.; Burke, A.; Bodai, Z.; Perdones-Montero, A.; et al. Automated High-Throughput Identification and Characterization of Clinically Important Bacteria and Fungi using Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 9419–9426. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Han, T.; Pei, H.; Ren, H.; Stead, S. A novel methodology for real-time identification of the botanical origins and adulteration of honey by rapid evaporative ionization mass spectrometry. Food Control 2019, 106, 106753. [Google Scholar] [CrossRef]
- Allen, D.R.; McWhinney, B.C. Quadrupole Time-of-Flight Mass Spectrometry: A Paradigm Shift in Toxicology Screening Applications. Clin. Biochem. Rev. 2019, 40, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Guitton, Y.; Dervilly-Pinel, G.; Jandova, R.; Stead, S.; Takats, Z.; Le Bizec, B. Rapid evaporative ionisation mass spectrometry and chemometrics for high-throughput screening of growth promoters in meat producing animals. Food Addit. Contam. Part A 2018, 35, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Rigano, F.; Stead, S.; Mangraviti, D.; Jandova, R.; Petit, D.; Marino, N.; Mondello, L. Use of an “Intelligent Knife” (iknife), Based on the Rapid Evaporative Ionization Mass Spectrometry Technology, for Authenticity Assessment of Pistachio Samples. Food Anal. Methods 2019, 12, 558–568. [Google Scholar] [CrossRef]
- Verplanken, K.; Stead, S.; Jandova, R.; Poucke, C.V.; Claereboudt, J.; Bussche, J.V.; Saeger, S.D.; Takats, Z.; Wauters, J.; Vanhaecke, L. Rapid evaporative ionization mass spectrometry for high-throughput screening in food analysis: The case of boar taint. Talanta 2017, 169, 30–36. [Google Scholar] [CrossRef]
- Kosek, V.; Uttl, L.; Jírů, M.; Black, C.; Chevallier, O.; Tomaniová, M.; Elliott, C.T.; Hajšlová, J. Ambient mass spectrometry based on REIMS for the rapid detection of adulteration of minced meats by the use of a range of additives. Food Control 2019, 104, 50–56. [Google Scholar] [CrossRef]
- Composition of Meat. Available online: http://www.fao.org/ag/againfo/themes/en/meat/backgr_composition.html (accessed on 5 March 2021).
- Sarsby, J.; McLean, L.; Harman, V.M.; Beynon, R.J. Monitoring recombinant protein expression in bacteria by rapid evaporative ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2019. [Google Scholar] [CrossRef]
- Black, C.; Chevallier, O.P.; Haughey, S.A.; Balog, J.; Stead, S.; Pringle, S.D.; Riina, M.V.; Martucci, F.; Acutis, P.L.; Morris, M.; et al. A real time metabolomic profiling approach to detecting fish fraud using rapid evaporative ionisation mass spectrometry. Metabolomics 2017, 13. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, J.; Li, S.; Rao, W.; Wang, Y.; Wang, H. In situ rapid evaporative ionization mass spectrometry method for real-time discrimination of Pelodiscus sinensis in different culturing modes without sample preparation. Food Anal. Methods 2019, 12, 2699–2708. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Zhang, Y.; Wang, H.; Li, S.; Dai, Z.; Shen, Q. In Situ Method for Real-Time Discriminating Salmon and Rainbow Trout without Sample Preparation Using iKnife and Rapid Evaporative Ionization Mass Spectrometry-Based Lipidomics. J. Agric. Food Chem. 2019, 67, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Gredell, D. Assessment of Rapid Evaporative Ionization Mass Spectrometry (REIMS) to Characterise Beef Quality and The Impact of Oven Temperature and Relative Humidity on Beef. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2018. [Google Scholar]
- Gifford, C.L. Capabilities of Rapid Evaporative Ionization Mass Spectrometry to Predict Lamb Flavor and Overview of Feeding Genetically Modified Grain to Livestock. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2019. [Google Scholar]
- Lin, Y.; Wang, H.; Rao, W.; Cui, Y.; Yu, X.; Dai, Z.; Shen, Q. Rapid Evaporative Ionization Mass Spectrometry-Based Lipidomics Tracking of Grass Carp (Ctenopharyngodon idellus) during In Vitro Multiple-Stage Digestion. J. Agric. Food Chem. 2018, 66, 6246–6253. [Google Scholar] [CrossRef]
- Gredell, D.A.; Schroeder, A.R.; Belk, K.E.; Broeckling, C.D.; Heuberger, A.L.; Kim, S.-Y.; King, D.A.; Shackelford, S.D.; Sharp, J.L.; Wheeler, T.L.; et al. Comparison of Machine Learning Algorithms for Predictive Modeling of Beef Attributes Using Rapid Evaporative Ionization Mass Spectrometry (REIMS) Data. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Zaitsu, K. Introduction to ambient ionization mass spectrometry. In Ambient Ionization Mass Spectrometry in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–32. [Google Scholar]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Nilles, J.M.; Connell, T.R.; Durst, H.D. Quantitation of Chemical Warfare Agents Using the Direct Analysis in Real Time (DART) Technique. Anal. Chem. 2009, 81, 6744–6749. [Google Scholar] [CrossRef]
- Flaudrops, C.; Armstrong, N.; Raoult, D.; Chabriere, E. Determination of the animal origin of meat and gelatin by MALDI-TOF-MS. J. Food Compos. Anal. 2015, 41, 104–112. [Google Scholar] [CrossRef]
- Pavlovic, M.; Huber, I.; Busch, U. MALDI-TOF MS Profiling Based Identification of Food Components. In Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 742–747. [Google Scholar]
- Sekimoto, K. Direct analysis in real time. In Ambient Ionization Mass Spectrometry in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–75. [Google Scholar]
- Sugiura, Y.; Sugiyama, E.; Suematsu, M. DESI-based imaging mass spectrometry in forensic science and clinical diagnosis. In Ambient Ionization Mass Spectrometry in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–118. [Google Scholar]
- Chernetsova, E.S.; Morlock, G.E. Ambient desorption ionization mass spectrometry (DART, DESI) and its bioanalytical applications. Bioanal. Rev. 2011, 3, 1–9. [Google Scholar] [CrossRef]
- Yong, W.; Guo, T.; Fang, P.; Liu, J.; Dong, Y.; Zhang, F. Direct determination of multi-pesticides in wine by ambient mass spectrometry. Int. J. Mass Spectrom. 2017, 417, 53–57. [Google Scholar] [CrossRef]
- Vaclavik, L.; Rosmus, J.; Popping, B.; Hajslova, J. Rapid determination of melamine and cyanuric acid in milk powder using direct analysis in real time-time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 4204–4211. [Google Scholar] [CrossRef]
- Shin, Y.-S.; Drolet, B.; Mayer, R.; Dolence, K.; Basile, F. Desorption Electrospray Ionization-Mass Spectrometry of Proteins. Anal. Chem. 2007, 79, 3514–3518. [Google Scholar] [CrossRef]
- Schulz, S.; Wagner, S.; Gerbig, S.; Wächter, H.; Sielaff, D.; Bohn, D.; Spengler, B. DESI MS based screening method for phthalates in consumer goods. Analyst 2015, 140, 3484–3491. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yong, W.; Jin, Y.; Zhang, L.; Liu, J.; Wang, S.; Chen, Q.; Dong, Y.; Su, H.; Tan, T. Applications of DART-MS for food quality and safety assurance in food supply chain. Mass Spectrom. Rev. 2017, 36, 161–187. [Google Scholar] [CrossRef]
- Nielen, M.W.F.; Hooijerink, H.; Zomer, P.; Mol, J.G.J. Desorption electrospray ionization mass spectrometry in the analysis of chemical food contaminants in food. Trac Trends Anal. Chem. 2011, 30, 165–180. [Google Scholar] [CrossRef]
- Cajka, T.; Danhelova, H.; Zachariasova, M.; Riddellova, K.; Hajslova, J. Application of direct analysis in real time ionization–mass spectrometry (DART–MS) in chicken meat metabolomics aiming at the retrospective control of feed fraud. Metabolomics 2013, 9, 545–557. [Google Scholar] [CrossRef]
- Cajka, T.; Danhelova, H.; Vavrecka, A.; Riddellova, K.; Kocourek, V.; Vacha, F.; Hajslova, J. Evaluation of direct analysis in real time ionization–mass spectrometry (DART–MS) in fish metabolomics aimed to assess the response to dietary supplementation. Talanta 2013, 115, 263–270. [Google Scholar] [CrossRef]
- Montowska, M.; Rao, W.; Alexander, M.R.; Tucker, G.A.; Barrett, D.A. Tryptic Digestion Coupled with Ambient Desorption Electrospray Ionization and Liquid Extraction Surface Analysis Mass Spectrometry Enabling Identification of Skeletal Muscle Proteins in Mixtures and Distinguishing between Beef, Pork, Horse, Chicken, and Turkey Meat. Anal. Chem. 2014, 86, 4479–4487. [Google Scholar] [CrossRef]
- Busman, M.; Bobell, J.R.; Maragos, C.M. Determination of the aflatoxin M1 (AFM1) from milk by direct analysis in real time—Mass spectrometry (DART-MS). Food Control 2015, 47, 592–598. [Google Scholar] [CrossRef]
- Bernier, M.C.; Li, F.; Musselman, B.; Newton, P.N.; Fernández, F.M. Fingerprinting of falsified artemisinin combination therapies via direct analysis in real time coupled to a compact single quadrupole mass spectrometer. Anal. Methods 2016, 8, 6616–6624. [Google Scholar] [CrossRef]
- Fiorino, G.M.; Losito, I.; De Angelis, E.; Logrieco, A.F.; Monaci, L. Direct analysis in real time coupled to high resolution mass spectrometry as a rapid tool to assess salmon (Salmo salar) freshness. J. Mass Spectrom. 2018, 53, 781–791. [Google Scholar] [CrossRef]
- Rigano, F.; Mangraviti, D.; Stead, S.; Martin, N.; Petit, D.; Dugo, P.; Mondello, L. Rapid evaporative ionization mass spectrometry coupled with an electrosurgical knife for the rapid identification of Mediterranean Sea species. Anal. Bioanal. Chem. 2019, 411, 6603–6614. [Google Scholar] [CrossRef]
- Wagner, I.; Koch, N.I.; Harris, J.; White, N.; Price, T.A.R.; Jones, S.; Hurst, J.L.; Beynon, R.J. Rapid Evaporative Ionisation Mass Spectrometry (REIMS) as a new technique for insect identification. Open Biol. 2020, 10. [Google Scholar] [CrossRef]
- Barney, D.; Bedford, L. Raw material selection: Fruit, vegetables and cereals. In Chilled Foods: A Comprehensive Guide, 3rd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherlands, 2008; pp. 25–41. [Google Scholar] [CrossRef]
- Cavin, C.; Cottenet, G.; Cooper, K.M.; Zbinden, P. Meat Vulnerabilities to Economic Food Adulteration Require New Analytical Solutions. CHIMIA Int. J. Chem. 2018, 72, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Independent Report: Elliot Review into the Integrity and Assurance of Food Supply Networks: Final Report. Available online: https://www.gov.uk/government/publications/elliott-review-into-the-integrity-and-assurance-of-food-supply-networks-final-report (accessed on 20 March 2020).
- Keskin, E.; Atar, H.H. Molecular identification of fish species from surimi-based products labeled as Alaskan pollock. J. Appl. Ichthyol. 2012, 28, 811–814. [Google Scholar] [CrossRef]
- Xiong, X.; Guardone, L.; Cornax, M.J.; Tinacci, L.; Guidi, A.; Gianfaldoni, D.; Armani, A. DNA barcoding reveals substitution of Sablefish (Anoplopoma fimbria) with Patagonian and Antarctic Toothfish (Dissostichus eleginoides and Dissostichus mawsoni) in online market in China: How mislabeling opens door to IUU fishing. Food Control 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Bingpeng, X.; Heshan, L.; Zhilan, Z.; Chunguang, W.; Yanguo, W.; Jianjun, W. DNA barcoding for identification of fish species in the Taiwan Strait. PLoS ONE 2018, 13, e0198109. [Google Scholar] [CrossRef]
- Song, G.; Chen, K.; Wang, H.; Zhang, M.; Yu, X.; Wang, J.; Shen, Q. In situ and real-time authentication of Thunnus species by iKnife rapid evaporative ionization mass spectrometry based lipidomics without sample pretreatment. Food Chem. 2020, 318, 126504. [Google Scholar] [CrossRef]
- Petney, T.N.; Andrews, R.H.; Saijuntha, W.; Wenz-Mucke, A.; Sithithaworn, P. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int. J. Parasitol. 2013, 43, 1031–1046. [Google Scholar] [CrossRef]
- Arena, K.; Rigano, F.; Mangraviti, D.; Cacciola, F.; Occhiuto, F.; Dugo, L.; Dugo, P.; Mondello, L. Exploration of Rapid Evaporative-Ionization Mass Spectrometry as a Shotgun Approach for the Comprehensive Characterization of Kigelia Africana (Lam) Benth. Fruit. Molecules 2020, 25, 962. [Google Scholar] [CrossRef]
- Galvin-King, P.; Haughey, S.A.; Montgomery, H.; Elliott, C.T. The Rapid Detection of Sage Adulteration Using Fourier Transform Infra-Red (FTIR) Spectroscopy and Chemometrics. J. AOAC Int. 2019, 102, 354–362. [Google Scholar] [CrossRef]
- AUS-MEAT. Chiller assessment language. In Chiller Assessment Requirements; AUS-MEAT Limited: Murarrie, Australia, 2014. [Google Scholar]
- Condon, J. Objective Carcase Grading: What’s Coming Down the Technology Super-Highway? Available online: https://www.beefcentral.com/processing/objective-carcase-grading-whats-coming-down-the-technology-super-highway/ (accessed on 18 March 2020).
- ALMTech. Research & Development. Available online: https://www.almtechau.com/research-development (accessed on 21 April 2020).
- Liu, X.; Trautmann, J.; Wigger, R.; Zhou, G.; Mörlein, D. Fatty acid composition and its association with chemical and sensory analysis of boar taint. Food Chem. 2017, 231, 301–308. [Google Scholar] [CrossRef]
- Meier-Dinkel, L.; Gertheiss, J.; Müller, S.; Wesoly, R.; Mörlein, D. Evaluating the performance of sensory quality control: The case of boar taint. Meat Sci. 2015, 100, 73–84. [Google Scholar] [CrossRef]
- Stead, S.; Hird, S.; Balog, J.; Hooper, A.; Pringle, S.; Wilson, M.; Morris, M. Rapid, Direct Technique for the Discrimination of Meat Tissues Originating from Different Animal Species for Food Authenticity. Available online: https://www.waters.com/webassets/cms/library/docs/2015bsms_hird_reims.pdf (accessed on 13 February 2021).
- Frank, D.; Ball, A.; Hughes, J.; Krishnamurthy, R.; Piyasiri, U.; Stark, J.; Watkins, P.; Warner, R. Sensory and Flavor Chemistry Characteristics of Australian Beef: Influence of Intramuscular Fat, Feed, and Breed. J. Agric. Food Chem. 2016, 64, 4299–4311. [Google Scholar] [CrossRef]
- McPhail, N.G.; Small, A.; Eustace, I. Meat Technology Update: Fat Composition of Beef & Sheepmeat: Opportunities for Manipulation. Available online: https://meatupdate.csiro.au/data/MEAT_TECHNOLOGY_UPDATE_08-2.pdf (accessed on 8 April 2020).
- Frank, D.; Hughes, J.; Piyasiri, U.; Zhang, Y.; Kaur, M.; Li, Y.; Mellor, G.; Stark, J. Volatile and non-volatile metabolite changes in 140-day stored vacuum packaged chilled beef and potential shelf life markers. Meat Sci. 2020, 161, 108016. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.M.; Flores, M.; McMillin, K.W.; Bidner, T.D. The effect of post-mortem aging on meat flavor quality in Brangus beef. Correlation of treatments, sensory, instrumental and chemical descriptors. Food Chem. 1997, 59, 531–538. [Google Scholar] [CrossRef]
- Lynch, N.M.; Kastner, C.L.; Kropf, D.H.; Caul, J.F. Flavour and aroma influences on acceptance of polyvinyl chloride versus vacuum packed ground beef. J. Food Sci. 1986, 51, 256. [Google Scholar] [CrossRef]
- Frank, D.; Zhang, Y.; Li, Y.; Luo, X.; Chen, X.; Kaur, M.; Mellor, G.; Stark, J.; Hughes, J. Shelf life extension of vacuum packaged chilled beef in the Chinese supply chain. A feasibility study. Meat Sci. 2019, 153, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Nissen, H.; Sørheim, O.; Dainty, R. Effects of vacuum, modified atmospheres and storage temperature on the microbial flora of packaged beef. Food Microbiol. 1996, 13, 183–191. [Google Scholar] [CrossRef]
- Khan, M.I.; Jung, S.; Nam, K.C.; Jo, C. Postmortem Aging of Beef with a Special Reference to the Dry Aging. Korean J. Food Sci. Anim. Resour. 2016, 36, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Babol, J.; Bredie, W.L.P.; Nielsen, B.; Tománková, J.; Lundström, K. A comparative study of beef quality after ageing longissimus muscle using a dry ageing bag, traditional dry ageing or vacuum package ageing. Meat Sci. 2014, 97, 433–442. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.G.; Hewett, B.; Fursey, G.A.J.; Wood, J.D.; Harrington, G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 1998, 49, 329–341. [Google Scholar] [CrossRef]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2003, 66, 21–32. [Google Scholar] [CrossRef]
- Australian Government, National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand. Available online: https://www.nrv.gov.au/nutrients/fats-total-fat-fatty-acids (accessed on 5 March 2020).
- Brewer, S. The Chemistry of Beef Flavour, Executive Summary. Available online: https://www.beefresearch.org/Media/BeefResearch/Docs/the_chemistry_of_beef_flavor_08-20-2020-98.pdf (accessed on 20 August 2020).
- Campo, M.M.; Nute, G.R.; Hughes, S.I.; Enser, M.; Wood, J.D.; Richardson, R.I. Flavour perception of oxidation in beef. Meat Sci. 2006, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yancey, E.J.; Dikeman, M.E.; Hachmeister, K.A.; Chambers, E.; Milliken, G.A. Flavor characterization of top-blade, top-sirloin, and tenderloin steaks as affected by pH, maturity, and marbling. J. Anim. Sci. 2005, 83, 2618–2623. [Google Scholar] [CrossRef]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Descalzo, A.M.; Sancho, A.M. A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Sci. 2008, 79, 423–436. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.J.; Lanari, M.C.; Tume, R.K. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture- and grain-fed cattle. Meat Sci. 2002, 60, 35–40. [Google Scholar] [CrossRef]
- Reicks, A.L.; Brooks, J.C.; Garmyn, A.J.; Thompson, L.D.; Lyford, C.L.; Miller, M.F. Demographics and beef preferences affect consumer motivation for purchasing fresh beef steaks and roasts. Meat Sci. 2011, 87, 403–411. [Google Scholar] [CrossRef]
- Hocquette, J.F.; Van Wezemael, L.; Chriki, S.; Legrand, I.; Verbeke, W.; Farmer, L.; Scollan, N.D.; Polkinghorne, R.; Rodbotten, R.; Allen, P.; et al. Modelling of beef sensory quality for a better prediction of palatability. Meat Sci. 2014, 97, 316–322. [Google Scholar] [CrossRef]
- Thompson, J.M.; Perry, D.; Daly, B.; Gardner, G.E.; Johnston, D.J.; Pethick, D.W. Genetic and environmental effects on the muscle structure response post-mortem. Meat Sci. 2006, 74, 59–65. [Google Scholar] [CrossRef]
- Young, O.A.; Hopkins, D.L.; Pethick, D.W. Critical control points for meat quality in the Australian sheep meat supply chain. Aust. J. Exp. Agric. 2005, 45, 593–601. [Google Scholar] [CrossRef][Green Version]
- Channon, H.A.; Warner, R. Delivering consistent quality Australian pork to consumers—A systems approach. Manip. Pig Prod. XIII 2011, 13, 262–293. [Google Scholar]
- Black, C.; Chevallier, O.P.; Elliott, C.T. The current and potential applications of Ambient Mass Spectrometry in detecting food fraud. Trac Trends Anal. Chem. 2016, 82, 268–278. [Google Scholar] [CrossRef]
- Montowska, M.; Alexander, M.R.; Tucker, G.A.; Barrett, D.A. Rapid detection of peptide markers for authentication purposes in raw and cooked meat using ambient liquid extraction surface analysis mass spectrometry. Anal. Chem. 2014, 86, 10257–10265. [Google Scholar] [CrossRef] [PubMed]
- Montowska, M.; Alexander, M.R.; Tucker, G.A.; Barrett, D.A. Authentication of processed meat products by peptidomic analysis using rapid ambient mass spectrometry. Food Chem. 2015, 187, 297–304. [Google Scholar] [CrossRef]
- USDA. United States Standards for Grades of Carcass Beef; United States Department of Agriculture: Washington, DC, USA, 1997; pp. 1–18.
- Meat and Livestock Australia. Australian Beef Eating Quality Insights. Available online: https://www.mla.com.au/globalassets/mla-corporate/marketing-beef-and-lamb/documents/meat-standards-australia/abeqi-2019-interactive.pdf (accessed on 17 September 2020).
- Hughes, J.; Kearney, G.; Warner, R.D. Improving beef meat colour scores at carcass grading. Anim. Prod. Sci. 2014, 54, 422–429. [Google Scholar] [CrossRef]
- Murray, A.C. Factors affecting beef color at time of grading. Can. J. Anim. Sci. 1989, 69, 347–355. [Google Scholar] [CrossRef]
- Hughes, J.; Bolumar, T.; Kanon, A.; Stark, J.; Tobin, A. Improving Beef Colour at Grading-Final Report; 2013/3005; CSIRO/AMPC: Canberra, Australia, 2017. [Google Scholar]
- Department of Agriculture and Water Resources. Microbiological Manual for Sampling and Testing of Export Meat and Meat Products; Version 1.03; Department of Agriculture and Water Resources: Canberra, Australia, 2018.
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Fang, J.; Dorrestein, P.C. Emerging mass spectrometry techniques for the direct analysis of microbial colonies. Curr. Opin. Microbiol. 2014, 19, 120–129. [Google Scholar] [CrossRef]
- Strittmatter, N. Development of Novel Mass Spectrometric Methods for the Characterisation and Identification of Microorganisms. Ph.D. Thesis, Imperial College, London, UK, 2016. [Google Scholar]
- Strittmatter, N.; Jones, E.A.; Veselkov, K.A.; Rebec, M.; Bundy, J.G.; Takats, Z. Analysis of intact bacteria using rapid evaporative ionisation mass spectrometry. Chem. Commun. 2013, 49, 6188. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Bolt, F.; Perdones-Montero, A.; Rickards, T.; Hardiman, K.; Abdolrasouli, A.; Burke, A.; Bodai, Z.; Karancsi, T.; Simon, D.; et al. Rapid Evaporative Ionisation Mass Spectrometry (REIMS) Provides Accurate Direct from Culture Species Identification within the Genus Candida. Sci. Rep. 2016, 6, 36788. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Alexander, J.L.; Bolt, F.; Burke, A.; Ashrafian, H.; Teare, J.; Marchesi, J.R.; Kinross, J.; Li, J.V.; Takáts, Z. Evaluation of Direct from Sample Metabolomics of Human Feces Using Rapid Evaporative Ionization Mass Spectrometry. Anal. Chem. 2019, 91, 13448–13457. [Google Scholar] [CrossRef]
- Bardin, E.E.; Cameron, S.J.S.; Perdones-Montero, A.; Hardiman, K.; Bolt, F.; Alton, E.W.F.W.; Bush, A.; Davies, J.C.; Takáts, Z. Metabolic Phenotyping and Strain Characterisation of Pseudomonas aeruginosa Isolates from Cystic Fibrosis Patients Using Rapid Evaporative Ionisation Mass Spectrometry. Sci. Rep. 2018, 8, 10952. [Google Scholar] [CrossRef]
- Antunes, L.C.M.; Ferreira, R.B.; Buckner, M.M.; Finlay, B.B. Quorum sensing in bacterial virulence. Microbiology 2010, 156, 2271–2282. [Google Scholar] [CrossRef]
- Marler Clark LLP. Petition for An Interpretive Rule Declaring ‘Outbreak’ Serotypes of SALMONELLA Enterica Subspecies Enterica to be Adulterants. Available online: https://www.fsis.usda.gov/wps/wcm/connect/d2a7c76e-dda9-475d-bf35-4cb69f5fca24/20-01-marler-011920.pdf?MOD=AJPERES (accessed on 17 April 2020).
- Meat and Livestock Australia in conjunction with Australian Feedlotters’ Association. Antimicrobial Stewardship Guidelines for the Australian Cattle Feedlot Industry. Available online: https://www.mla.com.au/globalassets/mla-corporate/research-and-development/program-areas/animal-health-welfare-and-biosecurity/mla_antimicrobial-stewardship-guidelines.pdf (accessed on 13 June 2020).
- Attalah, E.; Nasr, Y.S.; El-Gammal, H.A.; Nour El-Dien, F.A. Optimisation and validation of a new analytical method for the determination of four natural and synthetic hormones using LC-ESI-MS/MS. Food Addit. Contam. Part A 2016, 33, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Genangeli, M.; Caprioli, G.; Cortese, M.; Laus, F.; Matteucci, M.; Petrelli, R.; Ricciutelli, M.; Sagratini, G.; Sartori, S.; Vittori, S. Development and application of a UHPLC-MS/MS method for the simultaneous determination of 17 steroidal hormones in equine serum. J. Mass Spectrom. 2017, 52, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, M.; Romero-Gonzalez, R.; Garrido Frenich, A. Determination of steroid hormones and their metabolite in several types of meat samples by ultra high performance liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2018, 1540, 21–30. [Google Scholar] [CrossRef]
- Han, X.; Liu, D. Detection and analysis of 17 steroid hormones by ultra-high-performance liquid chromatography-electrospray ionization mass spectrometry (UHPLC-MS) in different sex and maturity stages of Antarctic krill (Euphausia superba Dana). PLoS ONE 2019, 14, e0213398. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, K.D.B.; Forbes, T.P.; Hulsbergen, A.W.C.; Verkouteren, J.; Krauss, S.T.; Koeberg, M.; Schoenmakers, P.J.; Gillen, G.; van Asten, A.C. Emerging techniques for the detection of pyrotechnic residues from seized postal packages containing fireworks. Forensic. Sci. Int. 2020, 308, 110160. [Google Scholar] [CrossRef] [PubMed]

| Species | Compound A | Reference |
|---|---|---|
| Beef | FAs, PLs | [7] |
| Cer, DAG, GluCer, MGDG, PL | [8] | |
| Pig | FAs, PLs | [16] |
| FAs, PLs, others | [4] | |
| FAs, PLs, others | [18] | |
| Pork/chicken | PLs | [19] |
| Turtle | PLs | [23] |
| Fish | Lipids—PLs | [27] |
| Lipids—PLs | [24] | |
| FAs, PLs | [24] | |
| PLs (FA as a dimer) | [22] |
| Fatty Acid | Class B | Beef | Sheep | Melting Point (°C) |
|---|---|---|---|---|
| Myristic (C14:0) | SFA | 2–4 | 2.5–4 | 53 |
| Palmitic (C16:0) | SFA | 22–28 | 22–27 | 63 |
| Palmitoleic (C16:1) | MUFA | 1–12 | 1–2 | 0 |
| Stearic (C18:0) | SFA | 4–30 | 17–30 | 70 |
| trans-Vaccenic (C18:1) | MUFA | 1–12 | 0.3–4 | 45 |
| Oleic (C18:1) | MUFA | 35–50 | 19–31 | 16 |
| Linoleic (C18:2) | PUFA | 1–2 | 2–4 | −9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barlow, R.S.; Fitzgerald, A.G.; Hughes, J.M.; McMillan, K.E.; Moore, S.C.; Sikes, A.L.; Tobin, A.B.; Watkins, P.J. Rapid Evaporative Ionization Mass Spectrometry: A Review on Its Application to the Red Meat Industry with an Australian Context. Metabolites 2021, 11, 171. https://doi.org/10.3390/metabo11030171
Barlow RS, Fitzgerald AG, Hughes JM, McMillan KE, Moore SC, Sikes AL, Tobin AB, Watkins PJ. Rapid Evaporative Ionization Mass Spectrometry: A Review on Its Application to the Red Meat Industry with an Australian Context. Metabolites. 2021; 11(3):171. https://doi.org/10.3390/metabo11030171
Chicago/Turabian StyleBarlow, Robert S., Adam G. Fitzgerald, Joanne M. Hughes, Kate E. McMillan, Sean C. Moore, Anita L. Sikes, Aarti B. Tobin, and Peter J. Watkins. 2021. "Rapid Evaporative Ionization Mass Spectrometry: A Review on Its Application to the Red Meat Industry with an Australian Context" Metabolites 11, no. 3: 171. https://doi.org/10.3390/metabo11030171
APA StyleBarlow, R. S., Fitzgerald, A. G., Hughes, J. M., McMillan, K. E., Moore, S. C., Sikes, A. L., Tobin, A. B., & Watkins, P. J. (2021). Rapid Evaporative Ionization Mass Spectrometry: A Review on Its Application to the Red Meat Industry with an Australian Context. Metabolites, 11(3), 171. https://doi.org/10.3390/metabo11030171






