Elemental and Isotopic Characterization of Tobacco from Umbria
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Description of the Isotopic and Elemental Content, According to the Geographical Origin
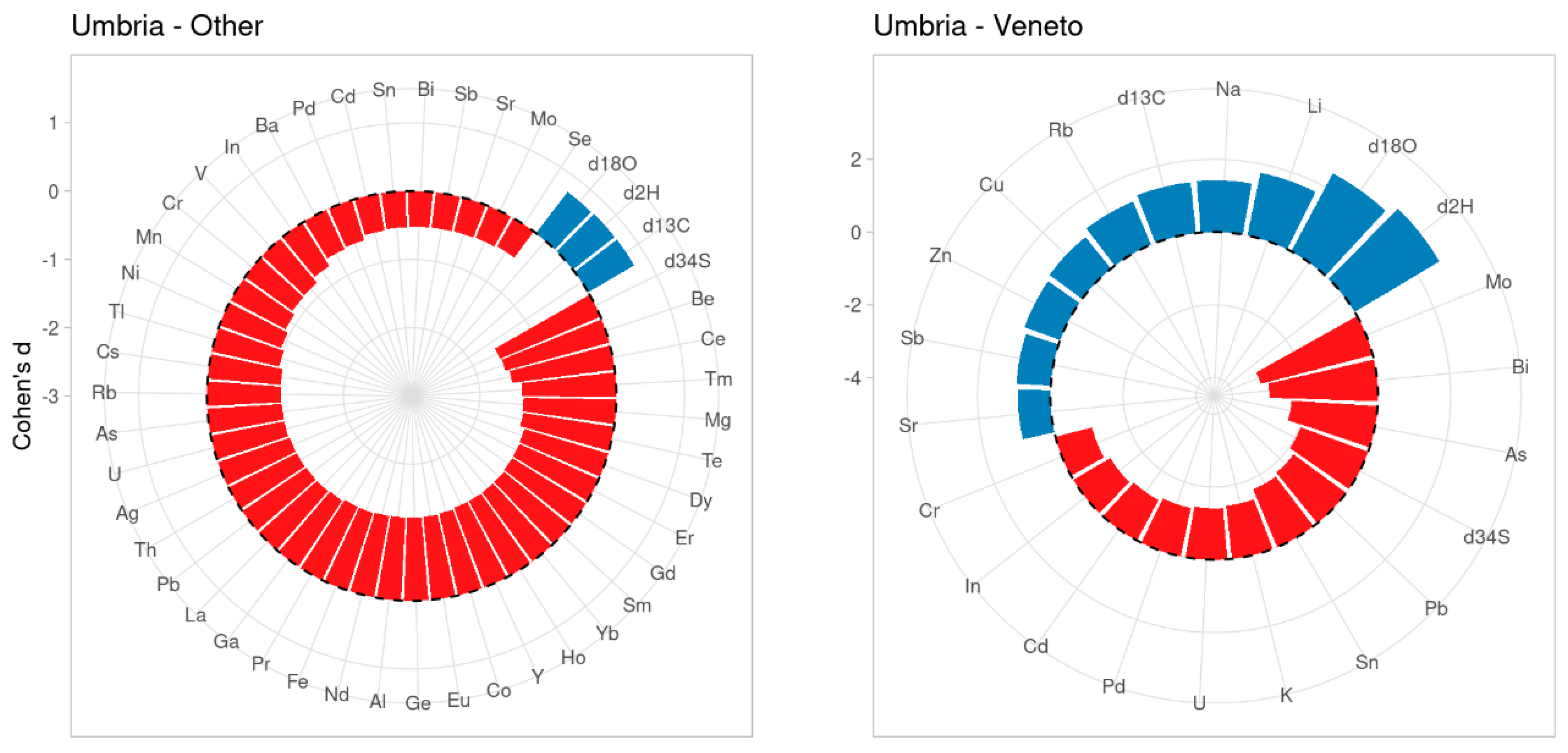
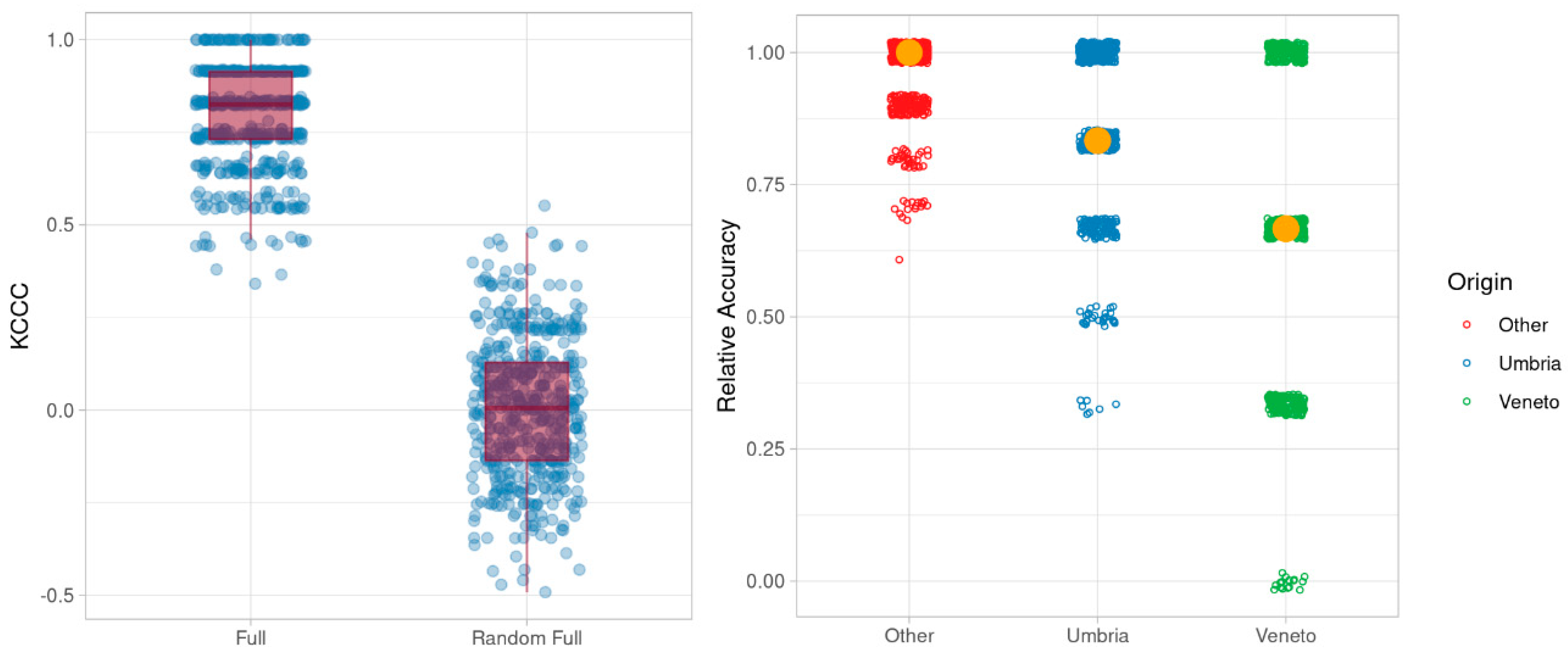
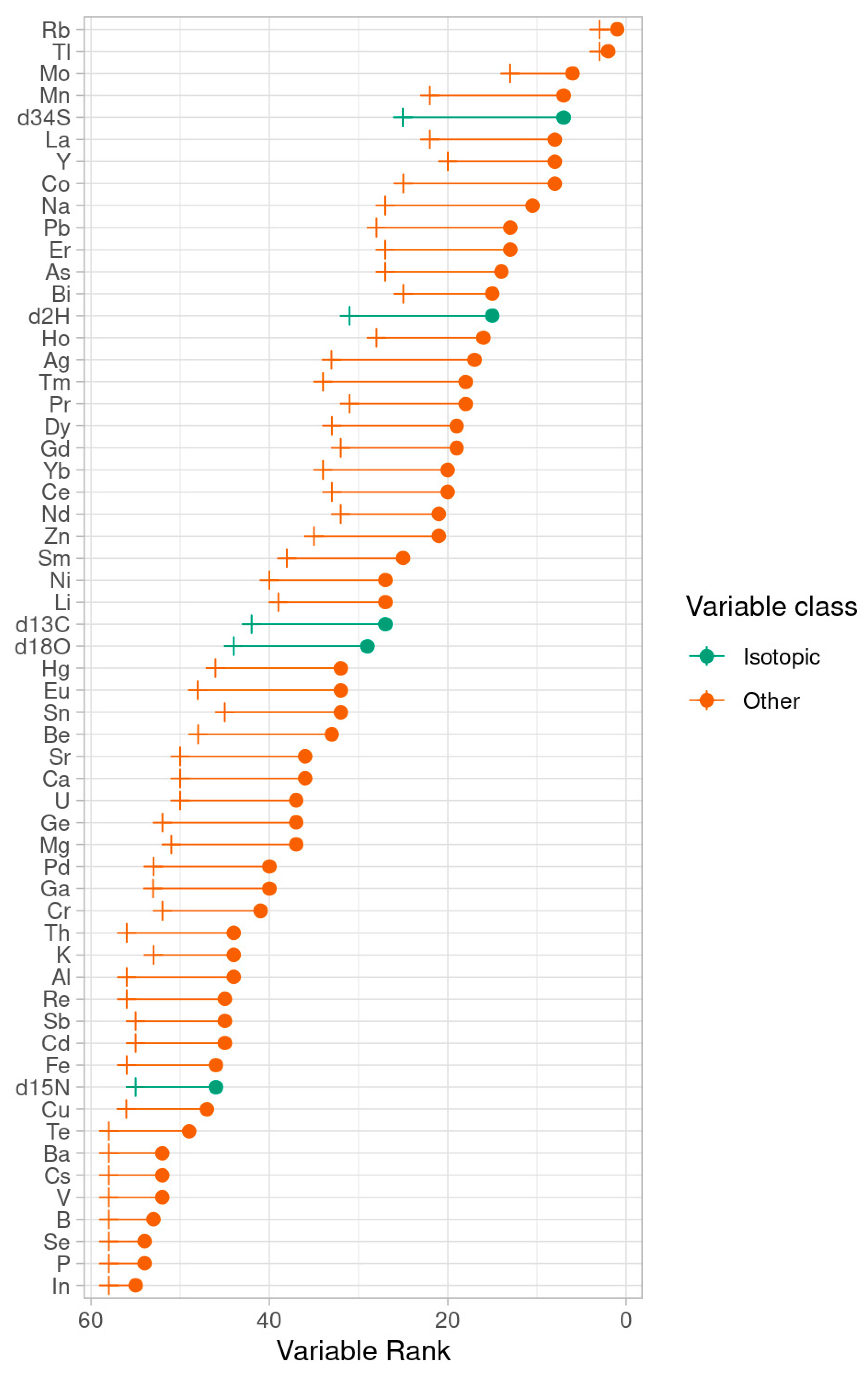
2.2. Characterization of Umbrian Tobacco Versus Tobacco from Veneto and Other Geographical Origins
3. Materials and Methods
3.1. Samples and Preparation
3.2. Analytical Methods
3.2.1. Stable Isotope Ratios Analysis
3.2.2. Elemental Analysis
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grisan, S.; Polizzotto, R.; Raiola, P.; Cristiani, S.; Ventura, F.; di Lucia, F.; Zuin, M.; Tommasini, S.; Morbidelli, R.; Damiani, F.; et al. Alternative use of tobacco as a sustainable crop for seed oil, biofuel, and biomass. Agron. Sustain. Dev. 2016, 36, 55. [Google Scholar] [CrossRef] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Nagaraj, G. Oilseeds: Properties, Processing, Products and Procedures; New India Publishing: New Delhi, India, 2009. [Google Scholar]
- Eshetu, B. Nicotiana tabacum L. Seed Oil; Springer: Heidelberg/Berlin, Germany, 2005. [Google Scholar]
- Usta, N.; Aydoğan, B.; Çon, A.H.; Uğuzdoğan, E.; Özkal, S.G. Properties and quality verification of biodiesel produced from tobacco seed oil. Energy Convers. Manag. 2011, 52, 2031–2039. [Google Scholar] [CrossRef]
- Rossi, L.; Fusi, E.; Baldi, G.; Fogher, C.; Cheli, F.; Baldi, A.; Dell’Orto, V. Tobacco Seeds By-Product as Protein Source for Piglets. Open J. Vet. Med. 2013, 3, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, D.; Badescu, C.; Acalovschi, I. Nicotine patch for the prevention of postoperative nausea and vomiting: A prospective randomised trial. Clin. Drug Investig. 2007, 27, 559–564. [Google Scholar] [CrossRef]
- Newhouse, P.A.; Hughes, J.R. The role of nicotine and nicotinic mechanisms in neuropsychiatric disease. Addiction 1991, 86, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A. Cigarette smoking and Parkinson’s disease. Neurology 1986, 36, 1490–1496. [Google Scholar] [CrossRef]
- Saitoh, F.; Noma, M.; Kawashima, N. The alkaloid contents of sixty Nicotiana species. Phytochemistry 1985, 24, 477–480. [Google Scholar] [CrossRef]
- Charlton, A. Medicinal uses of tobacco in history. J. R. Soc. Med. 2004, 97, 292–296. [Google Scholar] [CrossRef]
- Tobacco. Available online: https://ec.europa.eu/info/food-farming-fisheries/plants-and-plant-products/plant-products/tobacco_en (accessed on 9 March 2021).
- Mipaaf—Tabacco. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/3426 (accessed on 9 March 2021).
- Overview on Italian Tobacco Production. Available online: https://www.coresta.org/abstracts/overview-italian-tobacco-production-29005.html (accessed on 9 March 2021).
- Dluge, K.L.; Song, Z.; Wang, B.; Tyler Steede, W.; Xiao, B.; Liu, Y.; Dewey, R.E. Characterization of Nicotiana tabacum genotypes possessing deletion mutations that affect potyvirus resistance and the production of trichome exudates. BMC Genom. 2018, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, A. Characterising Tobacco Aquaporins: Towards Engineering Improved Photosynthesis and Plant Performance. 2020. Available online: https://openresearch-repository.anu.edu.au/bitstream/1885/206738/1/Annamaria%20De%20Rosa-%20PhD%20Thesis%2029July2020%20v_resubmission_FINAL_PDF.pdf (accessed on 9 March 2021).
- Santos, T.O.; Silva, C.F.; Damatto, S.R. Assessment of Trace Elements Concentration in Nicotiana tabacum L., Virginia Variety. Available online: http://repositorio.ipen.br/handle/123456789/28747 (accessed on 9 March 2021).
- Karabulut, B.; Karabulut, A.; Camas, N. Elemental analysis of some important tobacco varieties (Nicotiana tabacum L.) by WDXRF spectroscopy. Asian J. Chem. 2007, 19, 3971. [Google Scholar]
- Jordanoska, B.; Stafilov, T.; Pelivanoska, V. Accumulation and Availability of Trace Elements from Soil into Oriental Tobacco Grown in Macedonia. J. Environ. Eng. Landsc. Manag. 2018, 17, 1491–1500. [Google Scholar] [CrossRef]
- Silva, F.R.; Erdtmann, B.; Dalpiaz, T.; Nunes, E.; Ferraz, A.; Martins, T.L.C.; Dias, J.F.; da Rosa, D.P.; Porawskie, M.; Bona, S.; et al. Genotoxicity of Nicotiana tabacum leaves on Helix aspersa. Genet. Mol. Biol. 2013, 36, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Kazi, T.G.; Jalbani, N.; Arain, M.B.; Jamali, M.K.; Afridi, H.I.; Shah, A.Q. Determination of toxic elements in different brands of cigarette by atomic absorption spectrometry using ultrasonic assisted acid digestion. Environ. Monit. Assess. 2009, 154, 155–167. [Google Scholar] [CrossRef]
- Zumbado, M.; Luzardo, O.P.; Rodríguez-Hernández, Á.; Boada, L.D.; Henríquez-Hernández, L.A. Differential exposure to 33 toxic elements through cigarette smoking, based on the type of tobacco and rolling paper used. Environ. Res. 2019, 169, 368–376. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Talpur, F.N.; Brabazon, D. Evaluation of trace and toxic elements in the samples of different cigarettes and their impact on human health of Irish diabetes mellitus patients. Clin. Lab. 2015, 61, 123–140. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Guo, J.; Xia, Q.; Zhao, G.; Zhou, H.; Xie, F. Metabolic profiling of Chinese tobacco leaf of different geographical origins by GC-MS. J. Agric. Food Chem. 2013, 61, 2597–2605. [Google Scholar] [CrossRef]
- Jamin, E.; Naulet, N.; Martin, G.J. Multi-element and multi-site isotopic analysis of nicotine from tobacco leaves. Plant Cell Environ. 1997, 20, 589–599. [Google Scholar] [CrossRef]
- Camin, F.; Boner, M.; Bontempo, L.; Fauhl-Hassek, C.; Kelly, S.D.; Riedl, J.; Rossmann, A. Stable isotope techniques for verifying the declared geographical origin of food in legal cases. Trends Food Sci. Technol. 2017, 61, 176–187. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the geographical origin of food: The application of multi-element and multi-isotope analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. Trends Anal. Chem. 2016, 85, 123–132. [Google Scholar]
- Pustjens, A.M.; Muilwijk, M.; Weesepoel, Y.; van Ruth, S.M. Advances in Authenticity Testing of Geographical Origin of Food Products. Adv. Food Authent. Test. 2016, 339–367. [Google Scholar] [CrossRef]
- Binette, M.-J.; Lafontaine, P.; Vanier, M.; Ng, L.-K. Characterization of Canadian cigarettes using multi-stable isotope analysis by gas chromatography-isotope ratio mass spectrometry. J. Agric. Food Chem. 2009, 57, 1151–1155. [Google Scholar] [CrossRef]
- Judd, C.D.; Swami, K. ICP-MS determination of lead isotope ratios in legal and counterfeit cigarette tobacco samples. Isot. Environ. Health Stud. 2010, 46, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 436–468. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope Fractionation and Plant Water-Use Efficiency. Stable Isot. Ecol. Res. 1989, 21–40. [Google Scholar] [CrossRef]
- Tanz, N.; Schmidt, H.-L. δ34S-Value Measurements in Food Origin Assignments and Sulfur Isotope Fractionations in Plants and Animals. J. Agric. Food Chem. 2010, 3139–3146. [Google Scholar] [CrossRef]
- Spalla, S.; Baffi, C.; Barbante, C.; Turretta, C.; Cozzi, G.; Beone, G.M.; Bettinelli, M. Determination of rare earth elements in tomato plants by inductively coupled plasma mass spectrometry techniques. Rapid Commun. Mass Spectrom. 2009, 3285–3292. [Google Scholar] [CrossRef]
- Bontempo, L.; Camin, F.; Manzocco, L.; Nicolini, G.; Wehrens, R.; Ziller, L.; Larcher, R. Traceability along the production chain of Italian tomato products on the basis of stable isotopes and mineral composition. Rapid Commun. Mass Spectrom. 2011, 25, 899–909. [Google Scholar] [CrossRef]
- Bettinelli, M.; Spezia, S.; Pastorelli, N.; Terni, C. Trace elements in honey samples by means of ETV-ICP-MS. Plasma Source Mass Spectrom. 2007, 177–184. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef]
- Bontempo, L.; Camin, F.; Perini, M.; Ziller, L.; Larcher, R. Isotopic and elemental characterisation of Italian white truffle: A first exploratory study. Food Chem. Toxicol. 2020, 145, 111627. [Google Scholar] [CrossRef]
- Oddone, M.; Aceto, M.; Baldizzone, M.; Musso, D.; Osella, D. Authentication and Traceability Study of Hazelnuts from Piedmont, Italy. J. Agric. Food Chem. 2009, 3404–3408. [Google Scholar] [CrossRef]
- Bertoldi, D.; Santato, A.; Paolini, M.; Barbero, A.; Camin, F.; Nicolini, G.; Larcher, R. Botanical traceability of commercial tannins using the mineral profile and stable isotopes. J. Mass Spectrom. 2014, 49, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Wickham, M.H. Package Tidyverse. Easily Install and Load the ‘Tidyverse’. 2017. Available online: http://ftp.gr.xemacs.org/pub/CRAN/web/packages/tidyverse/tidyverse.pdf (accessed on 9 March 2021).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Ushey, K.; Allaire, J.J.; Tang, Y.; Eddelbuettel, D.; Lewis, B.; Keydana, S.; Hafen, R.; Geelnard, M. Reticulate: Interface to Python; R Package Version; R Core Team: Vienna, Austria, 2020; Volume 1. [Google Scholar]
- Torchiano, M.; Torchiano, M.M. Package “Effsize.” Package “Effsize”. 2020. Available online: https://pbil.univ-lyon1.fr/CRAN/web/packages/effsize/effsize.pdf (accessed on 9 March 2021).
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikitlearn: Machine Learning in Python. CoRR abs/1201.0490 (2012). arXiv 2012, arXiv:1201.0490.2012. [Google Scholar]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 597–599. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontempo, L.; Bertoldi, D.; Franceschi, P.; Rossi, F.; Larcher, R. Elemental and Isotopic Characterization of Tobacco from Umbria. Metabolites 2021, 11, 186. https://doi.org/10.3390/metabo11030186
Bontempo L, Bertoldi D, Franceschi P, Rossi F, Larcher R. Elemental and Isotopic Characterization of Tobacco from Umbria. Metabolites. 2021; 11(3):186. https://doi.org/10.3390/metabo11030186
Chicago/Turabian StyleBontempo, Luana, Daniela Bertoldi, Pietro Franceschi, Fabio Rossi, and Roberto Larcher. 2021. "Elemental and Isotopic Characterization of Tobacco from Umbria" Metabolites 11, no. 3: 186. https://doi.org/10.3390/metabo11030186
APA StyleBontempo, L., Bertoldi, D., Franceschi, P., Rossi, F., & Larcher, R. (2021). Elemental and Isotopic Characterization of Tobacco from Umbria. Metabolites, 11(3), 186. https://doi.org/10.3390/metabo11030186






