Feature-Based Molecular Network-Guided Dereplication of Natural Bioactive Products from Leaves of Stryphnodendron pulcherrimum (Willd.) Hochr
Abstract
:1. Introduction
2. Results
2.1. Stryphnodendron Pulcherrimum Extract Characterization by UHPLC-MS/MS
2.2. Structural Identification of Compounds Present in the Extract of S. pulcherrimum
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Collection and Extraction
4.3. Sample Preparation for UHPLC-MS/MS
4.4. UHPLC-MS/MS
4.5. Mass Spectrometry Data Treatment Parameters
4.6. Feature-Based Molecular Networking and Taxonomically Informed Metabolite Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, C.A.; Teixeira, A.L.; Rocha, R.O.; Baptista, A.C.; da Silva, A.A.S. Effect of temperature and pre-germination treatments on seed germination of juerana branca (Stryphnodendron pulcherrimum). Afr. J. AIDS Res. 2015, 10, 494–498. [Google Scholar] [CrossRef]
- Gonçalves, D.C.M.; de Gama, J.R.V.; de Oliveira, F.A.; de Junior, R.C.O.; Araujo, G.C.; de Almeida, L.S. Aspectos Mercadológicos dos Produtos não Madeireiros na Economia de Santarém-Pará, Brasil. Aspectos Mercadológicos dos Produtos não Madeireiros na Economia de Santarém-Pará Brasil 2012, 19, 9–16. [Google Scholar] [CrossRef] [Green Version]
- De Castilho, A.L.; da Silva, J.P.C.; Saraceni, C.H.C.; Díaz, I.E.C.; Paciencia, M.L.B.; Varella, A.D.; Suffredini, I.B. In vitro activity of Amazon plant extracts against Enterococcus faecalis. Braz. J. Microbiol. 2014, 45, 769–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.A. Medicinal Plants of the World: Volume 1: Chemical Constituents, Traditional and Modern Medicinal Uses; Humana Press: New York, NY, USA, 2010; ISBN 9781617374692. [Google Scholar]
- Ross, I.A. Medicinal Plants of the World: Chemical Constituents, Traditional and Modern Medicinal Uses, Volume 2; Humana Press: New York, NY, USA, 2013; ISBN 9781468497069. [Google Scholar]
- Spraker, J.E.; Luu, G.T.; Sanchez, L.M. Imaging mass spectrometry for natural products discovery: A review of ionization methods. Nat. Prod. Rep. 2020, 37, 150–162. [Google Scholar] [CrossRef]
- Liu, M.; Quinn, R.J. Fragment-based screening with natural products for novel anti-parasitic disease drug discovery. Expert Opin. Drug Discov. 2019, 14, 1283–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.; Choi, B.-R.; Ma, S.; Lee, J.W.; Jo, I.-H.; Lee, Y.-S.; Kim, G.-S.; Kim, S.; Lee, D.Y. Metabolomics for Age Discrimination of Ginseng Using a Multiplex Approach to HR-MAS NMR Spectroscopy, UPLC-QTOF/MS, and GC × GC-TOF/MS. Molecules 2019, 24, 2381. [Google Scholar] [CrossRef] [Green Version]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- De Mello, J.P.; Petereit, F.; Nahrstedt, A. Flavan-3-ols and prodelphinidins from Stryphnodendron adstringens. Phytochemistry 1996, 41, 807–813. [Google Scholar] [CrossRef]
- Palazzo de Mello, J.; Petereit, F.; Nahrstedt, A. Prorobinetinidins from Stryphnodendron adstringens. Phytochemistry 1996, 42, 857–862. [Google Scholar] [CrossRef]
- Ishida, K.; de Mello, J.C.P.; Cortez, D.A.G.; Filho, B.P.D.; Ueda-Nakamura, T.; Nakamura, C.V. Influence of tannins from Stryphnodendron adstringens on growth and virulence factors of Candida albicans. J. Antimicrob. Chemother. 2006, 58, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, G.C.; Vieira Machado, F.A.; Mendes de Toledo, C.E.; Sakuragui, C.M.; Palazzo de Mello, J.C. Chemotaxonomic significance of 5-deoxyproanthocyanidins in Stryphnodendron species. Biochem. Syst. Ecol. 2008, 36, 925–931. [Google Scholar] [CrossRef]
- Lima, J.C.S.; Martins, D.T.O.; de Souza, P.T., Jr. Experimental evaluation of stem bark of Stryphnodendron adstringens (Mart.) Coville for antiinflammatory activity. Phytother. Res. 1998, 12, 218–220. [Google Scholar] [CrossRef]
- Lopes, G.C.; Sanches, A.C.C.; Nakamura, C.V.; Dias Filho, B.P.; Hernandes, L.; de Mello, J.C.P. Influence of extracts of Stryphnodendron polyphyllum Mart. and Stryphnodendron obovatum Benth. on the cicatrisation of cutaneous wounds in rats. J. Ethnopharmacol. 2005, 99, 265–272. [Google Scholar] [CrossRef]
- Costa, M.A.; Ishida, K.; Kaplum, V.; Koslyk, E.D.A.; de Mello, J.C.P.; Ueda-Nakamura, T.; Dias Filho, B.P.; Nakamura, C.V. Safety evaluation of proanthocyanidin polymer-rich fraction obtained from stem bark of Stryphnodendron adstringens (BARBATIMAO) for use as a pharmacological agent. Regul. Toxicol. Pharmacol. 2010, 58, 330–335. [Google Scholar] [CrossRef]
- Pereira, E.M.; Gomes, R.T.; Freire, N.R.; Aguiar, E.G.; Brandão, M.d.G.L.; Santos, V.R. In vitro antimicrobial activity of Brazilian medicinal plant extracts against pathogenic microorganisms of interest to dentistry. Planta Med. 2011, 77, 401–404. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.R.; Ferreira Júnior, W.S.; de Bitu, V.C.N.; Pinheiro, P.G.; Menezes, C.D.A.; de Brito Junior, F.E.; de Albuquerque, U.P.; Kerntopf, M.R.; Coutinho, H.D.M.; Fachinetto, R.; et al. Ethnopharmacological study of Stryphnodendron rotundifoliumin two communities in the semi-arid region of northeastern Brazil. Rev. Bras. Farmacogn. 2014, 24, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.G.; Nascimento, A.M.; Henriques, B.O.; Chávez-Fumagalli, M.A.; Franca, J.R.; Duarte, M.C.; Lage, P.S.; Andrade, P.H.R.; Lage, D.P.; Rodrigues, L.B.; et al. Antileishmanial activity of standardized fractions of Stryphnodendron obovatum (Barbatimão) extract and constituent compounds. J. Ethnopharmacol. 2015, 165, 238–242. [Google Scholar] [CrossRef]
- Pinto, S.C.G.; Bueno, F.G.; Panizzon, G.P.; Morais, G.; Dos Santos, P.V.P.; Baesso, M.L.; de Leite-Mello, E.V.S.; de Mello, J.C.P. Stryphnodendron adstringens: Clarifying Wound Healing in Streptozotocin-Induced Diabetic Rats. Planta Med. 2015, 81, 1090–1096. [Google Scholar] [CrossRef]
- Vandesmet, V.C.S.; Felipe, C.F.B.; Kerntopf, M.R.; Rolón, M.; Vega, C.; Coronel, C.; Barbosa, A.G.R.; Coutinho, H.D.M.; Menezes, I.R.A. The use of herbs against neglected diseases: Evaluation of in vitro leishmanicidal and trypanocidal activity of Stryphnodendron rotundifolium Mart. Saudi J. Biol. Sci. 2017, 24, 1136–1141. [Google Scholar] [CrossRef] [Green Version]
- Penido, A.B.; De Morais, S.M.; Ribeiro, A.B.; Alves, D.R.; Rodrigues, A.L.M.; dos Santos, L.H.; de Menezes, J.E.S.A. Medicinal Plants from Northeastern Brazil against Alzheimer’s Disease. Evid. Based. Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef]
- De Freitas, A.L.D.; Kaplum, V.; Rossi, D.C.P.; da Silva, L.B.R.; de Melhem, M.S.C.; Taborda, C.P.; de Mello, J.C.P.; Nakamura, C.V.; Ishida, K. Proanthocyanidin polymeric tannins from Stryphnodendron adstringens are effective against Candida spp. isolates and for vaginal candidiasis treatment. J. Ethnopharmacol. 2018, 216, 184–190. [Google Scholar] [CrossRef]
- Trevisan, D.A.C.; da Silva, P.V.; Farias, A.B.P.; Campanerut-Sá, P.A.Z.; Ribeiro, T.D.V.R.; Faria, D.R.; de Mendonça, P.S.B.; de Mello, J.C.P.; Seixas, F.A.V.; Mikcha, J.M.G. Antibacterial activity of Barbatimão (Stryphnodendron adstringens) against Staphylococcus aureus: In vitro and in silico studies. Lett. Appl. Microbiol. 2020, 71, 259–271. [Google Scholar] [CrossRef]
- Giffoni de Carvalho, J.T.; Henao Agudelo, J.S.; Baldivia, D.D.S.; Carollo, C.A.; Silva, D.B.; de Picoli Souza, K.; Saraiva Câmara, N.O.; Dos Santos, E.L. Hydroethanolic stem bark extracts of Stryphnodendron adstringens impair M1 macrophages and promote M2 polarization. J. Ethnopharmacol. 2020, 254, 112684. [Google Scholar] [CrossRef]
- Tasdemir, D.; Lack, G.; Brun, R.; Rüedi, P.; Scapozza, L.; Perozzo, R. Inhibition of Plasmodium falciparum fatty acid biosynthesis: Evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J. Med. Chem. 2006, 49, 3345–3353. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Carvalho, C.P.; Carvalho, T.C.; Eberlin, M.N. Molecular ion: A more contemporary definition. J. Mass Spectrom. 2020, 55, e4598. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Allard, P.M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.L.; Allard, P.M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yokosuka, A.; Okabe, G.; Tatsuno, S.; Mimaki, Y. Stryphnosides G-P, 10 new triterpene glycosides from the pericarps of Stryphnodendron fissuratum. Carbohydr. Res. 2016, 434, 18–26. [Google Scholar] [CrossRef]
- Yokosuka, A.; Kawakami, S.; Haraguchi, M.; Mimaki, Y. Stryphnosides A–F, six new triterpene glycosides from the pericarps of Stryphnodendron fissuratum. Tetrahedron 2008, 64, 1474–1481. [Google Scholar] [CrossRef]
- Tursch, B.; Tursch, E.; Harrison, I.T.; Silva, G.B.C.T.D.C.B.D.; Monteiro, H.J.; Gilbert, B.; Mors, W.B.; Djerassi, C. Terpenoids. LIII.1a Demonstration of Ring Conformational Changes in Triterpenes of the β-Amyrin Class Isolated from Stryphnodendron coriaceum. J. Org. Chem. 1963, 28, 2390–2394. [Google Scholar] [CrossRef]
- Palazzo de Mello, J.C.; Petereit, F.; Nahrstedt, A. A dimeric proanthocyanidin from Stryphnodendron adstringens. Phytochemistry 1999, 51, 1105–1107. [Google Scholar] [CrossRef]
- do Nascimento, A.M.; Guedes, P.T.; Castilho, R.O.; Vianna-Soares, C.D. Stryphnodendron adstringens (Mart.) Coville (Fabaceae) proanthocyanidins quantitation by RP-HPLC. Braz. J. Pharm. Sci. 2013, 49, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, J.G.M.; de Leite, G.O.; Dubois, A.F.; Seeger, R.L.; Boligon, A.A.; Athayde, M.L.; Campos, A.R.; da Rocha, J.B.T. Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará State (Brazil): Potential involvement in its therapeutic use. Molecules 2012, 17, 934–950. [Google Scholar] [CrossRef]
- Santos, S.C.; Costa, W.F.; Ribeiro, J.P.; Guimarães, D.O.; Ferri, P.H.; Ferreira, H.D.; Seraphin, J.C. Tannin composition of barbatimão species. Fitoterapia 2002, 73, 292–299. [Google Scholar] [CrossRef]
- Henriques, B.O.; Corrêa, O.; Azevedo, E.P.C.; Pádua, R.M.; de Oliveira, V.L.S.; Oliveira, T.H.C.; Boff, D.; Dias, A.C.F.; de Souza, D.G.; Amaral, F.A.; et al. In Vitro TNF- Inhibitory Activity of Brazilian Plants and Anti-Inflammatory Effect of Stryphnodendron adstringens in an Acute Arthritis Model. Evid. Based. Complement. Alternat. Med. 2016, 2016, 9872598. [Google Scholar] [CrossRef] [Green Version]
- Felipe, A.M.M.; Rincão, V.P.; Benati, F.J.; Linhares, R.E.C.; Galina, K.J.; de Toledo, C.E.M.; Lopes, G.C.; de Mello, J.C.P.; Nozawa, C. Antiviral effect of Guazuma ulmifolia and Stryphnodendron adstringens on poliovirus and bovine herpesvirus. Biol. Pharm. Bull. 2006, 29, 1092–1095. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.N.; Pedroso, N.B.; Borges, L.L.; Oliveira, G.A.R.; Paula, J.R.; Conceição, E.C. Optimization of Ultrasound-assisted extraction of polyphenols, tannins and epigallocatechin gallate from barks of Stryphnodendron adstringens (Mart.) Coville bark extracts. Pharmacogn. Mag. 2014, 10, S318–S323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, D.A.J.; Amaral, J.G.; Garcia, L.B.; Dos Santos, M.S.; Silva, L.A.O.; Almeida, M.P.; Gomes, A.F.; Barros, D.R.P.; Lopes, N.P.; Pereira, G.R.; et al. Associating chitosan and microemulsion as a topical vehicle for the administration of herbal medicines. Carbohydr. Polym. 2021, 255, 117482. [Google Scholar] [CrossRef]
- Kind, T.; Fiehn, O. Seven Golden Rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry. BMC Bioinform. 2007, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Joseph, R.C.; Silva da Fonseca Diniz, M.; Magno do Nascimento, V.; de Barbosa Muribeca, A.J.; Costa Santiago, J.C.; da Cunha Borges, L.; da Costa Sá, P.R.; Portal Gomes, P.W.; da Silva Cardoso, J.C.; Rocha de Castro, M.N.; et al. Secure and Sustainable Sourcing of Plant Tissues for the Exhaustive Exploration of Their Chemodiversity. Molecules 2020, 25, 5992. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Seeram, N.P. Ultra-fast liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry for the rapid phenolic profiling of red maple (Acer rubrum) leaves. J. Sep. Sci. 2018, 41, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Sinosaki, N.B.M.; Tonin, A.P.P.; Ribeiro, M.A.S.; Poliseli, C.B.; Roberto, S.B.; da Silveira, R.; Visentainer, J.V.; Santos, O.O.; Meurer, E.C. Structural Study of Phenolic Acids by Triple Quadrupole Mass Spectrometry with Electrospray Ionization in Negative Mode and H/D Isotopic Exchange. J. Braz. Chem. Soc. 2020, 31, 402–408. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-targeted glycosidic profiling of international wines using neutral loss-high resolution mass spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef]
- Li, H.-J.; Deinzer, M.L. Tandem Mass Spectrometry for Sequencing Proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Guerrero, L.; Albertazzi, F.; Araya-Valverde, E.; Romero, R.M.; Villalobos, H.; Poveda, L.; Chavarría, M.; Tamayo-Castillo, G. Phenolic variation among Chamaecrista nictitans subspecies and varieties revealed through UPLC-ESI(-)-MS/MS chemical fingerprinting. Metabolomics 2019, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.D.; Brodbelt, J.S. An investigation of the homolytic saccharide cleavage of deprotonated flavonol 3-O-glycosides in a quadrupole ion trap mass spectrometer. J. Mass Spectrom. 2008, 43, 1045–1052. [Google Scholar] [CrossRef]
- Hvattum, E.; Ekeberg, D. Study of the collision-induced radical cleavage of flavonoid glycosides using negative electrospray ionization tandem quadrupole mass spectrometry. J. Mass Spectrom. 2003, 38, 43–49. [Google Scholar] [CrossRef]
- Demarque, D.P.; Crotti, A.E.M.; Vessecchi, R.; Lopes, J.L.C.; Lopes, N.P. Fragmentation reactions using electrospray ionization mass spectrometry: An important tool for the structural elucidation and characterization of synthetic and natural products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Kumar, B. LC-MS Identification of Proanthocyanidins in Bark and Fruit of six Terminalia species. Nat. Prod. Commun. 2018, 13, 1934578X1801300511. [Google Scholar] [CrossRef] [Green Version]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Gürler, S.B.; Kiraz, Y.; Baran, Y. Chapter 21—Flavonoids in cancer therapy: Current and future trends. In Biodiversity and Biomedicine; Ozturk, M., Egamberdieva, D., Pešić, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 403–440. ISBN 9780128195413. [Google Scholar]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Ow, Y.-Y.; Stupans, I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Kroes, B.H.; van den Berg, A.J.; Quarles van Ufford, H.C.; van Dijk, H.; Labadie, R.P. Anti-inflammatory activity of gallic acid. Planta Med. 1992, 58, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.P.; Nguyen, M.; Yow, T.T.; Chu, C.; Johnston, G.A.R.; Hanrahan, J.R.; Chebib, M. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem. Res. 2009, 34, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, X.; Cheng, Y.; Liu, Y. Myricitrin pretreatment ameliorates mouse liver ischemia reperfusion injury. Int. Immunopharmacol. 2020, 89, 107005. [Google Scholar] [CrossRef]
- He, N.; Wang, P.; Niu, Y.; Chen, J.; Li, C.; Kang, W.-Y. Evaluation antithrombotic activity and action mechanism of myricitrin. Ind. Crops Prod. 2019, 129, 536–541. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, Z.; Wu, Y.; Zhan, J.; Qi, W.; Lin, J.; Shen, J.; Xue, X.; Pan, X. The protective effect of myricitrin in osteoarthritis: An in vitro and in vivo study. Int. Immunopharmacol. 2020, 84, 106511. [Google Scholar] [CrossRef] [PubMed]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Q.; Chen, Y.; Pan, R.; Kuang, S.; Liu, G.; Sun, G.; Sun, X. Myricitrin Alleviates Oxidative Stress-induced Inflammation and Apoptosis and Protects Mice against Diabetic Cardiomyopathy. Sci. Rep. 2017, 7, 44239. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470741689. [Google Scholar]
- Wan, S.B.; Landis-Piwowar, K.R.; Kuhn, D.J.; Chen, D.; Dou, Q.P.; Chan, T.H. Structure-activity study of epi-gallocatechin gallate (EGCG) analogs as proteasome inhibitors. Bioorg. Med. Chem. 2005, 13, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.-H.; Cho, S.-Y.; Hong, Y.-D.; Chung, J.-O.; Kim, K.-S.; Shim, S.-M. Transport of gallocatechin gallate and catechin gallate in high-temperature-processed green tea extract from gastrointestinal tract to brain by an in vitro bio-mimic model system coupled with sequential cell cultures. J. Funct. Foods 2018, 47, 83–90. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tomiyama, D.; Yui, K.; Katsuki, M.; Ikeda, K.; Nonaka, A.; Miyamoto, T. Mechanism for the antibacterial action of epigallocatechin gallate (EGCg) on Bacillus subtilis. Biosci. Biotechnol. Biochem. 2015, 79, 845–854. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Salehi, B.; Sharopov, F.; Cho, W.C.; Sharifi-Rad, J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef] [Green Version]
- De Sousa-Fontoura, D.M.N.; Olinda, R.G.; Viana, G.A.; de Costa, K.M.F.M.; Batista, J.S.; Serrano, R.M.O.T.; Silva, O.M.D.; Camara, C.A.; Silva, T.M.S. Wound healing activity and chemical composition of geopropolis from Melipona subnitida. Rev. Bras. Farmacogn. 2020, 30, 367–373. [Google Scholar] [CrossRef]
- Santos, H.F.D.; Campos, J.F.; Santos, C.M.D.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; de Picoli Souza, K.; Estevinho, L.M.; Dos Santos, E.L. Chemical Profile and Antioxidant, Anti-Inflammatory, Antimutagenic and Antimicrobial Activities of Geopropolis from the Stingless Bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. [Google Scholar] [CrossRef]
- Wang, J.-B.; Qin, Y.; Kong, W.-J.; Wang, Z.-W.; Zeng, L.-N.; Fang, F.; Jin, C.; Zhao, Y.-L.; Xiao, X.-H. Identification of the antidiarrhoeal components in official rhubarb using liquid chromatography–tandem mass spectrometry. Food Chem. 2011, 129, 1737–1743. [Google Scholar] [CrossRef]
- Banerjee, P.; Erehman, J.; Gohlke, B.-O.; Wilhelm, T.; Preissner, R.; Dunkel, M. Super Natural II—A database of natural products. Nucleic Acids Res. 2014, 43, D935–D939. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.W.; Do, H.J.; Jeon, J.-H.; Kim, K. Quercitrin inhibits platelet activation in arterial thrombosis. Phytomedicine 2021, 80, 153363. [Google Scholar] [CrossRef]
- Dönder, Y.; Arikan, T.B.; Baykan, M.; Akyüz, M.; Öz, A.B. Effects of quercitrin on bacterial translocation in a rat model of experimental colitis. Asian J. Surg. 2018, 41, 543–550. [Google Scholar] [CrossRef]
- Cincin, Z.B.; Unlu, M.; Kiran, B.; Bireller, E.S.; Baran, Y.; Cakmakoglu, B. Molecular mechanisms of quercitrin-induced apoptosis in non-small cell lung cancer. Arch. Med. Res. 2014, 45, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.S.; Park, S.N. Cytoprotective effects and mechanisms of quercetin, quercitrin and avicularin isolated from Lespedeza cuneata G. Don against ROS-induced cellular damage. J. Ind. Eng. Chem. 2019, 71, 160–166. [Google Scholar] [CrossRef]
- Chambers, M.C.; MacLean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.I. Cytoscape: A Software Environment for Integrated Models. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
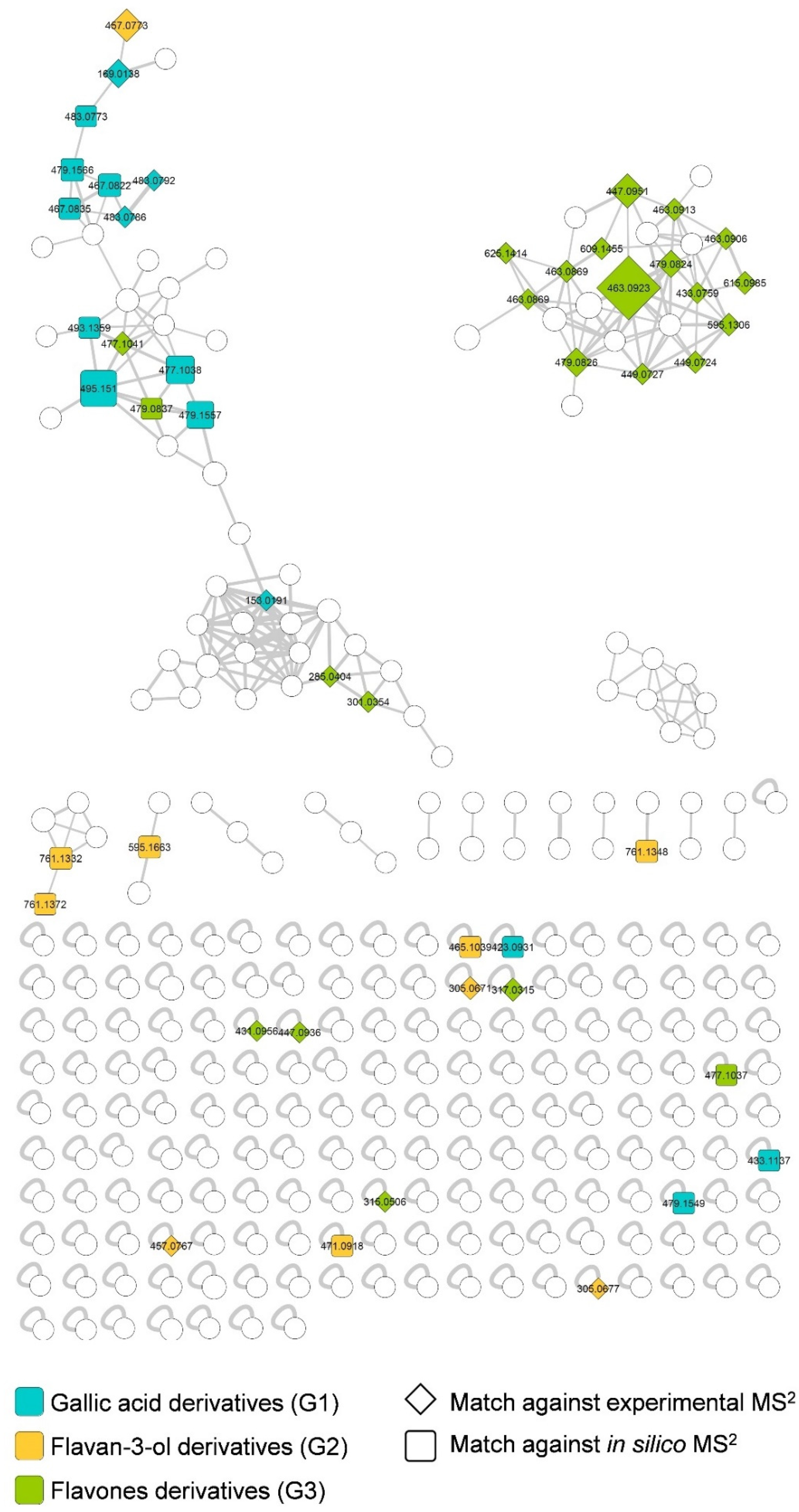
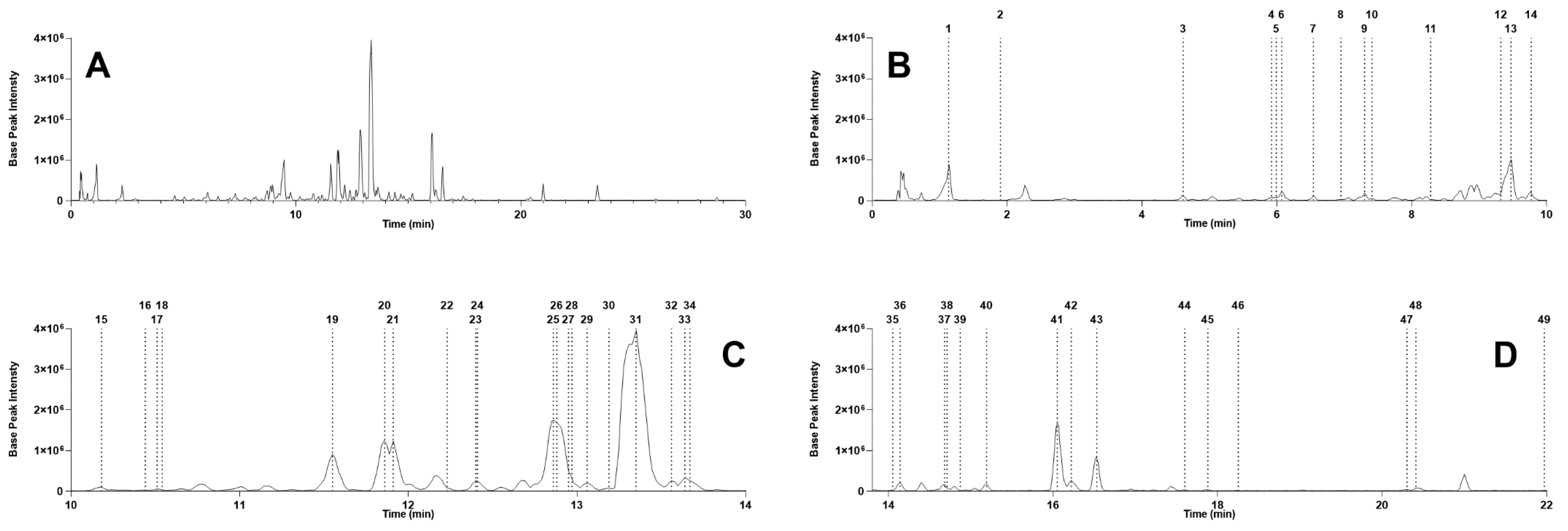

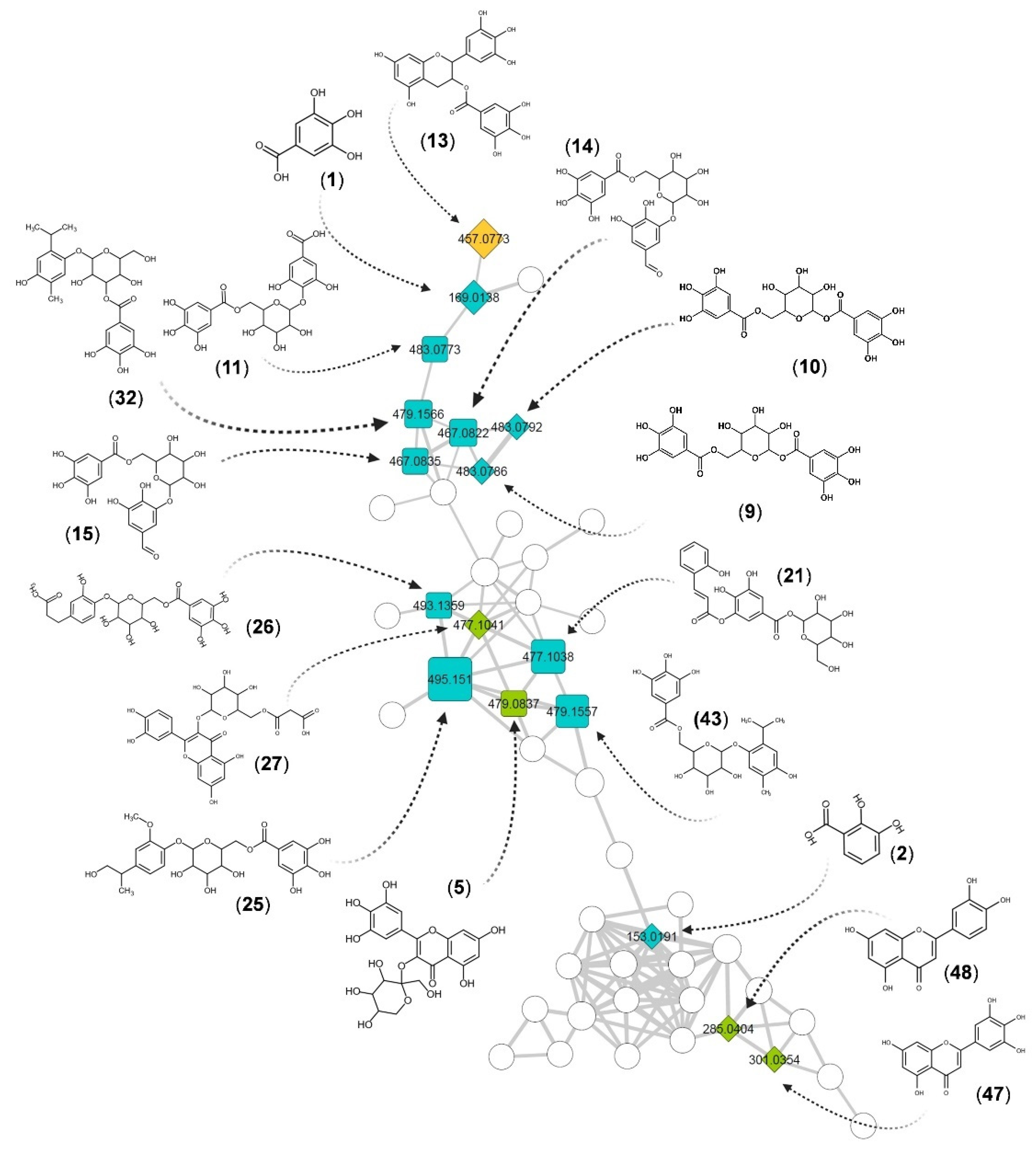


| Peak | RT (min) | [M–H]− | Molecular Formula | Error (ppm) | MS2 Fragments (m/z) | Compound Name | Spectrum Reference | Group | |
|---|---|---|---|---|---|---|---|---|---|
| Theoretical Mass (m/z) | Accurate Mass (m/z) | ||||||||
| 1 | 1.13 | 169.0137 | 169.0138 | C7H6O5 | 0.6 | 125 | gallic acid | CCMSLIB00004691622 | 1 |
| 2 | 1.90 | 153.0188 | 153.0191 | C7H6O4 | 2.0 | 109 | protocatechuic acid | CCMSLIB00000578339 | 1 |
| 3 | 4.61 | 761.1354 | 761.1332 | C37H30O18 | 2.9 | 609, 591, 573, 483, 465, 453, 441, 423, 355, 305, 285, 177, 125 | 3-O-galloyl-(epi)gallocatechin-(epi)gallocatechin | N/A | 2 |
| 4 | 5.92 | 305.0661 | 305.0677 | C15H14O7 | 5.2 | 261, 219, 179, 125 | gallocatechin | CCMSLIB00000081482 | 2 |
| 5 | 5.99 | 479.0826 | 479.0837 | C21H20O13 | 2.3 | 435, 389, 313, 250, 169, 125, 121 | myricetin 3-sorboside | N/A | 3 |
| 6 | 6.07 | 305.0661 | 305.0671 | C15H14O7 | 3.3 | 177, 150, 139, 125, 109 | (epi)gallocatechin | CCMSLIB00005787972 | 2 |
| 7 | 6.54 | 761.1354 | 761.1348 | C37H30O18 | 0.8 | 717, 635, 609, 593, 591, 575, 441, 423, 405, 305, 287, 243, 169, 125 | (epi)gallocatechin-3′-O-galloyl-(epi)gallocatechin | N/A | 2 |
| 8 | 6.95 | 423.0927 | 423.0931 | C19H20O11 | 0.9 | 313, 253, 169, 125 | 6-O-galloylarbutin | N/A | 1 |
| 9 | 7.30 | 483.0775 | 483.0786 | C20H20O14 | 2.3 | 331, 313, 287, 271, 211, 169, 125 | [3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyl)oxyoxan-2-yl]methyl 3,4,5-trihydroxybenzoate Isomer | CCMSLIB00000845139 | 1 |
| 10 | 7.41 | 483.0775 | 483.0792 | C20H20O14 | 3.5 | 331, 313, 287, 271, 211, 169, 125 | [3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyl)oxyoxan-2-yl]methyl 3,4,5-trihydroxybenzoate Isomer | CCMSLIB00000845139 | 1 |
| 11 | 8.28 | 483.0775 | 483.0773 | C20H20O14 | 0.4 | 169, 125 | gallic acid O-galloyl glucoside | N/A | 1 |
| 12 | 9.32 | 595.1663 | 595.1663 | C27H32O15 | 0.0 | 415, 385, 355, 343, 325, 313, 271, 235, 193, 119 | 3,5,7-trihydroxyflavanone-5-O-[galactopyranosyl-glucopyranoside] | N/A | 2 |
| 13 | 9.47 | 457.0771 | 457.0773 | C22H18O11 | 0.4 | 305, 261, 219, 179, 169, 125 | (epi)gallocatechin gallate | CCMSLIB00004702014 | 2 |
| 14 | 9.77 | 467.0826 | 467.0822 | C20H20O13 | 0.9 | 449, 423, 315, 313, 193, 169, 152, 125, 109 | 3,4,5-trihydroxybenzaldehyde-3-O-[6-O-(3,4,5-trihydroxybenzoyl)-glucopyranoside] Isomer | N/A | 1 |
| 15 | 10.18 | 467.0826 | 467.0835 | C20H20O13 | 1.9 | 449, 423, 315, 313, 169, 152, 125, 109 | 3,4,5-trihydroxybenzaldehyde-3-O-[6-O-(3,4,5-trihydroxybenzoyl)-glucopyranoside] Isomer | N/A | 1 |
| 16 | 10.44 | 465.1033 | 465.1039 | C21H22O12 | 1.3 | 447, 421, 357, 313, 285, 169, 151, 125 | taxifolin-4′-glucoside | N/A | 2 |
| 17 | 10.51 | 625.1405 | 625.1414 | C27H30O17 | 1.4 | 373, 316, 287, 271, 259, 242, 214, 178, 151, 116 | myricetin-3-O-hexosyl-deoxyhexoside | CCMSLIB00004678839 | 3 |
| 18 | 10.54 | 457.0771 | 457.0767 | C22H18O11 | 0.9 | 411, 383, 331, 305, 169, 125 | gallocatechin gallate | CCMSLIB00004702014 | 2 |
| 19 | 11.55 | 479.0826 | 479.0824 | C21H20O13 | 0.4 | 316, 287, 271, 259, 243, 214, 151, 124 | myricetin 3-galactoside Isomer | CCMSLIB00004706173 | 3 |
| 20 | 11.86 | 479.0826 | 479.0826 | C21H20O13 | 0.0 | 316, 287, 271, 259, 243, 214, 151, 124 | myricetin 3-galactoside Isomer | CCMSLIB00004706173 | 3 |
| 21 | 11.91 | 477.1033 | 477.1038 | C22H22O12 | 1.0 | 316, 313, 271, 169, 163, 151, 125, 119 | O-coumaroyl O-galloyl-hexoside | N/A | 1 |
| 22 | 12.23 | 761.1354 | 761.1372 | C37H30O18 | 2.4 | 609, 593, 591, 575, 545, 483, 465, 457, 441, 423, 305, 287, 243, 177, 125 | 3-O-galloyl-(epi)gallocatechin-(epi)gallocatechin | N/A | 2 |
| 23 | 12.40 | 595.1299 | 595.1306 | C26H28O16 | 1.2 | 431, 355, 316, 271, 219, 179, 151, 100 | myricetin-3-O-deoxyhexosyl-pentoside | CCMSLIB00004678827 | 3 |
| 24 | 12.41 | 471.0927 | 471.0918 | C23H20O11 | 1.9 | 429, 347, 305, 287, 183 | 3-O-galloyl-4′-O-methylepigallocatechin | N/A | 2 |
| 25 | 12.86 | 495.1503 | 495.1510 | C23H28O12 | 1.4 | 313, 181, 179, 169, 151, 125 | (3,4,5-trihydroxy-6-(4-(1-hydroxypropan-2-yl)-2-methoxyphenoxy)tetrahydro-2H-pyran-2-yl)methyl 3,4,5-trihydroxybenzoate | N/A | 1 |
| 26 | 12.88 | 493.1346 | 493.1359 | C23H26O12 | 2.6 | 313, 181, 179, 169, 151, 125 | (3,4,5-trihydroxy-6-(2-hydroxy-4-(3-oxobutyl)phenoxy)tetrahydro-2H-pyran-2-yl)methyl 3,4,5-trihydroxybenzoate | N/A | 1 |
| 27 | 12.95 | 477.1033 | 477.1041 | C22H22O12 | 1.7 | 433, 313, 253, 241, 211, 169, 163, 151, 125, 119, 107 | methoxy-quercetin-3-O-hexoside | CCMSLIB00004678842 | 3 |
| 28 | 12.97 | 609.1456 | 609.1455 | C27H30O16 | 0.2 | 495, 415, 373, 300, 271, 255, 243, 151, 119 | quercetin 3-O-neohesperidoside | CCMSLIB00004679290 | 3 |
| 29 | 13.06 | 449.0720 | 449.0724 | C20H18O12 | 0.9 | 359, 316, 287, 210, 178, 151 | myricetin-3-xyloside Isomer | CCMSLIB00000222475 | 3 |
| 30 | 13.19 | 449.0720 | 449.0727 | C20H18O12 | 1.6 | 316, 287, 271, 214, 178, 151 | myricetin-3-xyloside Isomer | CCMSLIB00000222475 | 3 |
| 31 | 13.35 | 463.0877 | 463.0923 | C21H20O12 | 9.9 | 316, 287, 271, 179, 151, 107 | myricitrin Isomer | CCMSLIB00004718497 | 3 |
| 32 | 13.56 | 479.1553 | 479.1566 | C23H28O11 | 2.7 | 313, 169, 165, 151, 125, 123 | 3,5-dihydroxy-2-(4-hydroxy-2-isopropyl-5-methylphenoxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-4-yl-3,4,5-trihydroxybenzoate | N/A | 1 |
| 33 | 13.64 | 615.0986 | 615.0985 | C28H24O16 | 0.2 | 463, 301, 169 | [(2S,3R,4S,5R,6R)-2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxochromen-3-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl] 3,4,5-trihydroxybenzoate | CCMSLIB00004706250 | 3 |
| 34 | 13.67 | 463.0877 | 463.0869 | C21H20O12 | 1.7 | 316, 300, 287, 271, 243, 178, 151 | myricetin-3-O-rhamnopyranoside | CCMSLIB00005463729 | 3 |
| 35 | 14.05 | 463.0877 | 463.0869 | C21H20O12 | 1.7 | 316, 300, 259, 218, 179, 117 | myricitrin Isomer | CCMSLIB00004706620 | 3 |
| 36 | 14.14 | 463.0877 | 463.0913 | C21H20O12 | 7.8 | 300, 271, 255, 179, 151, 104 | isoquercitrin | CCMSLIB00000077231 | 3 |
| 37 | 14.68 | 433.0771 | 433.0759 | C20H18O11 | 2.8 | 387, 348, 300, 249, 217, 178, 113 | avicularin | CCMSLIB00004706151 | 3 |
| 38 | 14.71 | 479.1553 | 479.1549 | C23H28O11 | 0.8 | 313, 300, 169, 151, 123 | 6″-O-galloylepirhododendrin | N/A | 1 |
| 39 | 14.87 | 477.1033 | 477.1037 | C22H22O12 | 0.8 | 405, 316, 300 | 3′,5,7,8-tetrahydroxy-4′-methoxyflavone-8-O-glucopyranoside | N/A | 3 |
| 40 | 15.19 | 463.0877 | 463.0906 | C21H20O12 | 6.2 | 391, 301, 272, 239, 151 | quercetin-4-glucoside | CCMSLIB00004683609 | 3 |
| 41 | 16.05 | 447.0927 | 447.0951 | C21H20O11 | 5.3 | 300, 271, 255, 243, 151 | quercitrin | CCMSLIB00004679288 | 3 |
| 42 | 16.22 | 317.0297 | 317.0315 | C15H10O8 | 5.7 | 178, 151, 137, 107 | myricetin | CCMSLIB00004705572 | 3 |
| 43 | 16.53 | 479.1553 | 479.1557 | C23H28O11 | 0.8 | 169, 165, 151, 125 | querglanin | N/A | 1 |
| 44 | 17.60 | 433.1135 | 433.1137 | C21H22O10 | 0.5 | 313, 169, 151, 125, 119 | phlorizin chalcone | N/A | 1 |
| 45 | 17.88 | 447.0927 | 447.0936 | C21H20O11 | 2.0 | 432, 405, 285, 199, 1 75, 151, 113 | luteolin-4′-O-glucoside | CCMSLIB00004720065 | 3 |
| 46 | 18.25 | 431.0978 | 431.0956 | C21H20O10 | 5.1 | 314, 285, 255, 227, 124 | kaempferol-7-O-deoxyhexoside | CCMSLIB00004678854 | 3 |
| 47 | 20.30 | 301.0348 | 301.0354 | C15H10O7 | 2.0 | 257, 178, 145, 116 | tricetin | CCMSLIB00004691766 | 3 |
| 48 | 20.41 | 285.0399 | 285.0404 | C15H10O6 | 1.7 | 251, 183, 116 | 3′,4′,5,7-tetrahydroxyflavone | CCMSLIB00004691238 | 3 |
| 49 | 21.97 | 315.0505 | 315.0506 | C16H12O7 | 0.3 | 300, 231, 188, 116 | 3′-O-methylquercetin | CCMSLIB00000081595 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, P.; Quirós-Guerrero, L.; Silva, C.; Pamplona, S.; Boutin, J.A.; Eberlin, M.; Wolfender, J.-L.; Silva, M. Feature-Based Molecular Network-Guided Dereplication of Natural Bioactive Products from Leaves of Stryphnodendron pulcherrimum (Willd.) Hochr. Metabolites 2021, 11, 281. https://doi.org/10.3390/metabo11050281
Gomes P, Quirós-Guerrero L, Silva C, Pamplona S, Boutin JA, Eberlin M, Wolfender J-L, Silva M. Feature-Based Molecular Network-Guided Dereplication of Natural Bioactive Products from Leaves of Stryphnodendron pulcherrimum (Willd.) Hochr. Metabolites. 2021; 11(5):281. https://doi.org/10.3390/metabo11050281
Chicago/Turabian StyleGomes, Paulo, Luis Quirós-Guerrero, Consuelo Silva, Sônia Pamplona, Jean A. Boutin, Marcos Eberlin, Jean-Luc Wolfender, and Milton Silva. 2021. "Feature-Based Molecular Network-Guided Dereplication of Natural Bioactive Products from Leaves of Stryphnodendron pulcherrimum (Willd.) Hochr" Metabolites 11, no. 5: 281. https://doi.org/10.3390/metabo11050281







