Characterizing Autophagy in the Cold Ischemic Injury of Small Bowel Grafts: Evidence from Rat Jejunum
Abstract
1. Introduction
2. Results
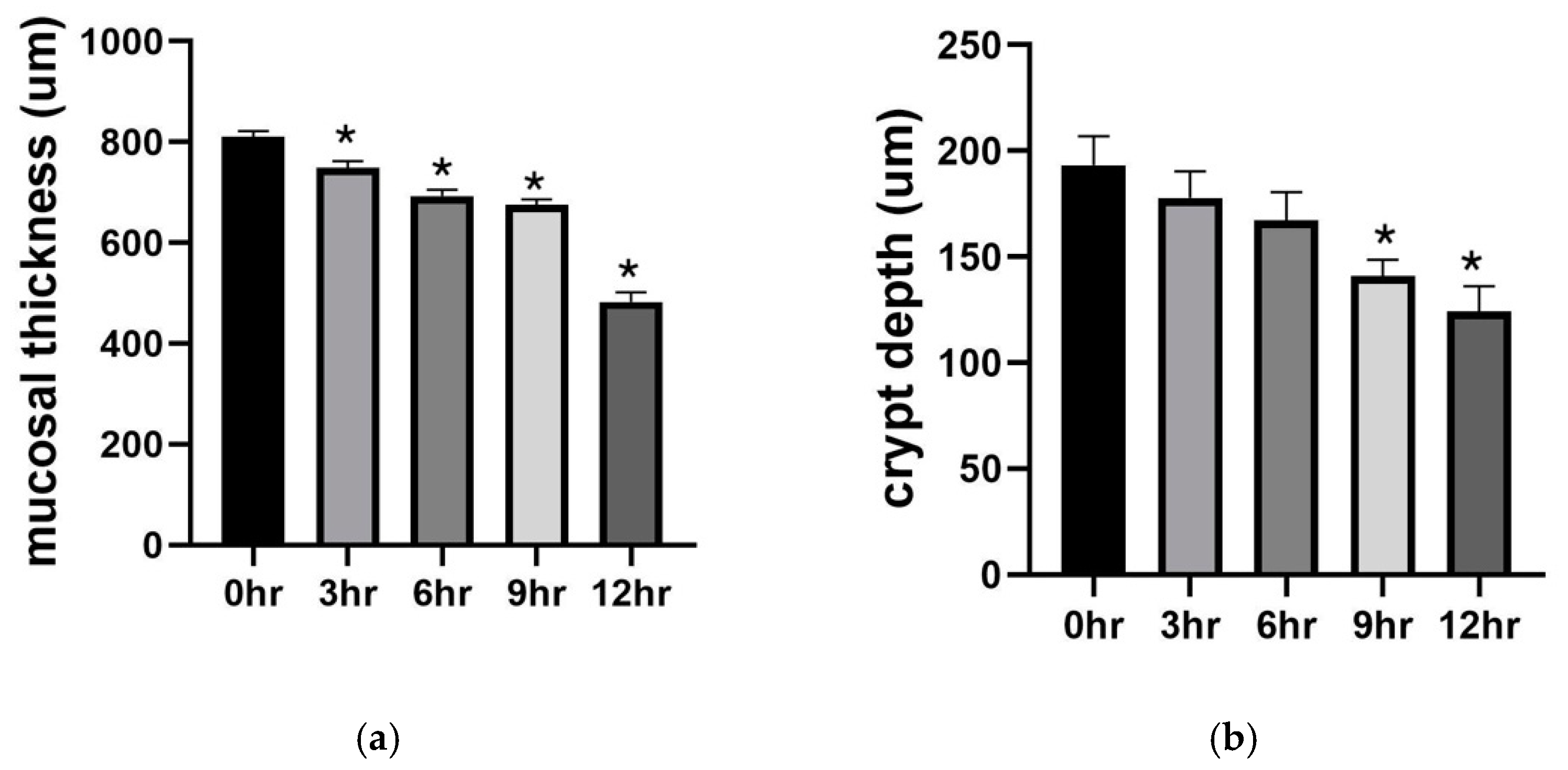
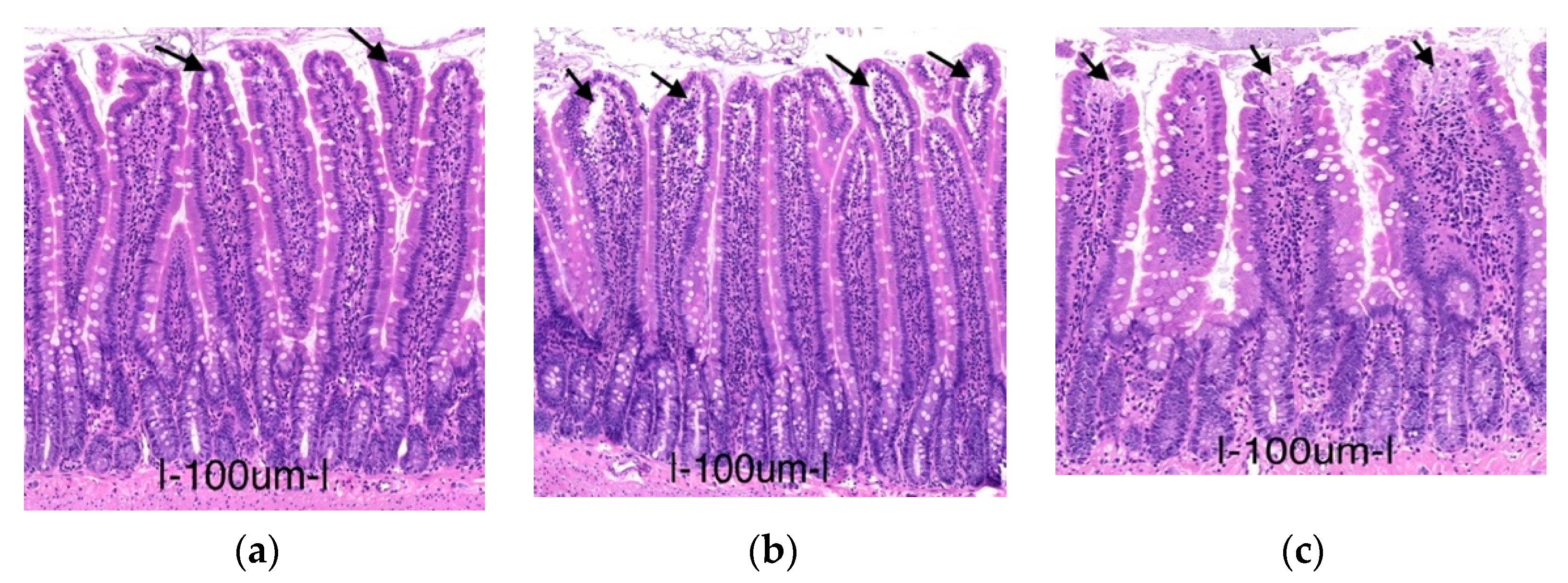
2.1. Preservation Injury of Small Bowel Grafts
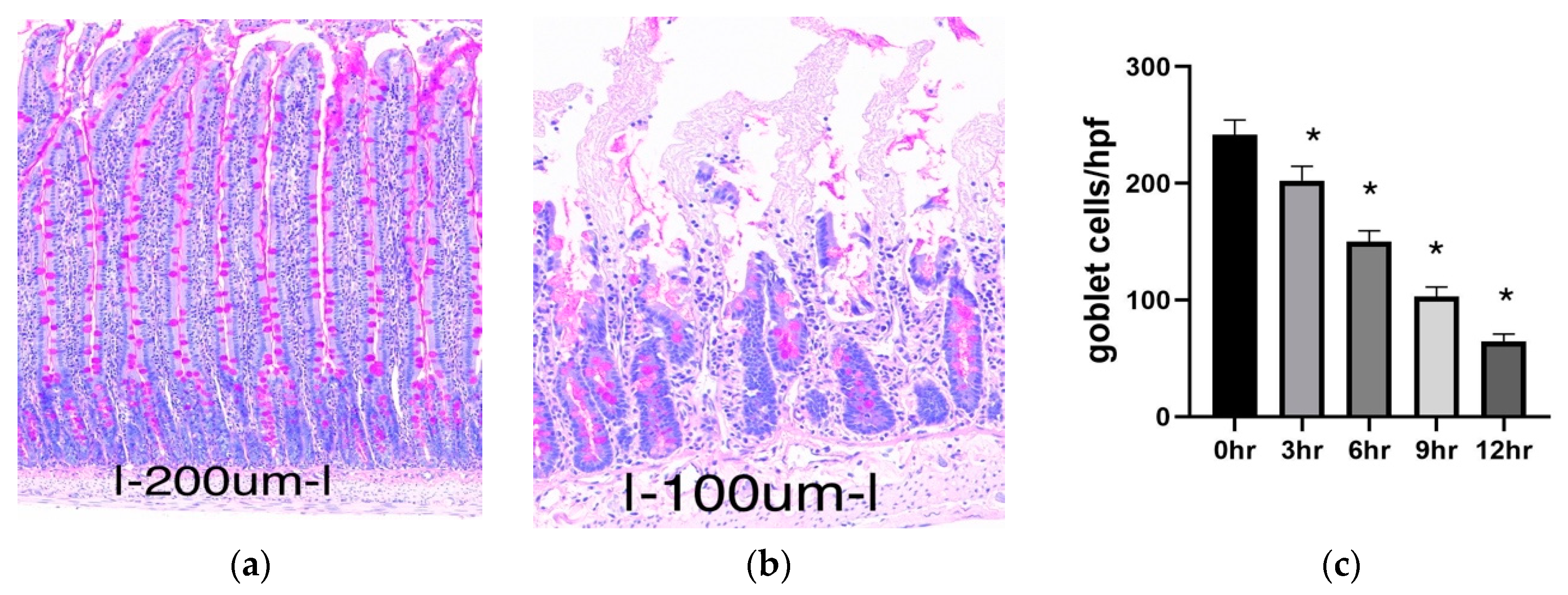
2.1.1. Histology
2.1.2. Goblet Cell Abundance
2.1.3. Biochemical Analysis
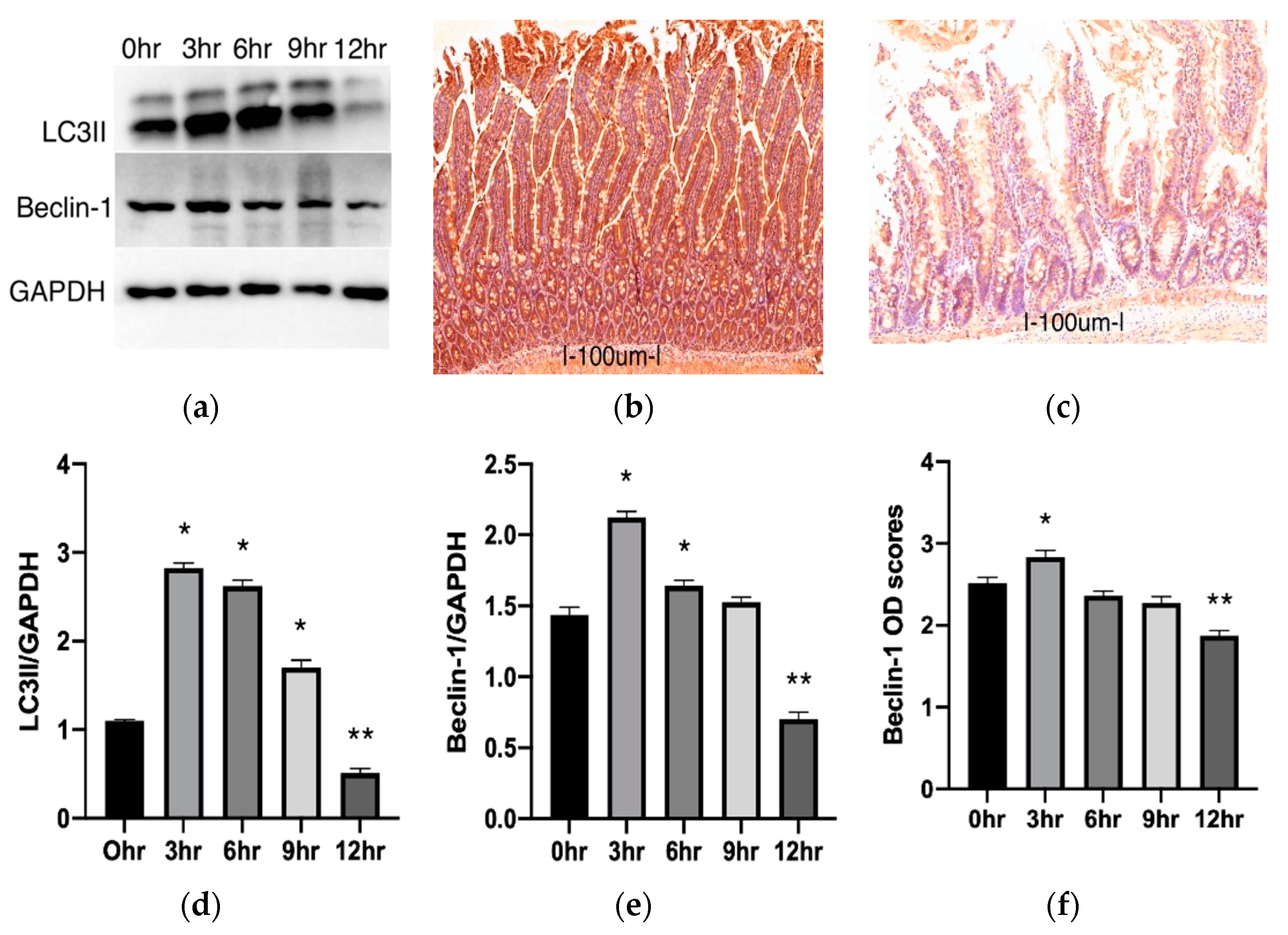
2.2. Autophagy Changes during Cold Preservation of Small Bowel Grafts
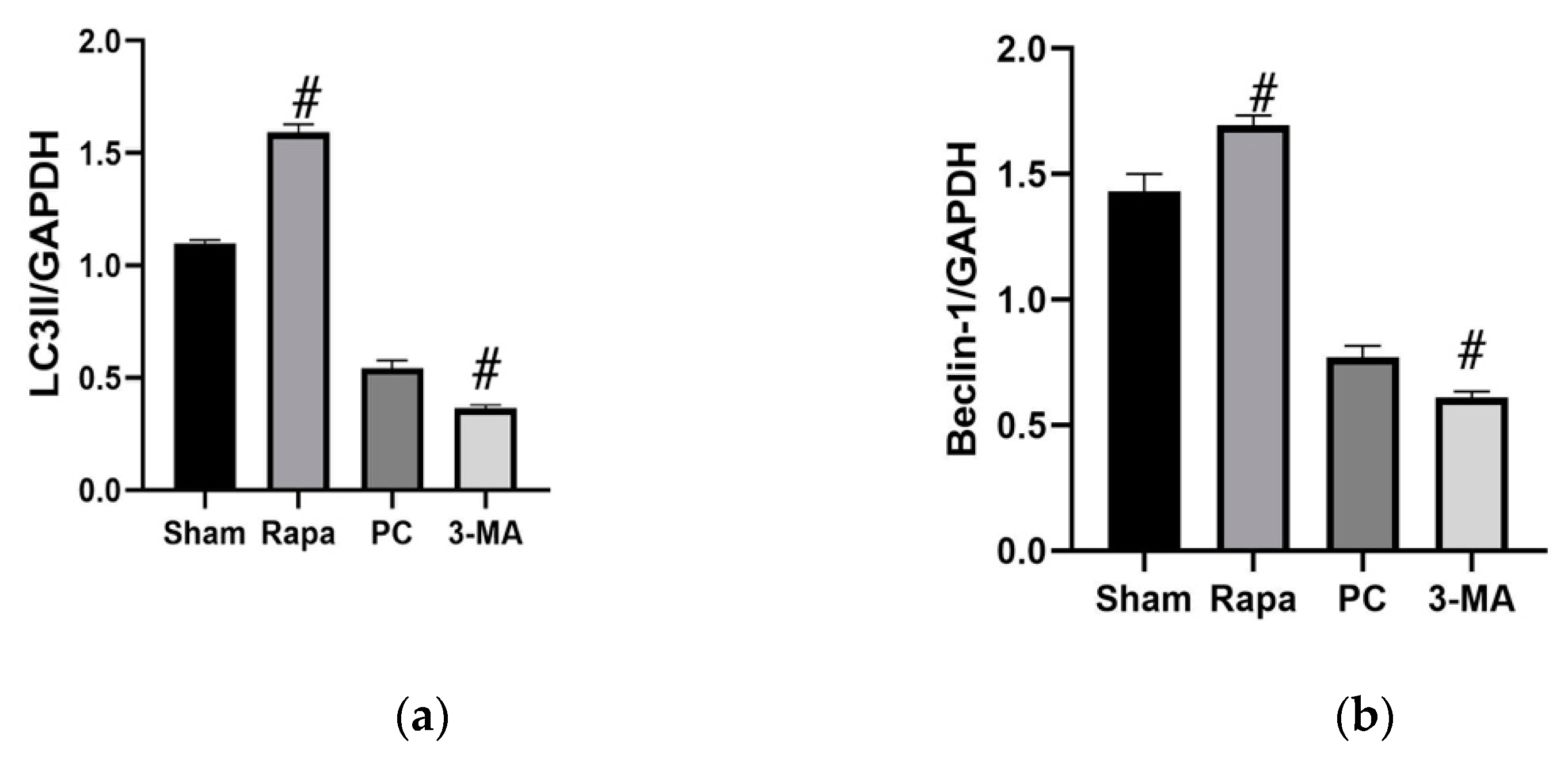
2.3. Effects of Regulating Autophagy during Cold Preservation of Small Bowel Grafts
2.3.1. Changes in Autophagic Activity
2.3.2. Effects on Apoptosis
2.3.3. Effects on Intestinal Mucosa
2.3.4. Effects on LDH and Lactate
2.3.5. Reperfusion Injury
2.3.6. The Protective Effects of Rapa-Enhanced Autophagy Are Attenuated by 3-MA
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Intestinal Procurement and Grouping
4.3. Histology (Hematoxylin–Eosin)
4.4. Goblet Cell Count
4.5. Immunohistochemistry (IHC)
4.6. Western Blot (wb)
4.7. TEM
4.8. Biochemical Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaz, P.; Matia, I.; Jackanin, S.; Rybarova, E.; Kron, I.; Pomfy, M.; Fronek, J.; Ryska, M. Preservation injury of jejunal grafts and its modulation by custodiol and University of Wisconsin perfusion solutions in Wistar rats. Eur. Surg. Res. 2004, 36, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Loo, L.; Vrakas, G.; Reddy, S.; Allan, P. Intestinal transplantation: A review. Curr. Opin. Gastroenterol. 2017, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Oltean, M. Intestinal preservation for transplantation: Current status and alternatives for the future. Curr. Opin. Organ Transplant. 2015, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elmagd, K.; Fung, J.; Bueno, J.; Martin, D.; Madariaga, J.R.; Mazariegos, G.; Bond, G.; Molmenti, E.; Corry, R.J.; Starzl, T.E.; et al. Logistics and technique for procurement of intestinal, pancreatic, and hepatic grafts from the same donor. Ann. Surg. 2000, 232, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Lysyy, T.; Finotti, M.; Maina, R.M.; Morotti, R.; Munoz-Abraham, A.S.; Bertacco, A.; Ibarra, C.; Barahona, M.; Agarwal, R.; D’Amico, F.; et al. Human Small Intestine Transplantation: Segmental Susceptibility to Ischemia Using Different Preservation Solutions and Conditions. Transplant. Proc. 2020, 52, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- Salehi, P.; Spratlin, J.; Chong, T.F.; Churchill, T.A. Beneficial effects of supplemental buffer and substrate on energy metabolism during small bowel storage. Cryobiology 2004, 48, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Stasko, P.; Toth, S.; Pristasova, Z.; Bujdos, M.; Pomfy, M. Development of jejunal graft damage during intestinal transplantation. Ann. Transplant. 2009, 14, 62–69. [Google Scholar] [PubMed]
- Park, P.O.; Haglund, U.; Bulkley, G.B.; Falt, K. The sequence of development of intestinal tissue-injury after strangulation ischemia and reperfusion. Surgery 1990, 107, 574–580. [Google Scholar]
- Leuvenink, H.G.D.; van Dijk, A.; Freund, R.L.; Ploeg, R.J.; van Goor, H. Luminal preservation of rat small intestine with University of Wisconsin or Celsior solution. Transplant. Proc. 2005, 37, 445–447. [Google Scholar] [CrossRef]
- Tesi, R.J.; Jaffe, B.M.; McBride, V.; Haque, S. Histopathologic changes in human small intestine during storage in viaspan organ preservation solution. Arch. Pathol. Lab. Med. 1997, 121, 714–718. [Google Scholar] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yang, Y.; Karsli-Uzunbas, G.; Poillet-Perez, L.; Sawant, A.; Hu, Z.S.; Zhao, Y.H.; Moore, D.; Hu, W.W.; White, E. Autophagy promotes mammalian survival by suppressing oxidative stress and p53. Genes Dev. 2020, 34, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Li, B.C.; Yao, X.; Luo, Y.H.; Niu, L.J.; Lin, L.; Li, Y.S. Inhibition of Autophagy Attenuated Intestinal Injury After Intestinal I/R via mTOR Signaling. J. Surg. Res. 2019, 243, 363–370. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Qiu, W.; Jin, P.; Wang, P.; Sun, Y. Role of autophagy and its molecular mechanisms in mice intestinal tract after severe burn. J. Trauma Acute Care Surg. 2017, 83, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Arozena, A.A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, J.X.; You, X.J.; Zhu, H.W.; Wei, J.L.; Xu, M.Y. Cold ischemia-induced autophagy in rat lung tissue. Mol. Med. Rep. 2015, 11, 2513–2519. [Google Scholar] [CrossRef][Green Version]
- Chen-Scarabelli, C.; Agrawal, P.R.; Saravolatz, L.; Abuniat, C.; Scarabelli, G.; Stephanou, A.; Loomba, L.; Narula, J.; Scarabelli, T.M.; Knight, R. The role and modulation of autophagy in experimental models of myocardial ischemia-reperfusion injury. J. Geriatr. Cardiol. 2014, 11, 338–348. [Google Scholar] [PubMed]
- Cursio, R.; Colosetti, P.; Gugenheim, J. Autophagy and Liver Ischemia-Reperfusion Injury. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.; Yu, B.T.; Huang, L.; Dai, B.; Liu, J.C.; Tang, J. The role of autophagy in lung ischemia/reperfusion injury after lung transplantation in rats. Am. J. Transl. Res. 2016, 8, 3593–3602. [Google Scholar] [PubMed]
- Mejlvang, J.; Olsvik, H.; Svenning, S.; Bruun, J.A.; Abudu, Y.P.; Larsen, K.B.; Brech, A.; Hansen, T.E.; Brenne, H.; Hansen, T.; et al. Starvation induces rapid degradation of selective autophagy receptors by endosomal microautophagy. J. Cell Biol. 2018, 217, 3640–3655. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef]
- Softeland, J.M.; Bagge, J.; Padma, A.M.; Casselbrant, A.; Zhu, C.L.; Wang, Y.F.; Hellstrom, M.; Olausson, M.; Oltean, M. Luminal polyethylene glycol solution delays the onset of preservation injury in the human intestine. Am. J. Transplant. 2021, 21, 2220–2230. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Marino, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef]
- Turkmen, K.; Martin, J.; Akcay, A.; Nguyen, Q.; Ravichandran, K.; Faubel, S.; Pacic, A.; Ljubanovic, D.; Edelstein, C.L.; Jani, A. Apoptosis and Autophagy in Cold Preservation Ischemia. Transplantation 2011, 91, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Autophagy and apoptosis in liver injury. Cell Cycle 2015, 14, 1631–1642. [Google Scholar] [CrossRef]
- Zhu, J.J.; Lu, T.F.; Yue, S.; Shen, X.D.; Gao, F.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Xia, Q.; Zhai, Y. Rapamycin Protection of Livers From Ischemia and Reperfusion Injury Is Dependent on Both Autophagy Induction and Mammalian Target of Rapamycin Complex 2-Akt Activation. Transplantation 2015, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, W.; Sun, Y.L.; Han, F.; Hu, C.A.A.; Wu, Z.L. Amino acid deprivation disrupts barrier function and induces protective autophagy in intestinal porcine epithelial cells. Amino Acids 2015, 47, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Softeland, J.M.; Casselbrant, A.; Biglarnia, A.R.; Linders, J.; Hellstrom, M.; Pesce, A.; Padma, A.M.; Jiga, L.P.; Hoinoiu, B.; Ionac, M.; et al. Intestinal Preservation Injury: A Comparison Between Rat, Porcine and Human Intestines. Int. J. Mol. Sci. 2019, 20, 3135. [Google Scholar] [CrossRef]
- Sola, A.; De Oca, J.; Gonzalez, R.; Prats, N.; Rosello-Catafau, J.; Gelpi, E.; Jaurrieta, E.; Hotter, G. Protective effect of ischemic preconditioning on cold preservation and reperfusion injury associated with rat intestinal transplantation. Ann. Surg. 2001, 234, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One Drug, Many Effects. Cell Metab. 2014, 19, 373–379. [Google Scholar] [CrossRef]
- Lopez-Garcia, P.; Pulido, J.C.; Colina, F.; Jimenez-Romero, C.; de Andres, C.I.; Lopez-Alonso, G.; Loinaz, C.; Gonzalez, M.A.M.; Alonso, I.J.; Molero, F.C.; et al. Histologic Evaluation of Organ Preservation Injury and Correlation With Cold Ischemia Time in 13 Intestinal Grafts. Transplant. Proc. 2014, 46, 2096–2098. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, W.; Li, L.; Wu, S.; Du, G.H. Pinocembrin Protects the Brain against Ischemia-Reperfusion Injury and Reverses the Autophagy Dysfunction in the Penumbra Area. Molecules 2014, 19, 15786–15798. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Pallet, N. Response Letter to “Autophagy in Renal Ischemia-Reperfusion Injury: Friend or Foe?”. Am. J. Transplant. 2014, 14, 1466–1467. [Google Scholar] [CrossRef] [PubMed]
- Cicalese, I.; Sileri, P.; Green, M.; Abu-Elmagd, K.; Fung, J.J.; Starzl, T.E.; Reyes, J. Bacterial translocation in clinical intestinal transplantation. Transplant. Proc. 2000, 32, 1210. [Google Scholar] [CrossRef]
- Decuypere, J.P.; Ceulemans, L.J.; Agostinis, P.; Monbaliu, D.; Naesens, M.; Pirenne, J.; Jochmans, I. Autophagy and the Kidney: Implications for Ischemia-Reperfusion Injury and Therapy. Am. J. Kidney Dis. 2015, 66, 699–709. [Google Scholar] [CrossRef]
- Brown, M.F.; Ross, A.J.; Dasher, J.; Turley, D.L.; Ziegler, M.M.; Oneill, J.A. The role of leukocytes in mediating mucosal injury of intestinal ischemia reperfusion. J. Pediatric Surg. 1990, 25, 214–217. [Google Scholar] [CrossRef]
- Jafari, S.M.S.; Hunger, R.E. IHC Optical Density Score: A New Practical Method for Quantitative Immunohistochemistry Image Analysis. Appl. Immunohistochem. Mol. Morphol. 2017, 25, E12–E13. [Google Scholar] [CrossRef] [PubMed]

| Groups | 0 h | 3 h | 6 h | 9 h | 12 h |
|---|---|---|---|---|---|
| Post-preservation | 0.16 ± 0.04 | 1.13 ± 0.09 * | 2.21 ± 0.12 * | 2.86 ± 0.10 * | 4.27 ± 0.10 * |
| Groups | 0 h | 6 h | 12 h |
|---|---|---|---|
| LDH (IU/L) | 27.44 ± 2.17 | 349.40 ± 15.86 * | 716.30 ± 34.02 * |
| Lactate (mmol/L) | 0.07 ± 0.01 | 0.68 ± 0.06 * | 1.55 ± 0.06 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caleb, I.; Erlitz, L.; Telek, V.; Vecsernyés, M.; Sétáló, G., Jr.; Hardi, P.; Takács, I.; Jancsó, G.; Nagy, T. Characterizing Autophagy in the Cold Ischemic Injury of Small Bowel Grafts: Evidence from Rat Jejunum. Metabolites 2021, 11, 396. https://doi.org/10.3390/metabo11060396
Caleb I, Erlitz L, Telek V, Vecsernyés M, Sétáló G Jr., Hardi P, Takács I, Jancsó G, Nagy T. Characterizing Autophagy in the Cold Ischemic Injury of Small Bowel Grafts: Evidence from Rat Jejunum. Metabolites. 2021; 11(6):396. https://doi.org/10.3390/metabo11060396
Chicago/Turabian StyleCaleb, Ibitamuno, Luca Erlitz, Vivien Telek, Mónika Vecsernyés, György Sétáló, Jr., Péter Hardi, Ildikó Takács, Gábor Jancsó, and Tibor Nagy. 2021. "Characterizing Autophagy in the Cold Ischemic Injury of Small Bowel Grafts: Evidence from Rat Jejunum" Metabolites 11, no. 6: 396. https://doi.org/10.3390/metabo11060396
APA StyleCaleb, I., Erlitz, L., Telek, V., Vecsernyés, M., Sétáló, G., Jr., Hardi, P., Takács, I., Jancsó, G., & Nagy, T. (2021). Characterizing Autophagy in the Cold Ischemic Injury of Small Bowel Grafts: Evidence from Rat Jejunum. Metabolites, 11(6), 396. https://doi.org/10.3390/metabo11060396






