Exploring Genotype-by-Environment Interactions of Chemical Composition of Raspberry by Using a Metabolomics Approach
Abstract
:1. Introduction
2. Results
2.1. Environment Data
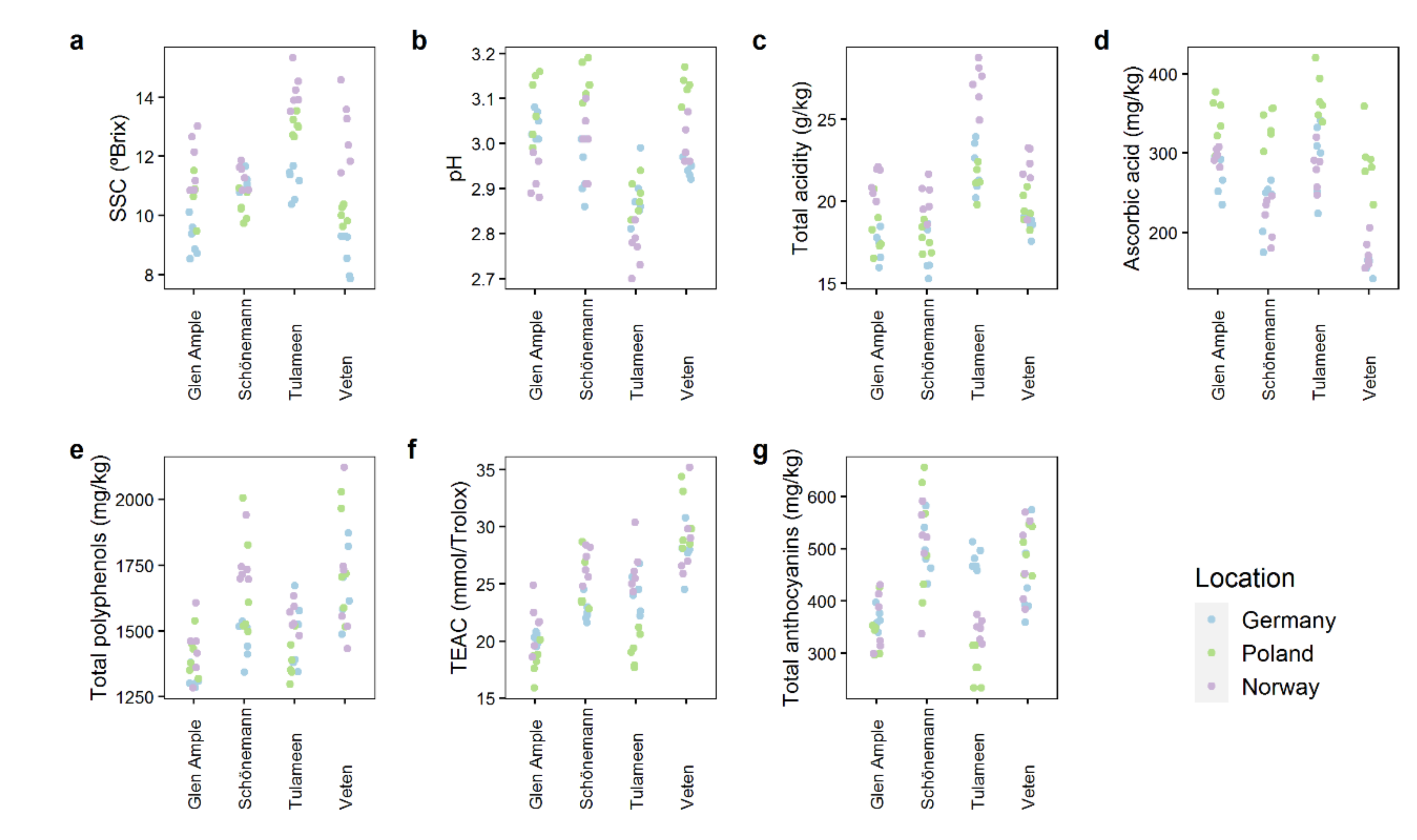
2.2. Quality Attributes Are Impacted by Both Genetic and Environmental Factors
2.3. Metabolomic Analyses
2.4. Key Metabolites for Fruit Quality Attributes Differentiate the Assessed Cultivars
2.5. Environment Impact on Important Aroma Volatiles
3. Discussion
3.1. Temperature, Radiation and Precipitation Impact Key Quality Attributes
3.2. Polyphenol Content Is Highly Genotype-Dependent
3.3. Flavor-Related Metabolites May Explain Differential Sensory Perception among Raspberry Cultivars
4. Material and Methods
4.1. Plant Material and Fruit Yield
4.2. SSC, pH, Total Acidity, Ascorbic Acid and TEAC Measurements
4.3. Metabolite Profile Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chironi, S.; Bacarella, S.; Altamore, L.; Ingrassia, M. Quality Factors Influencing Consumer Demand for Small Fruit by Focus Group and Sensory Test. J. Food Prod. Mark. 2017, 23, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Markowski, J. Grinding levels of raspberry pomace affect intestinal microbial activity, lipid and glucose metabolism in Wistar rats. Food Res. Int. 2019, 120, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Palonen, P.; Weber, C. Fruit color stability, anthocyanin content, and shelf life were not correlated with ethylene production rate in five primocane raspberry genotypes. Sci. Hortic. (Amsterdam) 2019, 247, 9–16. [Google Scholar] [CrossRef]
- McDougall, G.; Martinussen, I.; Stewart, D. Towards fruitful metabolomics: High throughput analyses of polyphenol composition in berries using direct infusion mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 362–369. [Google Scholar] [CrossRef]
- Aaby, K.; Skaret, J.; Røen, D.; Sønsteby, A. Sensory and instrumental analysis of eight genotypes of red raspberry (Rubus idaeus L.) fruits. J. Berry Res. 2019, 9, 483–498. [Google Scholar] [CrossRef] [Green Version]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Goff, S.A.; Klee, H.J. Plant Volatile Compounds: Sensory. Science 2006, 311, 815–820. [Google Scholar] [CrossRef]
- Hall, H.; Hummer, K.E.; Jamieson, A.; Jennings, S.N.; Weber, C. Raspberry breeding and genetics. In Plant Breeding Reviews Vol. 32; Janick, J., Ed.; Wiley-Blackwell: Hoboken, New Jersey, 2009. [Google Scholar]
- Sauvage, C.; Segura, V.; Bauchet, G.; Stevens, R.; Do, P.T.; Nikoloski, Z.; Fernie, A.R.; Causse, M. Genome-Wide Association in Tomato Reveals 44 Candidate Loci for Fruit Metabolic Traits. Plant Physiol. 2014, 165, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Bauchet, G.; Grenier, S.; Samson, N.; Segura, V.; Kende, A.; Beekwilder, J.; Cankar, K.; Gallois, J.L.; Gricourt, J.; Bonnet, J.; et al. Identification of major loci and genomic regions controlling acid and volatile content in tomato fruit: Implications for flavor improvement. New Phytol. 2017, 215, 624–641. [Google Scholar] [CrossRef] [Green Version]
- Zorrilla-Fontanesi, Y.; Rambla, J.-L.; Cabeza, A.; Medina, J.J.; Sanchez-Sevilla, J.F.; Valpuesta, V.; Botella, M.A.; Granell, A.; Amaya, I. Genetic Analysis of Strawberry Fruit Aroma and Identification of O-Methyltransferase FaOMT as the Locus Controlling Natural Variation in Mesifurane Content. Plant Physiol. 2012, 159, 851–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallarino, J.G.; Pott, D.M.; Cruz-rus, E.; Miranda, L.; Medina-minguez, J.J.; Valpuesta, V.; Fernie, A.R.; Sánchez-sevilla, J.F.; Osorio, S.; Amaya, I. Identification of quantitative trait loci and candidate genes for primary metabolite content in strawberry fruit. Hortic. Res. 2019, 6. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Rus, E.C.; Willmitzer, L.; Sevilla, J.F.S.; Amaya, I.; Osorio, S. Genetic analysis of phenylpropanoids and antioxidant capacity in strawberry fruit reveals mQTL hotspots and candidate genes. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Labadie, M.; Vallin, G.; Petit, A.; Ring, L.; Hoffmann, T.; Potier, A.; Schwab, W.G.; Denoyes, B. Metabolite Quantitative Trait Loci for flavonoids provide new insights into the genetic architecture of strawberry (Fragaria x ananassa) fruit quality. J. Agric. Food Chem. 2020, 68, 6927–6939. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide association of volatiles reveals candidate loci for blueberry flavor. New Phytol. 2020, 226, 1539–1540. [Google Scholar] [CrossRef]
- McClure, K.A.; Gong, Y.H.; Song, J.; Vinqvist-Tymchuk, M.; Campbell Palmer, L.; Fan, L.; Burgher-MacLellan, K.; Zhang, Z.Q.; Celton, J.M.; Forney, C.F.; et al. Genome-wide association studies in apple reveal loci of large effect controlling apple polyphenols. Hortic. Res. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Kassim, A.; Poette, J.; Paterson, A.; Zait, D.; McCallum, S.; Woodhead, M.; Smith, K.; Hackett, C.; Graham, J. Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traits loci (QTL). Mol. Nutr. Food Res. 2009, 53, 625–634. [Google Scholar] [CrossRef]
- McCallum, S.; Woodhead, M.; Hackett, C.A.; Kassim, A.; Paterson, A.; Graham, J. Genetic and environmental effects influencing fruit colour and QTL analysis in raspberry. Theor. Appl. Genet. 2010, 121, 611–627. [Google Scholar] [CrossRef]
- Dobson, P.; Graham, J.; Stewart, D.; Brennan, R.; Hackett, C.A.; McDougall, G.J. Over-seasons analysis of quantitative trait loci affecting phenolic content and antioxidant capacity in raspberry. J. Agric. Food Chem. 2012, 60, 5360–5366. [Google Scholar] [CrossRef]
- Bushakra, J.M.; Krieger, C.; Deng, D.; Stephens, M.J.; Allan, A.C.; Storey, R.; Symonds, V.V.; Stevenson, D.; McGhie, T.; Chagné, D.; et al. QTL involved in the modification of cyanidin compounds in black and red raspberry fruit. Theor. Appl. Genet. 2013, 126, 847–865. [Google Scholar] [CrossRef]
- Paterson, A.; Kassim, A.; McCallum, S.; Woodhead, M.; Smith, K.; Zait, D.; Graham, J. Environmental and seasonal influences on red raspberry flavour volatiles and identification of quantitative trait loci (QTL) and candidate genes. Theor. Appl. Genet. 2013, 126, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- Wang, S.Y.; Millner, P. Effect of different cultural systems on antioxidant capacity, phenolic content, and fruit quality of strawberries (Fragaria × aranassa Duch.). J. Agric. Food Chem. 2009, 57, 9651–9657. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.B.; Remberg, S.F.; Aaby, K. Quality and chemical composition of ten red raspberry (Rubus idaeus L.) genotypes during three harvest seasons. Food Chem. 2014, 160, 233–240. [Google Scholar] [CrossRef]
- Miele, A.; Rizzon, L.A.; De Queiroz, S.C.D.N.; Gianello, C. Physicochemical composition, minerals, and pesticide residues in organic grape juices. Food Sci. Technol. 2015, 35, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Ponder, A.; Hallmann, E. The effects of organic and conventional farm management and harvest time on the polyphenol content in different raspberry cultivars. Food Chem. 2019, 301, 125295. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Luan, G.; Zhang, S.; Zhuoma, Y.; Xie, J.; Zhou, W. Quantitative analyses of nine phenolic compounds and their antioxidant activities from thirty-seven varieties of raspberry grown in the Qinghai-Tibetan Plateau region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cui, J.; Chen, J.; Yao, J.; Hao, Y.; Fan, Y.; Liu, Y. Evaluation of physicochemical properties in three raspberries (Rubus idaeus) at five ripening stages in northern China. Sci. Hortic. (Amsterdam) 2020, 263, 109146. [Google Scholar] [CrossRef]
- Severo, J.; De Oliveira, I.R.; Bott, R.; Le Bourvellec, C.; Renard, C.M.G.C.; Page, D.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C radiation impacts strawberry metabolite content and volatile organic compound production. Lwt 2017, 85, 390–393. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Roussel, D.; Rolland, D. Potential link between fruit yield, quality parameters and phytohormonal changes in preharvest UV-C treated strawberry. Plant Physiol. Biochem. 2017, 116, 80–90. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Escobar, A.L.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Sarikhani, H. The effect of end of day far-red light on regulating flowering of short-day strawberry (Fragaria × ananassa Duch. cv. Paros) in a long-day situation. Russ. J. Plant Physiol. 2017, 64, 83–90. [Google Scholar] [CrossRef]
- Benbrook, C. The impacts of yield on nutritional quality: Lessons from organic farming. HortScience 2009, 44, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Frías-Moreno, M.N.; Parra-Quezada, R.A.; González-Aguilar, G.; Ruíz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, bioactive compounds, antioxidant capacity, and enzymes of raspberries at different maturity stages, effects of organic vs. Conventional fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Schmidt, P.; Hartung, J.; Rath, J.; Piepho, H.P. Estimating broad-sense heritability with unbalanced data from agricultural cultivar trials. Crop Sci. 2019, 59, 525–536. [Google Scholar] [CrossRef]
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lao, F.; Bi, S.; Pan, X.; Pang, X.; Hu, X.; Liao, X.; Wu, J. Insights into the major aroma-active compounds in clear red raspberry juice (Rubus idaeus L. cv. Heritage) by molecular sensory science approaches. Food Chem. 2021, 336, 127721. [Google Scholar] [CrossRef]
- Remberg, S.F.; Soønsteby, A.; Aaby, K.; Heide, O.M. Influence of postflowering temperature on fruit size and chemical composition of glen ample raspberry (Rubus Idaeus L.). J. Agric. Food Chem. 2010, 58, 9120–9128. [Google Scholar] [CrossRef]
- Pott, D.M.; Durán-Soria, S.; Osorio, S.; Vallarino, J.G. Combining metabolomic and transcriptomic approaches to assess and improve crop quality traits. CABI Agric. Biosci. 2021, 2, 1–20. [Google Scholar] [CrossRef]
- Almeida, J.; Perez-Fons, L.; Fraser, P.D. A transcriptomic, metabolomic and cellular approach to the physiological adaptation of tomato fruit to high temperature. Plant Cell Environ. 2020, 1–19. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 1–24. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits-making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigard, A.; Berhe, D.T.; Maoddi, E.; Sire, Y.; Boursiquot, J.M.; Ojeda, H.; Péros, J.P.; Doligez, A.; Romieu, C.; Torregrosa, L. Vitis vinifera L. fruit diversity to breed varieties anticipating climate changes. Front. Plant Sci. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, H.; Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolomics-Based Evaluation of Crop Quality Changes as a Consequence of Climate Change. Metabolites 2021, 11, 461. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Sønsteby, A.; Aaby, K.; Martinsen, B.K.; Heide, O.M.; Wold, A.B.; Remberg, S.F. Ascorbate pool, sugars and organic acids in black currant (Ribes nigrum L.) berries are strongly influenced by genotype and post-flowering temperature. J. Sci. Food Agric. 2017, 97, 1302–1309. [Google Scholar] [CrossRef]
- Tian, Y.; Laaksonen, O.; Haikonen, H.; Vanag, A.; Ejaz, H.; Linderborg, K.; Karhu, S.; Yang, B. Compositional Diversity among Blackcurrant (Ribes nigrum) Cultivars Originating from European Countries. J. Agric. Food Chem. 2019, 67, 5621–5633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Mackenzie, S.J.; Chandler, C.K.; Hasing, T.; Whitaker, V.M. The role of temperature in the late-season decline in soluble solids content of strawberry fruit in a subtropical production system. HortScience 2011, 46, 1562–1566. [Google Scholar] [CrossRef]
- Krüger, E.; Josuttis, M.; Nestby, R.; Toldam-Andersen, T.B.; Carlen, C.; Mezzetti, B. Influence of growing conditions at different latitudes of Europe on strawberry growth performance, yield and quality. J. Berry Res. 2012, 2, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Mazur, S.P.; Sønsteby, A.; Wold, A.B.; Foito, A.; Freitag, S.; Verrall, S.; Conner, S.; Stewart, D.; Heide, O.M. Post-flowering photoperiod has marked effects on fruit chemical composition in red raspberry (Rubus idaeus). Ann. Appl. Biol. 2014, 165, 454–465. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Walker, P.G.; Viola, R.; Woodhead, M.; Jorgensen, L.; Gordon, S.L.; Brennan, R.M.; Hancock, R.D. Ascorbic acid content of blackcurrant fruit is influenced by both genetic and environmental factors. Funct. Plant Sci. Biotechnol. 2010, 4, 40–52. [Google Scholar]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Wold, A.B.; Remberg, S.F. Yield and fruit quality of black currant (Ribes nigrum L.) are favoured by precipitation and cool summer conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 702–712. [Google Scholar] [CrossRef]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism : An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant, Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Lean, M.E.J.; Crozier, A. Rapid characterization of anthocyanins in red raspberry fruit by high-performance liquid chromatography coupled to single quadrupole mass spectrometry. J. Chromatogr. A 2002, 966, 63–70. [Google Scholar] [CrossRef]
- Josuttis, M.; Carlen, C.; Crespo, P.; Nestby, R.; Toldam-Andersen, T.B.; Dietrich, H.; Krüger, E. A comparison of bioactive compounds of strawberry fruit from Europe affected by genotype and latitude. J. Berry Res. 2012, 2, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Krüger, E.; Will, F.; Kumar, K.; Celejewska, K.; Chartier, P.; Masny, A.; Mott, D.; Petit, A.; Savini, G.; Sønsteby, A. Influence of post-flowering climate conditions on anthocyanin profile of strawberry cultivars grown from north to south Europe. Appl. Sci. 2021, 11, 1326. [Google Scholar] [CrossRef]
- Carbone, F.; Preuss, A.; De Vos, R.C.H.; D’Amico, E.; Perrotta, G.; Bovy, A.G.; Martens, S.; Rosati, C. Developmental, genetic and environmental factors affect the expression of flavonoid genes, enzymes and metabolites in strawberry fruits. Plant, Cell Environ. 2009, 32, 1117–1131. [Google Scholar] [CrossRef]
- Cocco, C.; Magnani, S.; Maltoni, M.L.; Quacquarelli, I.; Cacchi, M.; Antunes, L.E.C.; D’antuono, L.F.; Faedi, W.; Baruzzi, G. Effects of site and genotype on strawberry fruits quality traits and bioactive compounds. J. Berry Res. 2015, 5, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [Green Version]
- García, A.V.; Pérez, S.E.M.; Butsko, M.; Moya, M.S.P.; Sanahuja, A.B. Authentication of “adelita” raspberry cultivar based on physical properties, antioxidant activity and volatile profile. Antioxidants 2020, 9, 593. [Google Scholar] [CrossRef]
- Joubert, C.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. Field-grown grapevine berries use carotenoids and the associated xanthophyll cycles to acclimate to UV exposure differentially in high and low light (Shade) conditions. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Young, P.R.; Eyeghe-Bickong, H.A.; Du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine plasticity in response to an altered microclimate: Sauvignon Blanc modulates specific metabolites in response to increased berry exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, K.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. The transcriptional responses and metabolic consequences of acclimation to elevated light exposure in grapevine berries. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation strategies to improve oral bioavailability of ellagic acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins (Basel). 2016, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Krüger, E.; Schöpplein, E.; Rasim, S.; Cocca, G.; Fischer, H. Effects of Ripening Stage and Storage Time on Quality Parameters of Red Raspberry Fruit. Eur. J. Hortic. Sci. 2003, 68, 176–182. [Google Scholar]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; McGinn, J.; Lean, M.E.J.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; Van Der Meer, I.M.; De Vos, C.H.R. Antioxidants in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef]
- Kalt, W.; Forney, C.F.; Martin, A.; Prior, R.L. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J. Agric. Food Chem. 1999, 47, 4638–4644. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bakkalbasi, E.; Mentes, O.; Artik, N. Food ellagitannins-occurrence, effects of processing and storage. Crit. Rev. Food Sci. Nutr. 2009, 49, 283–298. [Google Scholar] [CrossRef]
- Stavang, J.A.; Freitag, S.; Foito, A.; Verrall, S.; Heide, O.M.; Stewart, D.; Sønsteby, A. Raspberry fruit quality changes during ripening and storage as assessed by colour, sensory evaluation and chemical analyses. Sci. Hortic. (Amsterdam) 2015, 195, 216–225. [Google Scholar] [CrossRef]
- Molder, K.; Moor, U.; Tonutare, T.; Poldma, P. Postharvest quality of ‘Glen Ample’ raspberry as affected by storage temperature and modified atmosphere packaging. J. Fruit Ornam. Plant Res. 2011, 19, 145–153. [Google Scholar]
- Tietel, Z.; Porat, R.; Weiss, K.; Ulrich, D. Identification of aroma-active compounds in fresh and stored “Mor” mandarins. Int. J. Food Sci. Technol. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.R.; Pannala, A.S.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur. Food Res. Technol. 2007, 224, 755–762. [Google Scholar] [CrossRef]
- Osorio, S.; Do, P.T.; Fernie, A.R. Profiling primary metabolites of tomato fruit with gas chromatography/mass spectrometry. Methods Mol. Biol. 2012, 860, 101–109. [Google Scholar]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. [email protected]: The Golm metabolome database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Profiling Volatile Compounds in Blackcurrant Fruit using Headspace Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry. J. Vis. Exp. 2021, 1–14. [Google Scholar] [CrossRef]
- Vallarino, J.G.; Kubiszewski-Jakubiak, S.; Ruf, S.; Rößner, M.; Timm, S.; Bauwe, H.; Carrari, F.; Rentsch, D.; Bock, R.; Sweetlove, L.J.; et al. Multi-gene metabolic engineering of tomato plants results in increased fruit yield up to 23%. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Perpiñá, G.; Esteras, C.; Gibon, Y.; Monforte, A.J.; Picó, B. A new genomic library of melon introgression lines in a cantaloupe genetic background for dissecting desirable agronomical traits. BMC Plant Biol. 2016, 16, 1–21. [Google Scholar] [CrossRef] [PubMed]






| Location | Month | Mean Temperature (°C) | Mean Radiation (W/m2) | Total Precipitation (mm3) |
|---|---|---|---|---|
| NIBIO (Norway) | March | −5.4 | 2365.6 | 17.5 |
| April | 5.6 | 4251.9 | 3.0 | |
| May | 8.5 | 4200.7 | 91.4 | |
| June | 14.4 | 4678.7 | 103.6 | |
| July | 20.7 | 6233.9 | 27.0 | |
| August | 14.7 | 3783.2 | 59.0 | |
| INHORT (Poland) | March | −1.6 | 2281.3 | 13.2 |
| April | 13.2 | 4842.3 | 28.6 | |
| May | 16.5 | 6092.0 | 51.6 | |
| June | 18.5 | 5880.7 | 30.0 | |
| July | 20.3 | 5467.4 | 155.0 | |
| HGU (Germany) | March | 4.8 | 2341.3 | 38.7 |
| April | 14.0 | 4705.9 | 19.4 | |
| May | 17.5 | 5982.0 | 71.6 | |
| June | 20.0 | 6013.3 | 74.8 | |
| July | 21.3 | 6085.0 | 41.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Soria, S.; Pott, D.M.; Will, F.; Mesa-Marín, J.; Lewandowski, M.; Celejewska, K.; Masny, A.; Żurawicz, E.; Jennings, N.; Sønsteby, A.; et al. Exploring Genotype-by-Environment Interactions of Chemical Composition of Raspberry by Using a Metabolomics Approach. Metabolites 2021, 11, 490. https://doi.org/10.3390/metabo11080490
Durán-Soria S, Pott DM, Will F, Mesa-Marín J, Lewandowski M, Celejewska K, Masny A, Żurawicz E, Jennings N, Sønsteby A, et al. Exploring Genotype-by-Environment Interactions of Chemical Composition of Raspberry by Using a Metabolomics Approach. Metabolites. 2021; 11(8):490. https://doi.org/10.3390/metabo11080490
Chicago/Turabian StyleDurán-Soria, Sara, Delphine M. Pott, Frank Will, Jennifer Mesa-Marín, Mariusz Lewandowski, Karolina Celejewska, Agnieszka Masny, Edward Żurawicz, Nikki Jennings, Anita Sønsteby, and et al. 2021. "Exploring Genotype-by-Environment Interactions of Chemical Composition of Raspberry by Using a Metabolomics Approach" Metabolites 11, no. 8: 490. https://doi.org/10.3390/metabo11080490
APA StyleDurán-Soria, S., Pott, D. M., Will, F., Mesa-Marín, J., Lewandowski, M., Celejewska, K., Masny, A., Żurawicz, E., Jennings, N., Sønsteby, A., Krüger, E., & Osorio, S. (2021). Exploring Genotype-by-Environment Interactions of Chemical Composition of Raspberry by Using a Metabolomics Approach. Metabolites, 11(8), 490. https://doi.org/10.3390/metabo11080490







