Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids
Abstract
:1. Introduction
2. Membrane Lipids: Response to Environmental Stress and How They Affect Metabolism, Ion Pumps and Channels
2.1. Temperature and Toxins
2.2. Diet
2.3. Membrane Pacemaker Theory of Metabolism
2.4. Potential Role for Membrane Regulation of ATP Use and Production in Chronic Hypoxia
2.4.1. Membrane Regulation of Na+/K+-ATPase (ATP Use)
2.4.2. Membrane Regulation of Glycolysis, β-Oxidation and the Tricarboxylic Acid (TCA) Cycle (ATP Production)
2.5. Evidence for Membrane Regulation of Ion Channels
2.6. Potential Role of Membranes in the Regulation of Mitochondrial Function
2.7. Potential Role of Membrane Lipids in Supporting Metabolic Suppression during Chronic Hypoxia
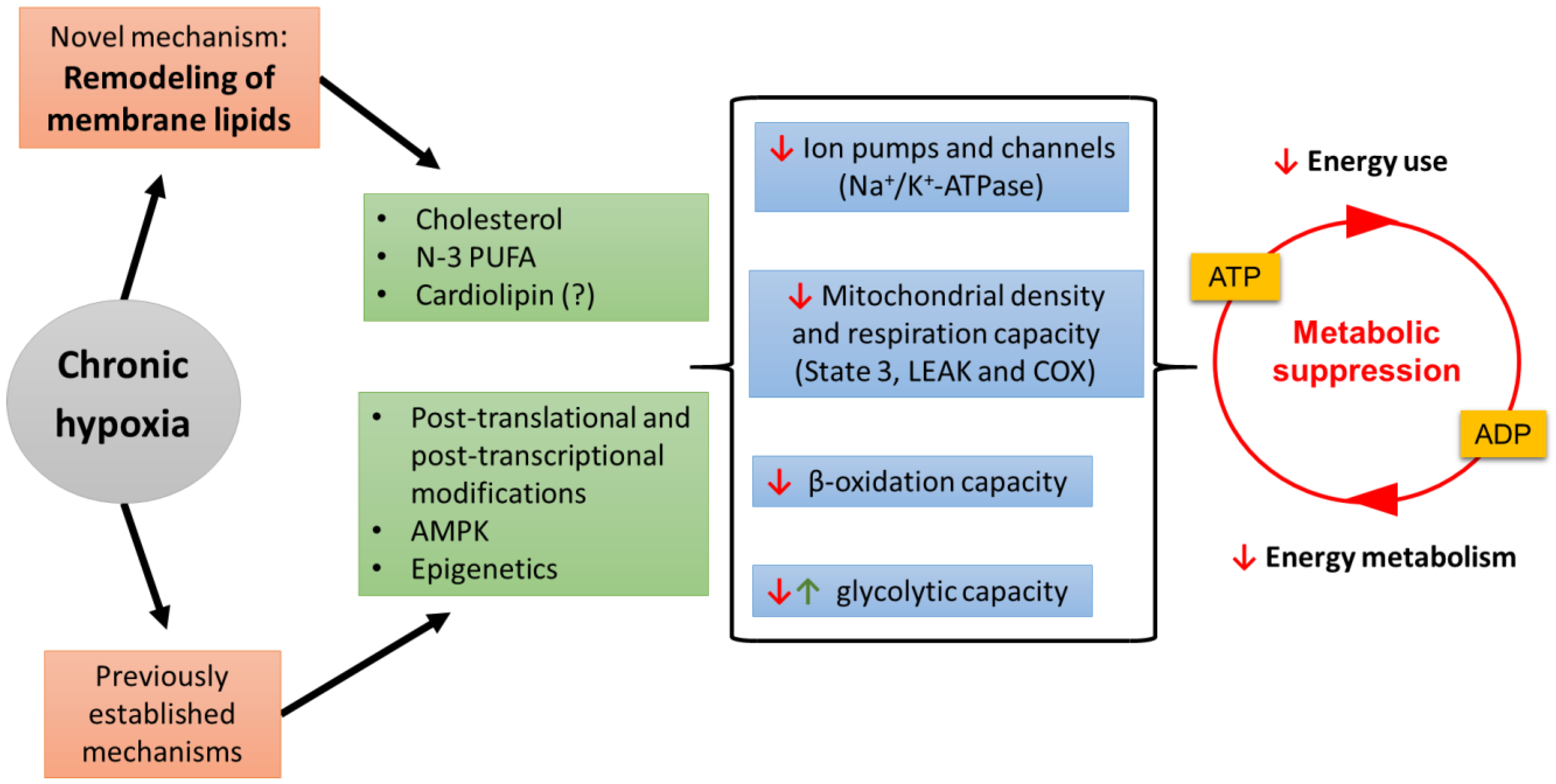
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Richards, J.G.; Farrell, A.P.; Brauner, C.J. Hypoxia, 1st ed.; Academic Press: London, UK, 2009; Volume 27, p. 528. [Google Scholar]
- Lutz, P.L.; Storey, K.B. Adaptations to variations in oxygen tension by vertebrates and invertebrates. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1479–1522. [Google Scholar]
- Diaz, R.J.; Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Stramma, L.; Johnson, G.C.; Sprintall, J.; Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science 2008, 320, 655–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochachka, P.W. Defense strategies against hypoxia and hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Lau, G.Y.; Richards, J.G.; Milsom, W.K. Naked mole rat brain mitochondria electron transport system flux and h+ leak are reduced during acute hypoxia. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, K.B. Metabolic regulation in mammalian hibernation: Enzyme and protein adaptations. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 1115–1124. [Google Scholar] [CrossRef]
- Martínez, M.L.; Landry, C.; Boehm, R.; Manning, S.; Cheek, A.O.; Rees, B.B. Effects of long-term hypoxia on enzymes of carbohydrate metabolism in the gulf killifish, fundulus grandis. J. Exp. Biol. 2006, 209, 3851–3861. [Google Scholar] [CrossRef] [Green Version]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1171–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storey, K.B.; Storey, J.M. Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Quart. Rev. Biol. 1990, 65, 145–174. [Google Scholar] [CrossRef]
- Pamenter, M.E. Mitochondria: A multimodal hub of hypoxia tolerance. Can. J. Zool. 2014, 92, 569–589. [Google Scholar] [CrossRef]
- Storey, K.B. Regulation of hypometabolism: Insights into epigenetic controls. J. Exp. Biol. 2015, 218, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhat, E.; Turenne, E.D.; Choi, K.; Weber, J.-M. Hypoxia-induced remodelling of goldfish membranes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 237, 110326. [Google Scholar] [CrossRef] [PubMed]
- Farhat, E.; Devereaux, M.E.M.; Pamenter, M.E.; Weber, J.-M. Naked mole-rats suppress energy metabolism and modulate membrane cholesterol in chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R148–R155. [Google Scholar] [CrossRef]
- Garcia, A.; Lev, B.; Hossain, K.R.; Gorman, A.; Pham, T.H.N.; Cornelius, F.; Allen, T.W.; Clarke, R.J. Cholesterol depletion inhibits na+, k+-atpase activity in a near-native membrane environment. J. Biol. Chem. 2019, 294, 5956–5969. [Google Scholar] [CrossRef]
- Yeagle, P.L.; Young, J.; Rice, D. Effects of cholesterol on sodium-potassium atpase atp hydrolyzing activity in bovine kidney. Biochemistry 1988, 27, 6449–6452. [Google Scholar] [CrossRef]
- Crockett, E.L.; Hazel, J.R. Cholesterol affects physical properties and (na+, k+)-atpase in basolateral membranes of renal and intestinal epithelia from thermally acclimated rainbow trout. J. Comp. Physiol. B 1997, 167, 344–351. [Google Scholar] [CrossRef]
- Bastiaanse, E.M.L.; Höld, K.M.; Van der Laarse, A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 1997, 33, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281. [Google Scholar] [CrossRef]
- Calhoon, E.A.; Ro, J.; Williams, J.B. Perspectives on the membrane fatty acid unsaturation/pacemaker hypotheses of metabolism and aging. Chem. Phys. Lipids 2015, 191, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Haga, K.L.; Hulbert, A.J.; Else, P.L. Relationship between body size, na+-k+-atpase activity, and membrane lipid composition in mammal and bird kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R301–R310. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, A.J.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Theor. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef]
- Hazel, J.R. Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Cossins, A.; Bowler, K.; Prosser, C. Homeoviscous adaptation and its effect upon membrane-bound proteins. J. Therm. Biol. 1981, 6, 183–187. [Google Scholar] [CrossRef]
- Anderson, R.L.; Minton, K.W.; Li, G.C.; Hahn, G.M. Temperature-induced homeoviscous adaptation of chinese hamster ovary cells. Biochim. Biophys. Acta Biomembr. 1981, 641, 334–348. [Google Scholar] [CrossRef]
- Lewis, R.; McElhaney, R.N. The mesomorphic phase behavior of lipid bilayers. Struct. Biol. Membr. 1992, 2. [Google Scholar] [CrossRef]
- Crockett, E.L. Cholesterol function in plasma membranes from ectotherms: Membrane-specific roles in adaptation to temperature1. Am. Zool. 1998, 38, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta Rev. Biomembr. 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Gonzalez, A.; Odjélé, A.; Weber, J.-M. Pcb-153 and temperature cause restructuring of goldfish membranes: Homeoviscous response to a chemical fluidiser. Aquat. Toxicol. 2013, 144–145, 11–18. [Google Scholar] [CrossRef]
- Martin, N.; Bureau, D.P.; Marty, Y.; Kraffe, E.; Guderley, H. Dietary lipid quality and mitochondrial membrane composition in trout: Responses of membrane enzymes and oxidative capacities. J. Comp. Physiol. B 2013, 183, 393–408. [Google Scholar] [CrossRef]
- Nagahuedi, S.; Popesku, J.T.; Trudeau, V.L.; Weber, J.-M. Mimicking the natural doping of migrant sandpipers in sedentary quails: Effects of dietary n-3 fatty acids on muscle membranes and ppar expression. J. Exp. Biol. 2009, 212, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Pierce, B.J.; McWilliams, S.R.; O’Connor, T.P.; Place, A.R.; Guglielmo, C.G. Effect of dietary fatty acid composition on depot fat and exercise performance in a migrating songbird, the red-eyed vireo. J. Exp. Biol. 2005, 208, 1277–1285. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S.K.; Else, P.L.; Hulbert, A.J. Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 pufa. Br. J. Nutr. 2010, 103, 522–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruf, T.; Arnold, W. Effects of polyunsaturated fatty acids on hibernation and torpor: A review and hypothesis. Am. J. Physiol Regul Integr Comp. Physiol 2008, 294, R1044–R1052. [Google Scholar] [CrossRef]
- Weber, J.-M. The physiology of long-distance migration: Extending the limits of endurance metabolism. J. Exp. Biol. 2009, 212, 593–597. [Google Scholar] [CrossRef] [Green Version]
- Frank, C.L. Short-term variations in diet fatty acid composition and torpor by ground squirrels. J. Mammal. 2002, 83, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Giroud, S.; Stalder, G.; Gerritsmann, H.; Kübber-Heiss, A.; Kwak, J.; Arnold, W.; Ruf, T. Dietary lipids affect the onset of hibernation in the garden dormouse (eliomys quercinus): Implications for cardiac function. Front. Physiol. 2018, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Maillet, D.; Weber, J.-M. Performance-enhancing role of dietary fatty acids in a long-distance migrant: The semipalmated sandpiper. J. Exp. Biol. 2006, 209, 2686–2695. [Google Scholar] [CrossRef] [Green Version]
- Maillet, D.; Weber, J.-M. Relationship between n-3 pufa content and energy metabolism in the flight muscles of a migrating shorebird: Evidence for natural doping. J. Exp. Biol. 2007, 210, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, A.J.; Else, P.L. Membranes and the setting of energy demand. J. Exp. Biol 2005, 208, 1593–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulbert, A.J. Membrane fatty acids as pacemakers of animal metabolism. Lipids 2007, 42, 811–819. [Google Scholar] [CrossRef]
- Valencak, T.G.; Ruf, T. N−3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell 2007, 6, 15–25. [Google Scholar] [CrossRef]
- Rodriguez, E.; Weber, J.-M.; Pagé, B.; Roubik, D.W.; Suarez, R.K.; Darveau, C.-A. Setting the pace of life: Membrane composition of flight muscle varies with metabolic rate of hovering orchid bees. Proc. R. Soc. B 2015, 282, 20142232. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Pagé, B.; Weber, J.-M. Membranes as a possible pacemaker of metabolism in cypriniform fish: Does phylogeny matter? J. Exp. Biol. 2015, 218, 2563–2572. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, D.F.S.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W.; Buck, L.T.; Doll, C.J.; Land, S.C. Unifying theory of hypoxia tolerance: Molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl. Acad. Sci. USA 1996, 93, 9493–9498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cáceda, R.; Gamboa, J.L.; Boero, J.A.; Monge-C, C.; Arregui, A. Energetic metabolism in mouse cerebral cortex during chronic hypoxia. Neurosci. Lett. 2001, 301, 171–174. [Google Scholar] [CrossRef]
- Benzi, G.; Gorini, A.; Arnaboldi, R.; Ghigini, B.; Villa, R. Synaptosomal non-mitochondrial atpase activities: Age-related alterations by chronic normobaric intermittent hypoxia. Neurochem. Int. 1994, 25, 61–67. [Google Scholar] [CrossRef]
- Paajanen, V.; Vornanen, M. Effects of chronic hypoxia on inward rectifier k+ current (ik1) in ventricular myocytes of crucian carp (carassius carassius) heart. J. Membr. Biol. 2003, 194, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Farhat, E.; Cheng, H.; Romestaing, C.; Pamenter, M.; Weber, J.-M. Goldfish response to chronic hypoxia: Mitochondrial respiration, fuel preference and energy metabolism. Metabolites 2021, 11, 187. [Google Scholar] [CrossRef]
- Turner, N.; Else, P.L.; Hulbert, A. Docosahexaenoic acid (dha) content of membranes determines molecular activity of the sodium pump: Implications for disease states and metabolism. Naturwissenschaften 2003, 90, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.J.; Hulbert, A.J.; Storlien, L.H.; Else, P.L. Membrane lipids and sodium pumps of cattle and crocodiles: An experimental test of the membrane pacemaker theory of metabolism. Am. J. Physiol. 2004, 287, R633–R641. [Google Scholar] [CrossRef] [PubMed]
- Else, P.; Wu, B. What role for membranes in determining the higher sodium pump molecular activity of mammals compared to ectotherms? J. Comp. Physiol. B 1999, 169, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. Lipid regulation of cell membrane structure and function. FASEB J. 1989, 3, 1833–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, D.S.; Connaty, A.D.; Mahalingam, S.; Wall, N.; Cheviron, Z.A.; Storz, J.F.; Scott, G.R.; McClelland, G.B. Acclimation to hypoxia increases carbohydrate use during exercise in high-altitude deer mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R400–R411. [Google Scholar] [CrossRef] [Green Version]
- Pastoris, O.; Dossena, M.; Foppa, P.; Arnaboldi, R.; Gorini, A.; Villa, R.; Benzi, G. Modifications by chronic intermittent hypoxia and drug treatment on skeletal muscle metabolism. Neurochem. Res. 1995, 20, 143–150. [Google Scholar] [CrossRef]
- Daneshrad, Z.; Garcia-Riera, M.; Verdys, M.; Rossi, A. Differential responses to chronic hypoxia and dietary restriction of aerobic capacity and enzyme levels in the rat myocardium. Mol. Cell Biochem. 2000, 210, 159–166. [Google Scholar] [CrossRef]
- Low, P.A.; Schmelzer, J.; Ward, K.; Yao, J. Experimental chronic hypoxic neuropathy: Relevance to diabetic neuropathy. Am. J. Physiol. Endocrinol. Metab. 1986, 250, E94–E99. [Google Scholar] [CrossRef]
- Waskova-Arnostova, P.; Kasparova, D.; Elsnicova, B.; Novotny, J.; Neckar, J.; Kolar, F.; Zurmanova, J. Chronic hypoxia enhances expression and activity of mitochondrial creatine kinase and hexokinase in the rat ventricular myocardium. Cell. Physiol. Biochem. 2014, 33, 310–320. [Google Scholar] [CrossRef]
- van den Thillart, G.; Smit, H. Carbohydrate metabolism of goldfish (Carassius auratus L.)—effects of long-term hypoxia-acclimation on enzyme patterns of red muscle, white muscle and liver. J. Comp. Physiol. B 1984, 154, 477–486. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bernard, L.M. Ultrastructure and metabolism of skeletal muscle fibres in the tench: Effects of long-term acclimation to hypoxia. Cell Tissue Res. 1982, 227, 179–199. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Li, W.; Zhang, X. Effects of acute and chronic hypoxia on the locomotion and enzyme of energy metabolism in chinese shrimp fenneropenaeus chinensis. Mar. Freshw. Behav. Physiol. 2018, 51, 275–291. [Google Scholar] [CrossRef]
- Mahfouz, M.E.; Hegazi, M.M.; El-Magd, M.A.; Kasem, E.A. Metabolic and molecular responses in nile tilapia, oreochromis niloticus during short and prolonged hypoxia. Mar. Freshw. Behav. Physiol. 2015, 48, 319–340. [Google Scholar] [CrossRef]
- Dukhande, V.V.; Sharma, G.C.; Lai, J.C.K.; Farahani, R. Chronic hypoxia-induced alterations of key enzymes of glucose oxidative metabolism in developing mouse liver are mtor dependent. Mol. Cell. Biochem. 2011, 357, 189–197. [Google Scholar] [CrossRef]
- Pillet, M.; Dupont-Prinet, A.; Chabot, D.; Tremblay, R.; Audet, C. Effects of exposure to hypoxia on metabolic pathways in northern shrimp (pandalus borealis) and greenland halibut (reinhardtius hippoglossoides). J. Exp. Mar. Biol. Ecol. 2016, 483, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Wu, R.; Randall, D.; Lam, P.; Ip, Y.; Chew, S. Metabolic adjustments in the common carp during prolonged hypoxia. J. Fish. Biol. 2000, 57, 1160–1171. [Google Scholar] [CrossRef]
- Lui, M.A.; Mahalingam, S.; Patel, P.; Connaty, A.D.; Ivy, C.M.; Cheviron, Z.A.; Storz, J.F.; McClelland, G.B.; Scott, G.R. High-altitude ancestry and hypoxia acclimation have distinct effects on exercise capacity and muscle phenotype in deer mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R779–R791. [Google Scholar] [CrossRef] [Green Version]
- Nikel, K.E.; Shanishchara, N.K.; Ivy, C.M.; Dawson, N.J.; Scott, G.R. Effects of hypoxia at different life stages on locomotory muscle phenotype in deer mice native to high altitudes. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2018, 224, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Moine, C.M.R.; Morash, A.J.; McClelland, G.B. Changes in hif-1α protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1098–R1104. [Google Scholar] [CrossRef] [Green Version]
- DiMauro, S.; Moraes, C.T. Mitochondrial encephalomyopathies. Arch. Neurol. 1993, 50, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Drynan, L.; Quant, P.A.; Zammit, V.A. Flux control exerted by mitochondrial outer membrane carnitine palmitoyltransferase over β-oxidation, ketogenesis and tricarboxylic acid cycle activity in hepatocytes isolated from rats in different metabolic states. Biochem. J. 1996, 317, 791–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galbes, O.; Goret, L.; Caillaud, C.; Mercier, J.; Obert, P.; Candau, R.; Py, G. Combined effects of hypoxia and endurance training on lipid metabolism in rat skeletal muscle. Acta Physiol. 2008, 193, 163–173. [Google Scholar] [CrossRef]
- Kennedy, S.L.; Stanley, W.C.; Panchal, A.R.; Mazzeo, R.S. Alterations in enzymes involved in fat metabolism after acute and chronic altitude exposure. J. Appl. Physiol. 2001, 90, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Vats, P.; Singh, V.K.; Sharma, Y.K.; Singh, S.N.; Singh, S.B. Impairment of mitochondrial β-oxidation in rats under cold-hypoxic environment. Int. J. Biometeorol. 2009, 53, 397. [Google Scholar] [CrossRef]
- Morash, A.J.; Kotwica, A.O.; Murray, A.J. Tissue-specific changes in fatty acid oxidation in hypoxic heart and skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R534–R541. [Google Scholar] [CrossRef]
- Templeman, N.M.; Beaudry, J.L.; Le Moine, C.M.R.; McClelland, G.B. Chronic hypoxia- and cold-induced changes in cardiac enzyme and gene expression in cd-1 mice. Biochim. Biophys. Acta (BBA) Gen. Subj. 2010, 1800, 1248–1255. [Google Scholar] [CrossRef]
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.J.; Parrish, C.C.; Gamperl, A.K. Cardiac mitochondrial function, nitric oxide sensitivity and lipid composition following hypoxia acclimation in sablefish. J. Exp. Biol. 2019, 222, jeb208074. [Google Scholar] [CrossRef]
- Alasnier, C.; Rémignon, H.; Gandemer, G. Lipid characteristics associated with oxidative and glycolytic fibres in rabbit muscles. Meat Sci. 1996, 43, 213–224. [Google Scholar] [CrossRef]
- Arnold, W.; Giroud, S.; Valencak, T.G.; Ruf, T. Ecophysiology of omega fatty acids: A lid for every jar. Physiology 2015, 30, 232–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Xie, W.; Lei, T.; Hamilton, J.A. Eicosapentaenoic acid, but not oleic acid, stimulates β-oxidation in adipocytes. Lipids 2005, 40, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.A.; Giudici, A.M.; Renart, M.L.; Molina, M.L.; Montoya, E.; Fernández-Carvajal, A.; Fernández-Ballester, G.; Encinar, J.A.; González-Ros, J.M. Lipid modulation of ion channels through specific binding sites. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1560–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tillman, T.S.; Cascio, M. Effects of membrane lipids on ion channel structure and function. Cell Biochem. Biophys. 2003, 38, 161–190. [Google Scholar] [CrossRef]
- Levitan, I.; Fang, Y.; Rosenhouse-Dantsker, A.; Romanenko, V. Cholesterol and ion channels. In Cholesterol binding and Cholesterol Transport Proteins; Springer: New York, NY, USA, 2010; pp. 509–549. [Google Scholar]
- D’Avanzo, N. Lipid regulation of sodium channels. In Current Topics in Membranes; Elsevier: Amsterdam, The Netherlands, 2016; Volume 78, pp. 353–407. [Google Scholar]
- Kang, J.X.; Leaf, A. Evidence that free polyunsaturated fatty acids modify na+ channels by directly binding to the channel proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 3542–3546. [Google Scholar] [CrossRef] [Green Version]
- Palomer, E.; Martín-Segura, A.; Baliyan, S.; Ahmed, T.; Balschun, D.; Venero, C.; Martin, M.G.; Dotti, C.G. Aging triggers a repressive chromatin state at bdnf promoters in hippocampal neurons. Cell Rep. 2016, 16, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- McElroy, G.S.; Chandel, N.S. Mitochondria control acute and chronic responses to hypoxia. Exp. Cell Res. 2017, 356, 217–222. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K. Oxygen is the high-energy molecule powering complex multicellular life: Fundamental corrections to traditional bioenergetics. ACS Omega 2020, 5, 2221–2233. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Perevoschikova, I.V.; Goncalves, R.L.; Hey-Mogensen, M.; Brand, M.D. The determination and analysis of site-specific rates of mitochondrial reactive oxygen species production. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 526, pp. 189–217. [Google Scholar]
- St-Pierre, J.; Brand, M.D.; Boutilier, R.G. Mitochondria as atp consumers: Cellular treason in anoxia. Proc. Natl. Acad. Sci. USA 2000, 97, 8670–8674. [Google Scholar] [CrossRef] [Green Version]
- Heather, L.C.; Cole, M.A.; Tan, J.-J.; Ambrose, L.J.; Pope, S.; Abd-Jamil, A.H.; Carter, E.E.; Dodd, M.S.; Yeoh, K.K.; Schofield, C.J. Metabolic adaptation to chronic hypoxia in cardiac mitochondria. Basic Res. Cardiol. 2012, 107, 268. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.C.; Pichiule, P.; Boero, J.; Arregui, A. Reduced mitochondrial respiration in mouse cerebral cortex during chronic hypoxia. Neurosci. Lett. 1995, 193, 169–172. [Google Scholar] [CrossRef]
- Costa, L.E.; Boveris, A.; Koch, O.R.; Taquini, A.C. Liver and heart mitochondria in rats submitted to chronic hypobaric hypoxia. Am. J. Physiol. Cell Physiol. 1988, 255, C123–C129. [Google Scholar] [CrossRef] [PubMed]
- Dawson, N.J.; Lyons, S.A.; Henry, D.A.; Scott, G.R. Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol. 2018, 223, e13030. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; McClelland, G.B.; Scott, G.R. Evolved changes in the intracellular distribution and physiology of muscle mitochondria in high-altitude native deer mice. J. Physiol. 2017, 595, 4785–4801. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Tattersall, G.J.; Boutilier, R.G. Metabolic depression and enhanced o2 affinity of mitochondria in hypoxic hypometabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1205–R1214. [Google Scholar] [CrossRef]
- Sokolova, I. Mitochondrial adaptations to variable environments and their role in animals’ stress tolerance. Integr. Comp. Biol. 2018, 58, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Du, S.N.; Mahalingam, S.; Borowiec, B.G.; Scott, G.R. Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (fundulus heteroclitus). J. Exp. Biol. 2016, 219, 1130–1138. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.G.; Iftikar, F.I.; Baker, D.W.; Hickey, A.J.; Herbert, N.A. Low-o2 acclimation shifts the hypoxia avoidance behaviour of snapper (pagrus auratus) with only subtle changes in aerobic and anaerobic function. J. Exp. Biol. 2013, 216, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Guderley, H.; Kraffe, E.; Bureau, W.; Bureau, D.P. Dietary fatty acid composition changes mitochondrial phospholipids and oxidative capacities in rainbow trout red muscle. J. Comp. Physiol. B 2008, 178, 385–399. [Google Scholar] [CrossRef]
- Staples, J.F.; Brown, J.C.L. Mitochondrial metabolism in hibernation and daily torpor: A review. J. Comp. Physiol. B 2008, 178, 811–827. [Google Scholar] [CrossRef]
- Frank, C.L.; Storey, K.B. The optimal depot fat composition for hibernation by golden-mantled ground squirrels (spermophilus lateralis). J. Comp. Physiol. B. 1995, 164, 536–542. [Google Scholar] [CrossRef]
- Frank, C.L. The influence of dietary fatty acids on hibernation by golden-mantled ground squirrels (spermophilus lateralis). Physiol. Zool. 1992, 65, 906–920. [Google Scholar] [CrossRef]
- Staples, J.F. Metabolic suppression in mammalian hibernation: The role of mitochondria. J. Exp. Biol. 2014, 217, 2032–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerson, A.R.; Brown, J.C.L.; Thomas, R.; Bernards, M.A.; Staples, J.F. Effects of dietary polyunsaturated fatty acids on mitochondrial metabolism in mammalian hibernation. J. Exp. Biol 2008, 211, 2689–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.; Lloyd, G.P.; Thomas, R.H.; Guglielmo, C.G.; Staples, J.F. Mitochondrial respiration and succinate dehydrogenase are suppressed early during entrance into a hibernation bout, but membrane remodeling is only transient. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2011, 181, 699–711. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive oxygen species affect mitochondrial electron transport complex i activity through oxidative cardiolipin damage. Gene 2002, 286, 135–141. [Google Scholar] [CrossRef]
- Frick, N.T.; Bystriansky, J.S.; Ip, Y.K.; Chew, S.F.; Ballantyne, J.S. Cytochrome c oxidase is regulated by modulations in protein expression and mitochondrial membrane phospholipid composition in estivating african lungfish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R608–R616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraffe, E.; Marty, Y.; Guderley, H. Changes in mitochondrial oxidative capacities during thermal acclimation of rainbow trout oncorhynchus mykiss: Roles of membrane proteins, phospholipids and their fatty acid compositions. J. Exp. Biol. 2007, 210, 149–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]

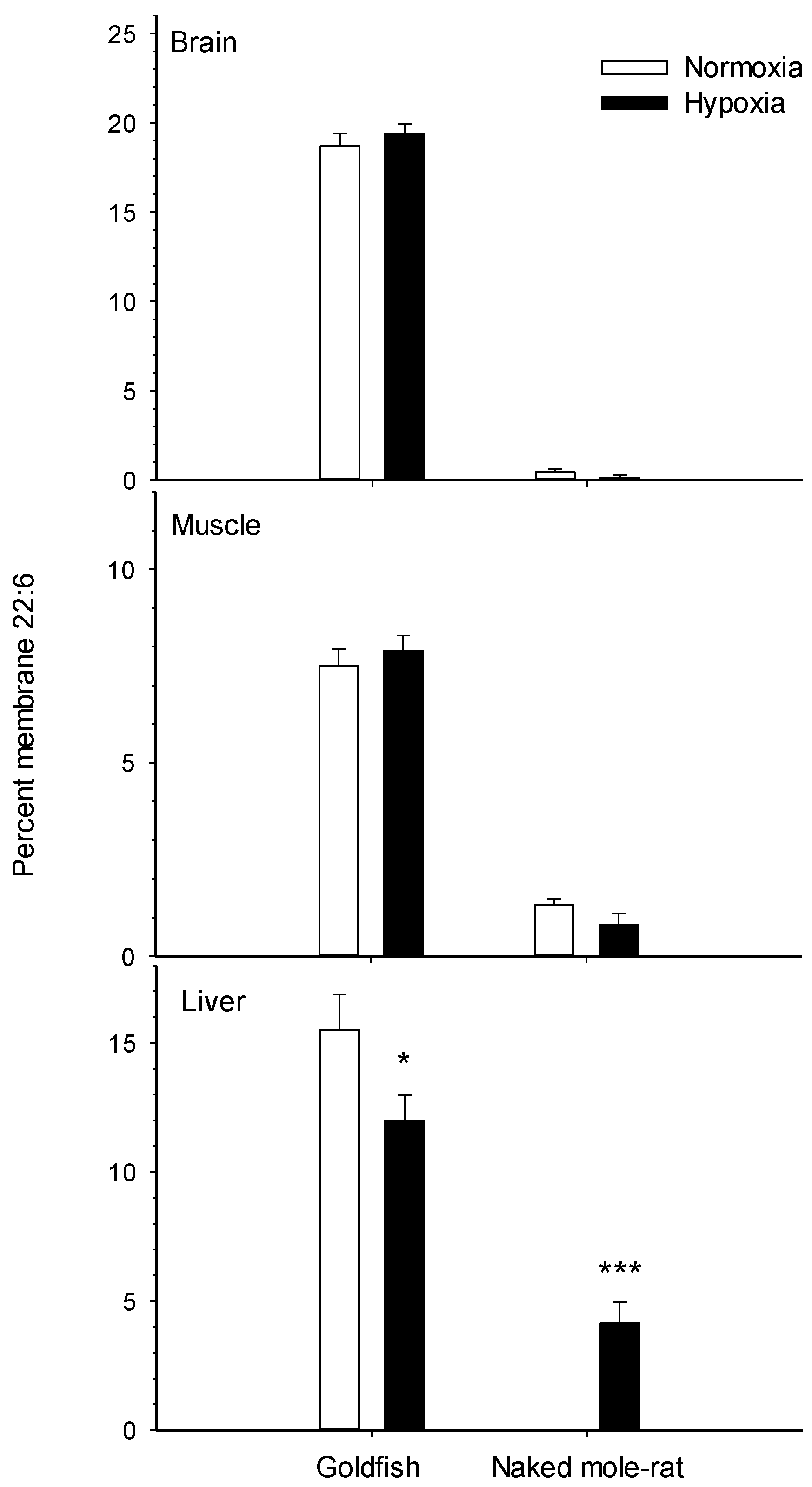
| Species | Tissue | Na+/K+-ATPase Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Mouse (Mus musculus) | Brain | - | 3 | [48] |
| Rat (Rattus norvegicus) | Brain | ~30–40% ↓ | 4 | [49] |
| Naked mole rat (Heterocephalus glaber) | Brain | 77% ↓ | 4 | [15] |
| Liver | 41% ↑ | [15] | ||
| Temporalis, heart | - | [15] | ||
| Crucian carp (Carassius carassius) | Heart | 33% ↓ | 3 | [50] |
| Goldfish (Carassius auratus) | Brain | 40% ↓ | 4 | [51] |
| Liver, white muscle | - | [51] |
| Species | Tissue | HK Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Gastrocnemius | 35% ↑ | 6–8 | [56] |
| Gastrocnemius | - | [56] | ||
| Mouse (Mus musculus) | Brain | - | 3 | [48] |
| Rat (Rattus norvegicus) | Gastrocnemius, soleus, heart, brain | 8–105% ↑ | 3–10 | [57,58,59,60] |
| Gulf killifish (Fundulus grandis) | Heart, brain | 16–28% ↑ | 4 | [9] |
| Liver | - | [9] | ||
| Goldfish (Carassius auratus) | Brain | 12% ↓ | 4 | [51] |
| White muscle | 82% ↑ | |||
| White muscle, red muscle, liver | - | 4–6 | [51,61] | |
| Tench (Tinca tinca) | White muscle | 67% ↓ | 6 | [62] |
| Red muscle, liver | - | [62] | ||
| Chinese shrimp (Fenneropenaeus chinensis) | pancreas, pleopod, abdominal muscle | 24–26% ↓ | 2 | [63] |
| Species | Tissue | PFK Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Gastrocnemius | - | 6–8 | [56] |
| Rat (Rattus norvegicus) | Heart, soleus, gastrocnemius, caudal nerve | - | 3–10 | [57,58,59] |
| Gulf killifish (Fundulus grandis) | White muscle | 25% ↓ | 4 | [9] |
| Liver | 63% ↑ | [9] | ||
| Heart, brain | - | [9] | ||
| Nile tilapia (Oreochromis niloticus) | Liver, white muscle | 59–123 ↑ | 4 | [64] |
| Tench (Tinca tinca) | White muscle | - | 6 | [62] |
| Red muscle, liver | 98–120% ↑ | [62] | ||
| Chinese shrimp (Fenneropenaeus chinensis) | hepatopancreas, pleopod, abdominal muscle | 16–31% ↓ | 2 | [63] |
| Species | Tissue | PK Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Gastrocnemius | - | 6–8 | [56] |
| Rat (Rattus norvegicus) | Heart, soleus | - | 3 | [58] |
| Gastrocnemius | - | 4 | [57] | |
| Naked mole-rat (Heterocephalus glaber) | Liver, temporalis muscle, brain, heart, kidney | 61–99% ↓ | 4 | [15] |
| Mouse (Mus musculus) | Liver | 65% ↓ | 4 | [65] |
| Northern shrimp (Pandalus borealis) | White muscle | - | 1 | [66] |
| Greenland halibut (Reinhardtius hippoglossoides) | White muscle | 46% ↓ | [66] | |
| Common carp (Cyprinus carpio) | White muscle | - | 1 | [67] |
| Nile tilapia (Oreochromis niloticus) | Liver | 61–96% ↑ | 4 | [64] |
| White muscle | - | [64] | ||
| Gulf killifish (Fundulus grandis) | White muscle | 23% ↓ | 4 | [9] |
| Heart | 24% ↑ | [9] | ||
| Liver, brain | - | [9] | ||
| Goldfish (Carassius auratus) | White and red muscle, liver | - | 6 | [61] |
| Liver | 47% ↓ | 4 | [51] | |
| White muscle, brain | - | [51] | ||
| Tench (Tinca tinca) | White and red muscle | - | 6 | [62] |
| Liver | 86% ↑ | [62] | ||
| Chinese shrimp (Fenneropenaeus chinensis) | hepatopancreas, pleopod, abdominal muscle | 14–39% ↓ | 2 | [63] |
| Species | Tissue | LDH Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Gastrocnemius, diaphragm | - | 6–8 | [68,69] |
| Mouse (Mus musculus) | Hindlimb muscles | 28% ↓ | 1 | [70] |
| Brain, liver | - | 3–4 | [48,65] | |
| Rat (Rattus norvegicus) | Soleus | - | 3 | [58] |
| Gastrocnemius, heart, gastrocnemius and liver mitochondria | 25–54% ↑ | 1–3 | [65,66,71] | |
| Naked mole-rat (Heterocephalus glaber) | Brain, liver, temporalis | 62–82% ↓ | 4 | [15] |
| Kidney | 81% ↑ | [15] | ||
| Heart | - | [15] | ||
| Northern Shrimp (Pandalus borealis) | White muscle | 45–88% ↓ | 1 | [66] |
| Greenland halibut (Reinhardtius hippoglossoides) | White muscle | 58% ↓ | [66] | |
| Common carp (Cyprinus carpio) | White muscle | - | 1 | [67] |
| Liver | ~60% ↑ | [67] | ||
| Nile tilapia (Oreochromis niloticus) | Liver, white muscle | 80–176% ↑ | 4 | [64] |
| Gulf killifish (Fundulus grandis) | White muscle | 30% ↓ | 4 | [9] |
| Liver | 30% ↑ | [9] | ||
| Heart, brain | - | [9] | ||
| Goldfish (Carassius auratus) | White muscle, red muscle, liver, brain | - | 4–6 | [51,61] |
| Tench (Tinca tinca) | White and red muscles | - | 6 | [62] |
| Liver | 116% ↑ | [62] | ||
| Chinese shrimp (Fenneropenaeus chinensis) | hepatopancreas, pleopod, abdominal muscle | 26–33% ↓ | 2 | [63] |
| Species | Tissue | CPT Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Rat (Rattus norvegicus) | Muscle, heart | 16–34% ↓ | 5 | [73,74] |
| Liver, gastrocnemius mitochondria | - | 1–5 | [74,75] | |
| Naked mole-rat (Heterocephalus glaber) | Liver, temporalis muscle | 89–98% ↓ | 4 | [15] |
| Brain, heart, kidney | - | [15] | ||
| Mouse (Mus musculus) | Skeletal muscle | 65% ↓ | 1 | [76] |
| Heart | - | [76] | ||
| Goldfish (Carassius auratus) | Brain | 18% ↓ | 4 | [51] |
| Liver, white muscle | - | [51] | ||
| Tench (Tinca tinca) | Red muscle, liver | 162–236% ↑ | 6 | [62] |
| White muscle | - | [62] |
| Species | Tissue | HOAD Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Gastrocnemius, liver | - | 6–8 | [56] |
| Mouse (Mus musculus) | Left ventricle | 36% ↓ | 4 | [77] |
| Rat (Rattus norvegicus) | Heart, skeletal, liver and liver mitochondria | 20–71% ↓ | 1–5 | [58,73,74,75] |
| Soleus, gastrocnemius mitochondria | - | 1–3 | [58,75] | |
| Naked mole-rat (Heterocephalus glaber) | Liver, temporalis muscle | 69–93% ↓ | 4 | [15] |
| Brain, heart, kidney | - | [15] | ||
| Mouse (Mus musculus) | Heart, skeletal | - | 1 | [76] |
| Goldfish (Carassius auratus) | Brain | 70% ↑ | 4 | [51] |
| Liver, White muscle | - | [51] |
| Species | Tissue | CS Response | Hypoxia Duration (Weeks) | Reference |
|---|---|---|---|---|
| Deer mouse (Peromyscus maniculatus) | Liver, gastrocnemius, diaphragm | - | 6–8 | [64,76,77] |
| Mouse (Mus musculus) | Liver mitochondria | 34% ↓ | 1 | [75] |
| Hindlimb muscles, heart | - | 1–4 | [70,77] | |
| Gastrocnemius mitochondria | - | 1 | [75] | |
| Brain, liver | - | 3–4 | [48,65] | |
| Rat (Rattus norvegicus) | Gastrocnemius | 34–39% ↓ | 4 | [57] |
| Gastrocnemius, heart, liver | - | 3–5 | [66,79,80] | |
| Naked mole-rat (Heterocephalus glaber) | Brain, liver, temporalis, kidney | 25–78% ↓ | 4 | [15] |
| Heart | 94–115% ↑ | [15] | ||
| Goldfish (Carassius auratus) | Brain, liver, white muscle | - | 4 | [51] |
| Common carp (Cyprinus carpio) | White muscle | ~25% ↓ | 1 | [67] |
| Liver | - | [67] | ||
| Northern shrimp (Pandalus borealis) | White muscle | 40% ↓ | 1 | [66] |
| Greenland halibut (Reinhardtius hippoglossoides) | White muscle | 33% ↓ | [66] | |
| Chinese shrimp (Fenneropenaeus chinensis) | pancreas, pleopod, abdominal muscle | 31–70% ↓ | 2 | [63] |
| Sablefish (Anoplopoma fimbria) | Heart | 20% ↑ | 3 | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhat, E.; Weber, J.-M. Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids. Metabolites 2021, 11, 503. https://doi.org/10.3390/metabo11080503
Farhat E, Weber J-M. Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids. Metabolites. 2021; 11(8):503. https://doi.org/10.3390/metabo11080503
Chicago/Turabian StyleFarhat, Elie, and Jean-Michel Weber. 2021. "Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids" Metabolites 11, no. 8: 503. https://doi.org/10.3390/metabo11080503
APA StyleFarhat, E., & Weber, J.-M. (2021). Hypometabolic Responses to Chronic Hypoxia: A Potential Role for Membrane Lipids. Metabolites, 11(8), 503. https://doi.org/10.3390/metabo11080503






