Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Antioxidant Activities of Noodles
2.2.1. GABA Analysis
2.2.2. FRAP Analysis
2.2.3. Total Polyphenol Content
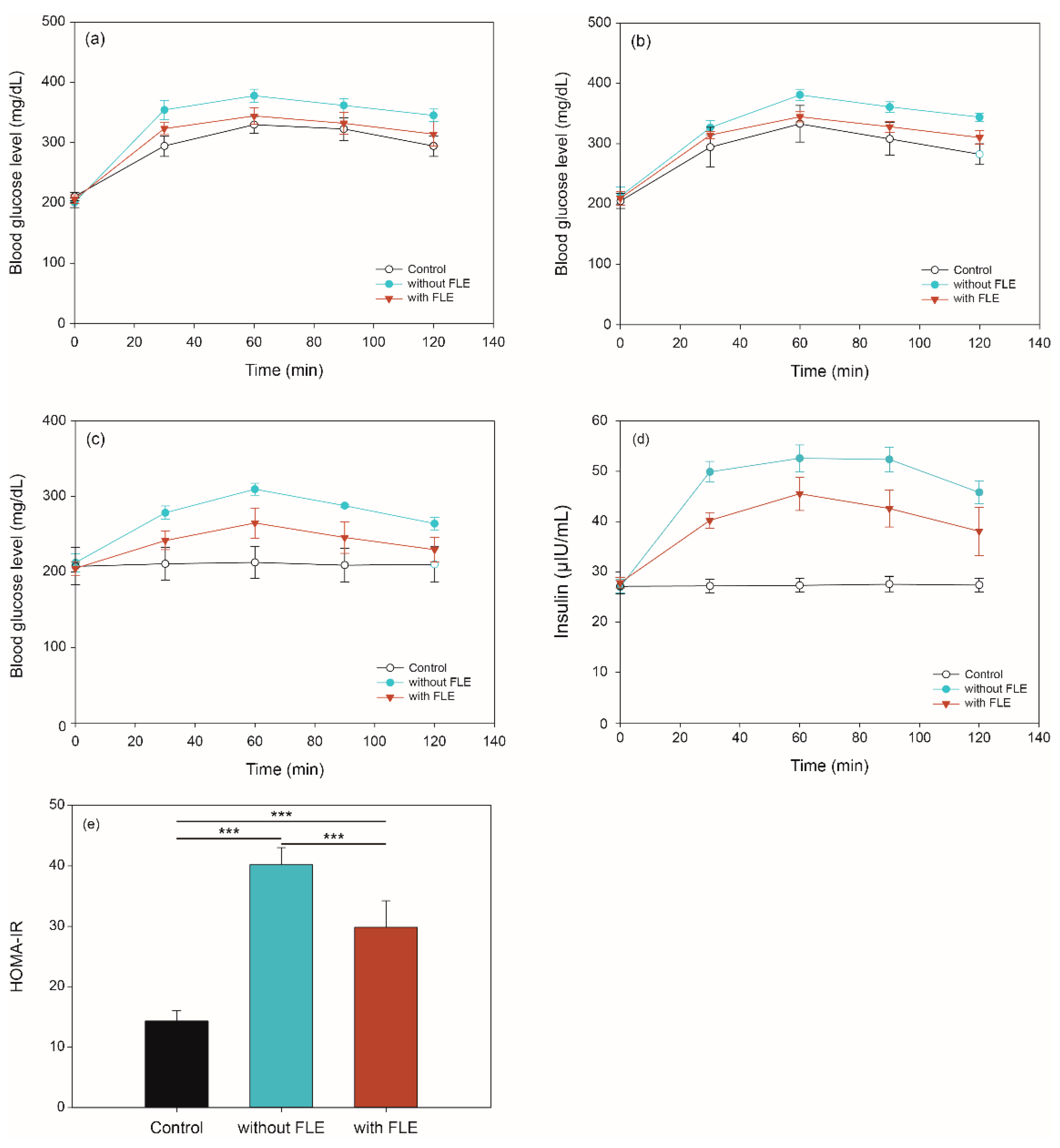
2.3. Blood Glucose and Insulin Concentration via In Vivo Digestion of Noodles Using OGTT, OSTT, FBGT, and HOMA-IR
2.4. Metabolite Profiling of Serum In Vivo Digestion
2.4.1. Identification of Metabolites
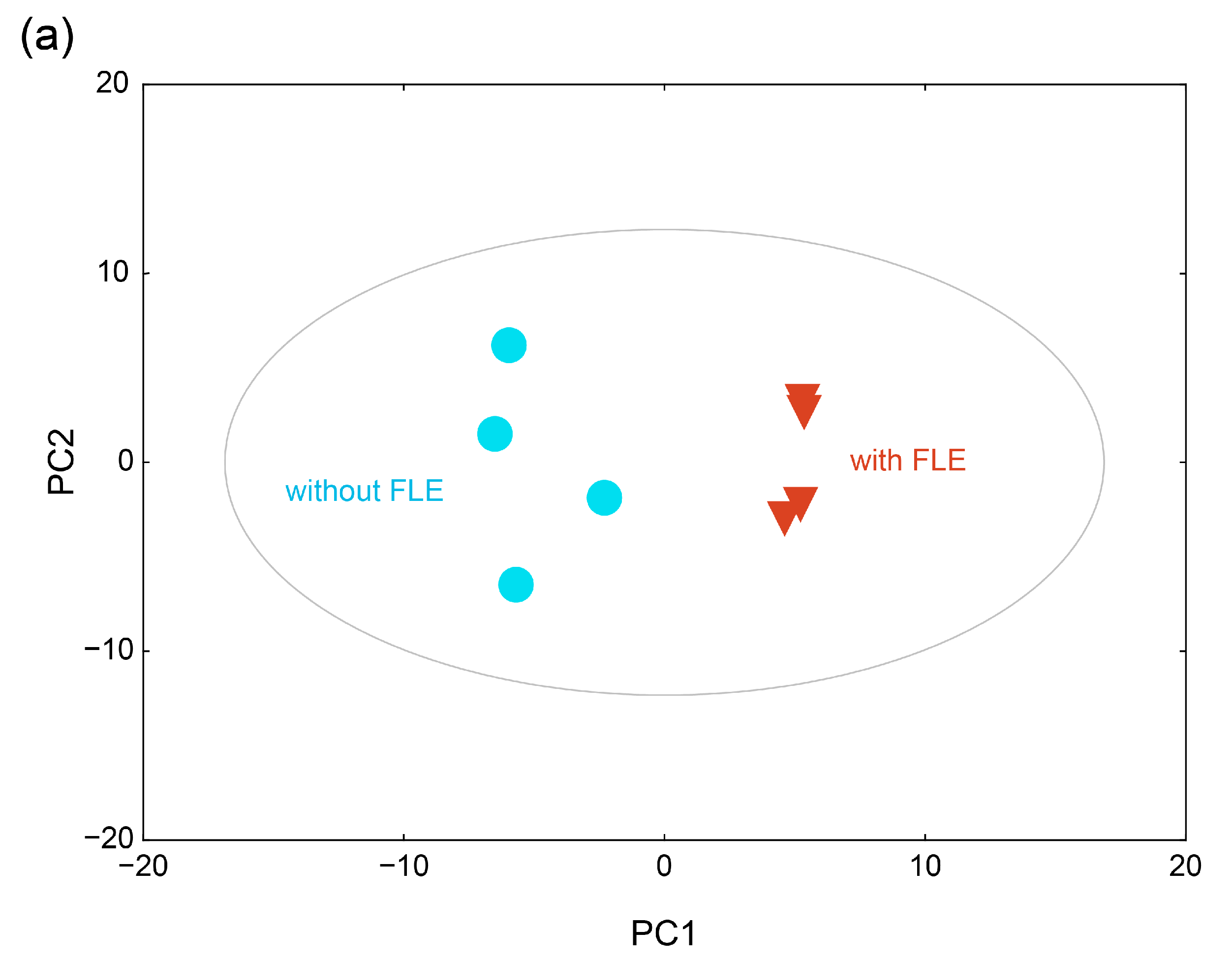
2.4.2. Metabolite Profiles of the Fermented Extract by PCA and HCA
2.4.3. Difference in Metabolite Changes Owing to the Fermented Extract
3. Materials and Methods
3.1. Wheat Noodle Preparation
3.2. FLE
3.3. Antioxidant Test
3.3.1. Extraction of Noodles
3.3.2. GABA Analysis
3.3.3. FRAP Analysis
3.3.4. Total Phenolic Content
3.4. Animal Experiments and Blood Glucose Concentration
3.4.1. Type 2 Diabetes Induction
3.4.2. OGTT, OSTT, FBGT, and HOMA-IR
3.5. Metabolite Profiling of Mice Serum
3.5.1. Extraction of Metabolites in the Mice Serum
3.5.2. GC–TOF-MS Analysis of Metabolites in Mice Serum
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guasch-Ferre, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Hu, F.B. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care 2016, 39, 833–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B.; Satija, A.; Manson, J.E. Curbing the diabetes pandemic the need for global policy solutions. JAMA 2015, 313, 2319–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sami, W.; Ansari, T.; Butt, N.S.; Ab Hamid, M.R. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. IJHS 2017, 11, 67–71. [Google Scholar]
- Zuniga, Y.L.M.; Rebello, S.A.; Oi, P.L.; Zheng, H.L.; Lee, J.; Tai, E.S.; Van Dam, R.M. Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br. J. Nutr. 2014, 111, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Wandee, Y.; Uttapap, D.; Puncha-arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N. Enrichment of rice noodles with fibre-rich fractions derived from cassava pulp and pomelo peel. Int. J. Food Sci. Technol. 2014, 49, 2348–2355. [Google Scholar] [CrossRef]
- Cao, Z.F.; Liu, Y.; Zhu, H.; Li, Y.S.; Xiao, Q.; Yi, C.P. Effect of soy protein isolate on textural properties, cooking properties and flavor of whole-grain flat rice noodles. Foods 2021, 10, 1085. [Google Scholar] [CrossRef]
- Suk, W.; Kim, J.; Kim, D.-Y.; Lim, H.; Choue, R. Effect of wheat flour noodles with Bombyx mori powder on glycemic response in healthy subjects. Prev. Nutr. Food Sci. 2016, 21, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Srikaeo, K.; Sangkhiaw, J. Effects of amylose and resistant starch on glycaemic index of rice noodles. LWT-Food Sci. Technol. 2014, 59, 1129–1135. [Google Scholar] [CrossRef]
- Geng, D.H.; Zhou, S.M.; Wang, L.L.; Zhou, X.R.; Liu, L.; Lin, Z.X.; Qin, W.Y.; Liu, L.Y.; Tong, L.T. Effects of slight milling combined with cellulase enzymatic treatment on the textural and nutritional properties of brown rice noodles. LWT-Food Sci. Technol. 2020, 128, 109520. [Google Scholar] [CrossRef]
- Geng, D.-H.; Lin, Z.; Qin, W.; Wang, A.; Wang, F.; Tong, L.-T. Effects of ultrasound-assisted cellulase enzymatic treatment on the textural properties and in vitro starch digestibility of brown rice noodles. LWT-Food Sci. Technol. 2021, 146, 111543. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of ginseng anti-diabetic studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Miura, T.; Takagi, S.; Ishida, T. Management of diabetes and its complications with banaba (Lagerstroemia speciosa L.) and corosolic acid. Evid. Based Complement Altern. Med. 2012, 2012, 871495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, X.M.; Lan, H.L.; Wang, W.J. Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): A meta-analysis of randomized controlled trials. Food Nutr. Res. 2017, 61, 1377571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A mini review on antidiabetic properties of fermented foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadpour, E.; Ghorbani, A.; Sadeghnia, H.R. Water-soluble compounds of lettuce inhibit DNA damage and lipid peroxidation induced by glucose/serum deprivation in N2a cells. Acta Pol. Pharm. 2014, 71, 409–413. [Google Scholar] [PubMed]
- Ghorbani, A.; Sadeghnia, H.R.; Asadpour, E. Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr. Neurosci. 2015, 18, 103–109. [Google Scholar] [CrossRef]
- Han, Y.Y.; Zhao, C.H.; He, X.Y.; Sheng, Y.; Ma, T.S.; Sun, Z.F.; Liu, X.Y.; Liu, C.J.; Fan, S.X.; Xu, W.T.; et al. Purple lettuce (Lactuca sativa L.) attenuates metabolic disorders in diet induced obesity. J. Funct. Foods 2018, 45, 462–470. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, J.L.; Li, F. Application of Metabolomics in the Study of Natural Products. Nat. Prod. Bioprospect. 2018, 8, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Hasanpour, M.; Iranshahy, M.; Iranshahi, M. The application of metabolomics in investigating anti-diabetic activity of medicinal plants. Biomed. Pharmacother. 2020, 128, 110263. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, Y.Q. Metabolic effects of mulberry branch bark powder on diabetic mice based on GC-MS metabolomics approach. Nutr. Metab. 2019, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.Y.; Cong, W.J.; Shen, L.; Wei, H.; Wang, Y.; Wang, L.Y.; Ruan, K.F.; Wu, F.; Feng, Y. Fecal metabonomic study of a polysaccharide, MDG-1 from Ophiopogon japonicus on diabetic mice based on gas chromatography/time-of-flight mass spectrometry (GC TOF/MS). Mol. Biosyst. 2014, 10, 304–312. [Google Scholar] [CrossRef]
- Purwana, I.; Zheng, J.; Li, X.M.; Deurloo, M.; Son, D.O.; Zhang, Z.Y.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.P.; et al. GABA promotes human beta-cell proliferation and modulates glucose homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef] [Green Version]
- Bansal, P.; Wang, S.L.; Liu, S.H.; Xiang, Y.Y.; Lu, W.Y.; Wang, Q.H. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 beta-cells. PLoS ONE 2011, 6, e26225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeap, S.K.; Ali, N.M.; Yusof, H.M.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic effects of fermented and nonfermented mung bean extracts on alloxan-induced-diabetic mice. J. Biomed. Biotechnol. 2012, 2012, 285430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.M.; Weltman, A.L.; Vincent, K.R.; Barrett, E.; Innes, K.E.; Taylor, A.G. Effects of antioxidant supplementation on insulin sensitivity, endothelial adhesion molecules, and oxidative stress in normal-weight and overweight young adults. Metab. Clin. Exp. 2009, 58, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. 2012, 12, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Martinez, M.M. Unraveling the inhibition of intestinal glucose transport by dietary phenolics: A review. Curr. Pharm. Des. 2019, 25, 3418–3433. [Google Scholar] [CrossRef]
- Hanhineva, K.; Torronen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkanan, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Oh, C.H.; Oh, S.H. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J. Med. Food 2004, 7, 19–23. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Daily, J.W.; Kim, H.J.; Park, S. Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 2010, 30, 1–13. [Google Scholar] [CrossRef]
- Cheon, J.M.; Kim, D.I.; Kim, K.S. Insulin sensitivity improvement of fermented Korean Red Ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob mice. J. Ginseng. Res. 2015, 39, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Kwon, D.Y.; Kim, M.J.; Kang, S.; Kim, D.S.; Park, S. Jerusalem artichoke and chungkookjang additively improve insulin secretion and sensitivity in diabetic rats. Nutr. Metab. 2012, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Ha, B.J. Antihyperglycemic and antihyperlipidemic effects of fermented rhynchosia nulubilis in alloxan-induced diabetic rats. Toxicol. Res. 2013, 29, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Song, I.B.; Gu, H.J.; Na, J.Y.; Kim, S.; Yang, H.S.; Lee, S.C.; Huh, C.K.; Kwon, J. Anti-diabetic effect of fermented milk containing conjugated linoleic acid on type II diabetes mellitus. Korean J. Food Sci. Anim. Resour. 2016, 36, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulkawi, N.; Ng, K.H.; Zamberi, N.R.; Yeap, S.K.; Satharasinghe, D.A.; Tan, S.W.; Ho, W.Y.; Abd Rashid, N.Y.; Lazim, M.I.M.; Jamaluddin, A.; et al. Antihyperglycemic and anti-inflammatory effects of fermented food paste in high-fat diet and streptozotocin-challenged mice. Drug Des. Dev. Ther. 2018, 12, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Tam, Z.Y.; Ng, S.P.; Tan, L.Q.; Lin, C.H.; Rothenbacher, D.; Klenk, J.; Boehm, B.O.; Team, S.P.C.; Acti, F.E.S.G. Metabolite profiling in identifying metabolic biomarkers in older people with late-onset type 2 diabetes mellitus. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Liu, J.X.; Morningstar, J.; Heckman-Stoddard, B.M.; Lee, C.G.; Dagogo-Jack, S.; Ferguson, J.F.; Hamman, R.F.; Knowler, W.C.; Mather, K.J.; et al. Metabolite profiles of incident diabetes and heterogeneity of treatment effect in the diabetes prevention program. Diabetes 2019, 68, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Ahola-Olli, A.V.; Mustelin, L.; Kalimeri, M.; Kettunen, J.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Havulinna, A.S.; Lehtimaki, T.; Kahonen, M.; et al. Circulating metabolites and the risk of type 2 diabetes: A prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia 2019, 62, 2298–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, S.O.; Hoogeveen, R.C.; Brancati, F.L.; Astor, B.C.; Ballantyne, C.M.; Schmidt, M.I.; Young, J.H. Association of blood lactate with type 2 diabetes: The atherosclerosis risk in communities carotid MRI study. Int. J. Epidemiol. 2010, 39, 1647–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umetrics, A.B. User‘s Guide to SIMCA-P, SIMCA-P+ Version 11.0; Umetics AB: Umea, Sweden, 2005. [Google Scholar]
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2012, 2, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Henly, D.C.; Phillips, J.W.; Berry, M.N. Suppression of glycolysis is associated with an increase in glucose cycling in hepatocytes from diabetic rats. J. Biol. Chem. 1996, 271, 11268–11271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.I.; Lee, B.Y. Anti-diabetic effect of material fermented using rice bran and soybean as the main ingredient by Bacillus sp. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 222–229. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Tsukioka, T.; Shinkawa, H.; Kobayashi, F.; Nishikawa, Y.; Kodai, S.; Mizuguchi, S.; Suehiro, S.; Okada, S. Effect of AOB, a fermented-grain food supplement, on oxidative stress in type 2 diabetic rats. Biofactors 2007, 30, 91–104. [Google Scholar] [CrossRef]
- Tamaya, K.; Matsui, T.; Toshima, A.; Noguchi, M.; Ju, Q.; Miyata, Y.; Tanaka, T.; Tanaka, K. Suppression of blood glucose level by a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves in disaccharide-loaded Sprague-Dawley rats. J. Sci. Food Agric. 2010, 90, 779–783. [Google Scholar] [CrossRef]
- Jung, Y.M.; Lee, S.H.; Lee, D.S.; You, M.J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.S.; Lee, Y.J.; Ku, S.K. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Varadaiah, Y.G.C.; Sivanesan, S.; Nayak, S.B.; Thirumalarao, K.R. Purine metabolites can indicate diabetes progression. Arch. Physiol. Biochem. 2019, 1–15. [Google Scholar] [CrossRef]
- Papandreou, C.; Li, J.; Liang, L.M.; Bullo, M.; Zheng, Y.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferre, M.; Razquin, C.; Clish, C.; et al. Metabolites related to purine catabolism and risk of type 2 diabetes incidence; modifying effects of the TCF7L2-rs7903146 polymorphism. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Xia, J.F.; Wang, Z.H.; Zhang, F.F. Association between related purine metabolites and diabetic retinopathy in Type 2 diabetic patients. Int. J. Endocrinol. 2014, 2014, 651050. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Han, Q.; Liu, Y.J.; Sun, C.L.; Gang, X.K.; Wang, G.X. The relationship between branched-chain amino acid related metabolomic signature and insulin resistance: A systematic review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef]
- Siddik, M.A.; Shin, A.C. Recent progress on branched-chain amino acids in obesity, diabetes, and beyond. Endocrinol. Meta. 2019, 34, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Akter, S.; Kuwahara, K.; Matsushita, Y.; Nakagawa, T.; Konishi, M.; Honda, T.; Yamamoto, S.; Hayashi, T.; Noda, M.; et al. Serum amino acid profiles and risk of type 2 diabetes among Japanese adults in the Hitachi Health Study. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Park, N.Y.; No, H.K. Quality and shelf life of noodles containing onion powder. Korean J. Food Preserv. 2016, 23, 218–224. [Google Scholar] [CrossRef]
- Park, W.-P. Quality characteristics of noodles added with Houttuynia cordata Thunb. powder. Korean J. Food Preserv. 2014, 21, 34–39. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemical: Arlington, VA, USA, 1995. [Google Scholar]
- Chun, H.S. Manufacturing Method of Natural Fermented-Composition Having Fixed Nitric Oxide and Natural Fermented-Composition Thereof. 10-2018-0002526. 8 January 2018. [Google Scholar] [CrossRef]
- Lu, Y.G.; Zhang, H.; Meng, X.Y.; Wang, L.; Guo, X.N. A validated HPLC method for the determination of GABA by pre-column derivatization with 2,4-dinitrofluorodinitrobenzene and its application to plant GAD activity study. Anal. Lett. 2010, 43, 2663–2671. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Park, K.M.; Hussein, K.H.; Nam, H.S.; Kim, H.M.; Kang, B.M.; Lee, D.G.; Han, H.J.; Woo, H.M. A novel mouse model of diabetes mellitus using unilateral nephrectomy. Lab. Anim. 2016, 50, 88–93. [Google Scholar] [CrossRef]
- Deeds, M.C.; Anderson, J.M.; Armstrong, A.S.; Gastineau, D.A.; Hiddinga, H.J.; Jahangir, A.; Eberhardt, N.L.; Kudva, Y.C. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Lab. Anim. 2011, 45, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Sniderman, A.D.; Cianflone, K. Acylation-stimulating protein (ASP) deficiency induces obesity resistance and increased energy expenditure in ob/ob mice. J. Biol. Chem. 2002, 277, 45874–45879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiumi, S.; Shinohara, M.; Ikeda, A.; Yoshie, T.; Hatano, N.; Kakuyama, S.; Mizuno, S.; Sanuki, T.; Kutsumi, H.; Fukusaki, E.; et al. Serum metabolomics as a novel diagnostic approach for pancreatic cancer. Metabolomics 2010, 6, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, J.; Song, J.H.; Jung, Y.H.; Choi, I.S.; Choi, W.; Park, Y.C.; Seo, J.H.; Kim, K.H. Elucidation of ethanol tolerance mechanisms in Saccharomyces cerevisiae by global metabolite profiling. Biotechnol. J. 2016, 11, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Saeed, A.I.; Hagabati, N.K.; Braisted, J.C.; Liang, W.; Sharov, V.; Howe, E.A.; Li, J.W.; Thiagarajan, M.; White, J.A.; Quackenbush, J. TM4 microarray software suite. In DNA Microarrays, Part B: Databases and Statistics; Kimmel, A., Oluver, B., Eds.; Methods in Enzymology; Elsevier Academic Press Inc.: San Diego, CA, USA, 2006; Volume 411, pp. 134–193. [Google Scholar]



| Feature (%) | Standard Noodles | FLE Noodles | p-Value |
|---|---|---|---|
| Moisture | 12.30 ± 0.56 | 12.10 ± 0.74 | 0.49 |
| Ash | 3.41 ± 0.05 | 3.44 ± 0.06 | 0.28 |
| Crude fat | 1.25 ± 0.11 | 1.40 ± 0.02 | 0.39 |
| Crude protein | 9.29 ± 0.58 | 9.31 ± 0.57 | 1.00 |
| Carbohydrate | 73.74 ± 0.07 | 73.75 ± 0.19 | 0.06 |
| Chemical Class | Identified Metabolites | p-Value | Identified Metabolites | p-Value |
|---|---|---|---|---|
| organic acid | 2-ketoisocaproic acid | 0.535 | glyceric acid | 0.200 |
| 5-aminovaleric acid | 0.069 | glycolic acid | 0.024 | |
| α-ketoglutarate | 0.105 | lactic acid | 0.031 | |
| aminomalonic acid | 0.416 | oxalic acid | 0.072 | |
| aminovalerate | 0.015 | oxamic acid | 0.003 | |
| benzoic acid | 0.802 | phthalic acid | 0.032 | |
| β-hydroxybutyric acid | 0.610 | pyruvate | 0.044 | |
| citric acid | 0.303 | succinic acid | 0.440 | |
| galactonic acid | 0.337 | uric acid | 0.001 | |
| amino acid | alanine | 0.518 | methionine | 0.083 |
| aminoisobutyric acid | 0.088 | ornithine | 0.614 | |
| aspartic acid | 0.493 | oxoproline | 0.301 | |
| citrulline | 0.663 | phenylalanine | 0.330 | |
| glutamate | 0.062 | serine | 0.454 | |
| glutamine | 0.574 | threonine | 0.817 | |
| isoleucine | 0.001 | tyrosine | 0.281 | |
| leucine | 0.588 | valine | 0.003 | |
| lysine | 0.004 | |||
| sugar | 1,5-anhydroglucitol | <0.001 | mannose | 0.029 |
| arabitol | 0.102 | myo-inositol | 0.011 | |
| fructose | 0.047 | phosphogluconic acid | 0.205 | |
| fucose | 0.483 | sorbitol | 0.289 | |
| glucose | 0.154 | tagatose | 0.033 | |
| glycerol | 0.003 | threose | 0.065 | |
| lactose | 0.219 | trehalose | 0.002 | |
| malonic acid | 0.027 | xylose | 0.527 | |
| mannitol | 0.851 | |||
| fatty acid | 1-monopalmitin | 0.789 | myristic acid | 0.321 |
| 1-monostearin | 0.963 | nonanoate | 0.557 | |
| arachidic acid | 0.343 | oleic acid | 0.244 | |
| dodecanoate | 0.021 | palmitate | 0.121 | |
| heptadecanoic acid | 0.165 | pentadecanoic acid | 0.157 | |
| hexonic acid | 0.301 | squalene | 0.238 | |
| lignoceric acid | 0.204 | stearic acid | 0.239 | |
| methyl palmitoleate | 0.153 | |||
| amine | adenosine | 0.002 | nicotinamide | 0.013 |
| ethanolamine | 0.131 | taurine | 0.010 | |
| guanine | 0.054 | thymine | 0.265 | |
| guanosine | 0.481 | uracil | 0.892 | |
| inosine | 0.162 | |||
| phosphate | glycerol-1-phosphate | 0.876 | phosphate | <0.001 |
| mannose-6-phosphate | 0.790 | |||
| other | 2-hydroxypyridine | 0.315 | salicylic acid | 0.407 |
| salicylaldehyde | 0.001 | urea | 0.088 |
| PC1 | PC2 | ||
|---|---|---|---|
| Metabolites | Loadings | Metabolites | Loadings |
| oxamic acid | 0.955 | stearic acid | 0.824 |
| 1,5-anhydroglucitol | 0.931 | palmitate | 0.760 |
| adenosine | 0.899 | citrulline | 0.736 |
| salicylaldehyde | 0.892 | oleic acid | 0.687 |
| aminovalerate | 0.886 | 1-monopalmitin | 0.662 |
| glycerol | 0.850 | inosine | 0.591 |
| tagatose | 0.736 | ethanolamine | 0.583 |
| pyruvate | 0.721 | phthalic acid | 0.555 |
| trehalose | −0.977 | threonine | −0.831 |
| phosphate | −0.976 | ornithine | −0.823 |
| isoleucine | −0.975 | myristic acid | −0.810 |
| uric acid | −0.956 | benzoic acid | −0.766 |
| lysine | −0.953 | 2-hydroxypyridine | −0.752 |
| myo-inositol | −0.896 | squalene | −0.740 |
| valine | −0.890 | aspartic acid | −0.730 |
| taurine | −0.812 | serine | −0.725 |
| glutamate | −0.794 | pentadecanoic acid | −0.669 |
| malonic acid | −0.790 | nonanoate | −0.661 |
| malonic acid | −0.790 | glycerol-1-phosphate | −0.645 |
| phthalic acid | −0.780 | α-ketoglutarate | −0.635 |
| Group | Material | Dose | Material | Dose | |
| 1 | Control | Distilled water | - | Glucose | 2 g/kg |
| 2 | Without FLE | Standard noodle | 300 mg/kg | Glucose | 2 g/kg |
| 3 | With FLE | Noodles with fermented lettuce extract (FLE) | 300 mg/kg | Glucose | 2 g/kg |
| (a) | |||||
| Group | Material | Dose | Material | Dose | |
| 1 | Control | Distilled water | - | Sucrose | 2 g/kg |
| 2 | Without FLE | Standard noodle | 300 mg/kg | Sucrose | 2 g/kg |
| 3 | With FLE | Noodles with fermented lettuce extract (FLE) | 300 mg/kg | Sucrose | 2 g/kg |
| (b) | |||||
| Group | Material | Dose | |||
| 1 | Control | Distilled water | - | ||
| 2 | Without FLE | Standard noodle | 300 mg/kg | ||
| 3 | With FLE | Noodles with fermented lettuce extract (FLE) | 300 mg/kg | ||
| (c) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.Y.; Kim, E.; Zhang, M.; Lee, Y.-S.; Ji, B.; Lee, S.-H.; Cheong, Y.E.; Yun, S.-I.; Kim, Y.-S.; Kim, K.H.; et al. Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts. Metabolites 2021, 11, 520. https://doi.org/10.3390/metabo11080520
Jeong SY, Kim E, Zhang M, Lee Y-S, Ji B, Lee S-H, Cheong YE, Yun S-I, Kim Y-S, Kim KH, et al. Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts. Metabolites. 2021; 11(8):520. https://doi.org/10.3390/metabo11080520
Chicago/Turabian StyleJeong, Soon Yeon, Eunjin Kim, Ming Zhang, Yun-Seong Lee, Byeongjun Ji, Sun-Hee Lee, Yu Eun Cheong, Soon-Il Yun, Young-Soo Kim, Kyoung Heon Kim, and et al. 2021. "Antidiabetic Effect of Noodles Containing Fermented Lettuce Extracts" Metabolites 11, no. 8: 520. https://doi.org/10.3390/metabo11080520







