Heartwood Extract of Pterocarpus marsupium Roxb. Offers Defense against Oxyradicals and Improves Glucose Uptake in HepG2 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Preparation of Plant Extracts
2.3. Physicochemical Analysis
2.4. Estimation of Total Phenolic and Flavonoid Content
2.5. Phytochemical Profiling by GCMS
2.6. HPTLC Fingerprinting and Quantitative Analysis
2.7. Ultraperformance Liquid Chromatography-Mass Spectroscopy (UPLC-MS) Studies
2.8. Cell Culture and Treatments
2.8.1. MTT {3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide} Assay
2.8.2. Measurement of Glucose-Uptake
2.8.3. Detection of ROS in HepG2 Cells by Flow Cytometry Using 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFDA)
2.8.4. Assessment of Apoptosis and Necrosis
2.8.5. Morphological Changes in HepG2 Cells
Fluorescence Microscopy
Confocal Microscopy
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Analysis
3.2. Total Phenolic and Flavonoid Content of the Extract
3.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.4. HPTLC Fingerprinting and Quantitative Analysis
3.5. Ultra-Performance Liquid Chromatography-Mass Spectroscopy (UPLC-MS) Studies
3.6. Studies Using the HepG2 Cell Line
3.6.1. Cytotoxic Effect of MPME on HepG2 Cell Line
3.6.2. Glucose Uptake Assay
3.6.3. Detection of ROS in H2O2 Induced HepG2 Cells Using 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFDA) Probe by Flow Cytometry
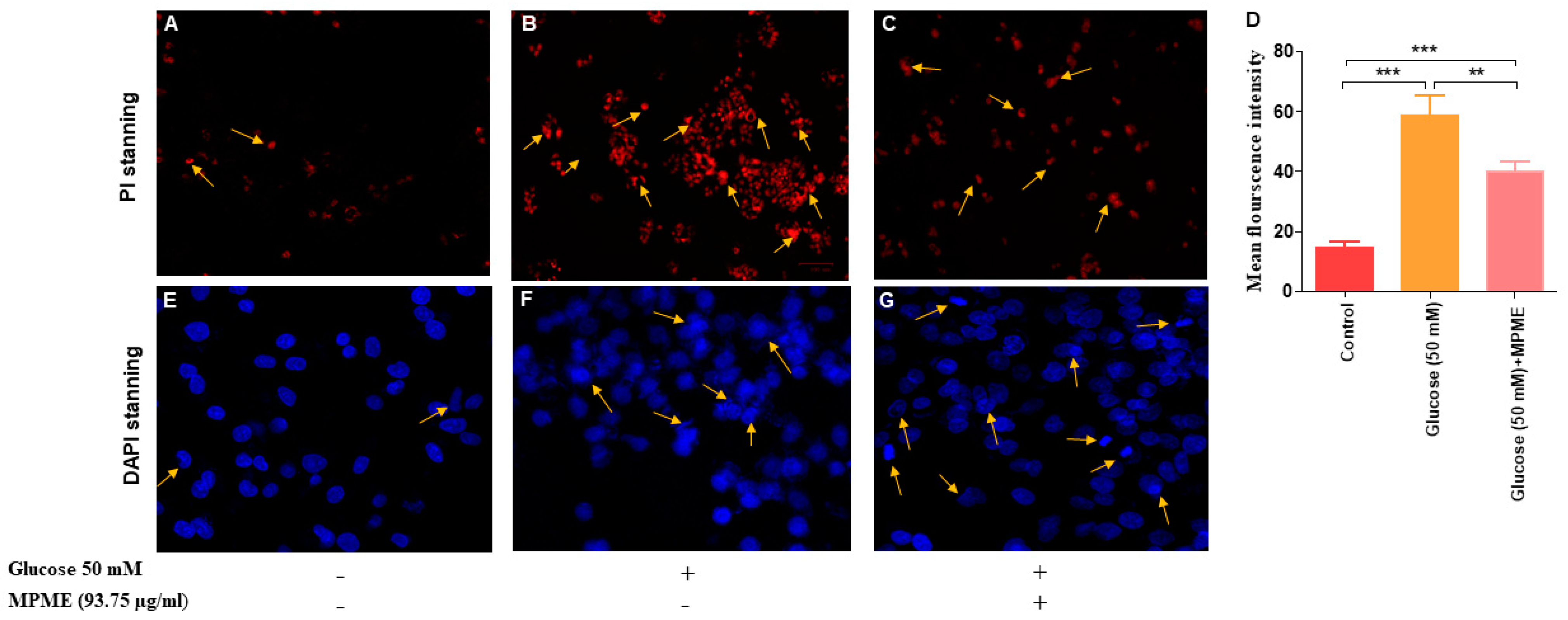
3.6.4. Assessment of Apoptosis and Necrosis by Fluorescence Microscopy
3.6.5. Assessment of Cellular Morphology on High Glucose (50 mM)-Induced HepG2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of Diabetes Mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; ISBN 9782930229874. [Google Scholar]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Kaabi, J. Al Epidemiology of type 2 Diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.I.; Srivastava, N. Erythrocyte plasma membrane redox system in first degree relatives of type 2 diabetic patients. Int. J. Diabetes Mellit. 2010, 2, 119–121. [Google Scholar] [CrossRef]
- Robertson, A.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in Diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef]
- Parhar, G.; Kalaji, W.; Sargi, J.; Adarkwah, O.; Zaman, K.; Gerolemou, L. A case of erythematous and vesicular rash in a critically ill patient admitted with Sars-CoV2: Viral or multi-drug effect? Chest 2020, 158, A719. [Google Scholar] [CrossRef]
- Gondi, M.; Prasada Rao, U.J.S. Ethanol extract of mango (Mangifera Indica L.) peel inhibits α-amylase and α-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J. Food Sci. Technol. 2015, 52, 7883–7893. [Google Scholar] [CrossRef]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant effects of vitamins in type 2 Diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Noh, S.; Lim, S.; Kim, B. Plant extracts for Type 2 Diabetes: From traditional medicine to modern drug discovery. Antioxidants 2021, 10, 81. [Google Scholar] [CrossRef]
- Dhanabal, S.P.; Kokate, C.K.; Ramanathan, M.; Kumar, E.P.; Suresh, B. Hypoglycaemic activity of Pterocarpus marsupium Roxb. Phytother. Res. 2006, 20, 4–8. [Google Scholar] [CrossRef]
- Sheehan, E.W.; Zemaitis, M.A.; Slatkin, D.J.; Schiff, P.L. A constituent of Pterocarpus marsupium, (-)-epicatechin, as a potential antidiabetic agent. J. Nat. Prod. 1983, 46, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Ip. Indian Pharmacopoeia. Appendix 9.4; Controller of Publication: Delhi, India, 1996. [Google Scholar]
- Khan, W.; Parveen, R.; Chester, K.; Parveen, S.; Ahmad, S. Hypoglycemic potential of aqueous extract of Moringa oleifera Leaf and in vivo GC-MS metabolomics. Front. Pharmacol. 2017, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Zahiruddin, S.; Khan, W.; Nehra, R.; Alam, M.J.; Mallick, M.N.; Parveen, R.; Ahmad, S. Pharmacokinetics and comparative metabolic profiling of iridoid enriched fraction of Picrorhiza kurroa—An ayurvedic herb. J. Ethnopharmacol. 2017, 197, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rab, R.A.; Zahiruddin, S.; Ibrahim, M.; Husain, F.; Parveen, R.; Khan, W.; Ahmad, F.J.; Khan, A.A.; Ahmad, S. HPTLC and UPLC-MS/MS methods for quality control analysis of itrifal formulations of unani system of medicine. J. AOAC Int. 2021, 103, 649–658. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Xu, Z.H.; Chen, W.Q. Neurotoxic effects of iron overload under high glucose concentration. Neural Regen. Res. 2013, 8, 3423–3433. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.; Yue, Z.; Ding, L.; Zhou, Y.; Huang, Z.; Huang, H. Rosmarinic acid ameliorates H2O2-induced oxidative stress in L02 cells through MAPK and Nrf2 pathways. Rejuvenation Res. 2019, 22, 289–298. [Google Scholar] [CrossRef]
- Venkatachalapathy, D.; Shivamallu, C.; Prasad, S.K.; Saradha, G.T.; Rudrapathy, P.; Amachawadi, R.G.; Patil, S.S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; et al. Assessment of chemopreventive potential of the plant extracts against liver cancer using Hepg2 cell line. Molecules 2021, 26, 4593. [Google Scholar] [CrossRef]
- Brana, C.; Benham, C.; Sundstrom, L. A Method for characterising cell death in vitro by combining propidium iodide staining with immunohistochemistry. Brain Res. Protoc. 2002, 10, 109–114. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef]
- Arbain, D.; Saputri, G.A.; Syahputra, G.S.; Widiyastuti, Y.; Susanti, D.; Taher, M. Genus Pterocarpus: A review of ethnopharmacology, phytochemistry, biological activities, and clinical evidence. J. Ethnopharmacol. 2021, 278, 114316. [Google Scholar] [CrossRef]
- Picton, S.F.; Flatt, P.R.; McClenaghan, N.H. Differential acute and long term actions of succinic acid monomethyl ester exposure on insulin-secreting BRIN-BD11 cells. Int. J. Exp. Diabetes Res. 2001, 2, 19–27. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab. Res. Rev. 2020, 36, e3319. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Kaur, G.; Kaur, N.; Sharma, C.; Batra, K.; Singh, D. The traditional uses, phytochemistry and pharmacology of genus Hibiscus: A review. Eur. J. Med. Plants 2021, 32, 1–37. [Google Scholar] [CrossRef]
- Kim, N.; Lee, J.O.; Lee, H.J.; Lee, Y.W.; Kim, H.I.; Kim, S.J.; Park, S.H.; Lee, C.S.; Ryoo, S.W.; Hwang, G.S.; et al. AMPK, a metabolic sensor, is involved in isoeugenol-induced glucose uptake in muscle cells. J. Endocrinol. 2016, 228, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Romero Rocamora, C.; Ramasamy, K.; Meng Lim, S.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bajpai, V.; Gupta, A.; Gaikwad, A.N.; Maurya, R.; Kumar, B. Identification and quantification of secondary metabolites of Pterocarpus marsupium by LC–MS techniques and its in-vitro lipid lowering activity. Ind. Crops Prod. 2019, 127, 26–35. [Google Scholar] [CrossRef]
- Bule, M.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502. [Google Scholar] [CrossRef]
- Kim, M.J.; Ryu, G.R.; Chung, J.S.; Sim, S.S.; Min, D.S.; Rhie, D.J.; Yoon, S.H.; Hahn, S.J.; Kim, M.S.; Jo, Y.H. Protective effects of epicatechin against the toxic effects of streptozotocin on rat pancreatic islets: In Vivo and in vitro. Pancreas 2003, 26, 292–299. [Google Scholar] [CrossRef]
- Raafat, K.M.; Omar, A.G. Phytotherapeutic activity of curcumol: Isolation, GC–MS identification, and assessing potentials against acute and subchronic hyperglycemia, tactile allodynia, and hyperalgesia. Pharm. Biol. 2016, 54, 1334–1344. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Mitra, J.; Joshi, T. Isoflavonoids from the heartwood of Pterocarpus marsupium. Phytochemistry 1983, 22, 2326–2327. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Bourebaba, N.; Kornicka-Garbowska, K.; Marycz, K.; Bourebaba, L.; Kowalczuk, A. Laurus nobilis ethanolic extract attenuates hyperglycemia and hyperinsulinemia-induced insulin resistance in HepG2 Cell line through the reduction of oxidative stress and improvement of mitochondrial biogenesis—Possible implication in pharmacotherapy. Mitochondrion 2021, 59, 190–213. [Google Scholar] [CrossRef] [PubMed]
- Gulati, V.; Gulati, P.; Harding, I.H.; Palombo, E.A. Exploring the anti-diabetic potential of australian aboriginal and indian ayurvedic plant extracts using cell-based assays. BMC Complement. Altern. Med. 2015, 15, 8. [Google Scholar] [CrossRef]
- Wu, S.F.; Hwang, T.L.; Chen, S.L.; Wu, C.C.; Ohkoshi, E.; Lee, K.H.; Chang, F.R.; Wu, Y.C. Bioactive components from the heartwood of Pterocarpus santalinus. Bioorganic Med. Chem. Lett. 2011, 21, 5630–5632. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of Diabetes Mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Yen Nguyen, T.K.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced glucose uptake in human liver cells and inhibition of carbohydrate hydrolyzing enzymes by Nordic berry extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, R.; Srivastava, S.P.; Gautam, S.; Tamrakar, A.K.; Maurya, R.; Srivastava, A.K. Antidiabetic activity of heart wood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J. Exp. Biol. 2013, 51, 363–374. [Google Scholar]
- Dabur, R. Identification of molecular pathways affected by treatment with heartwood water extract of Pterocarpus marsupium in MCF 7 cancer cell line. J. Herb. Med. 2017, 9, 42–52. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMP-activated protein kinase (AMPK) signaling pathway coordinates cell growth, autophagy, & metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. MTORC1 as the main gateway to autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in Diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Swaminathan, K.; Chatterjee, S.; Dey, A. Apoptosis in HepG2 cells exposed to high glucose. Toxicol. In Vitro 2010, 24, 387–396. [Google Scholar] [CrossRef]
- Maradagi, T.; Kumar, R.; Ponesakki, G. Hyperglycaemia-induced human hepatocellular carcinoma (HepG2) cell proliferation through ROS-mediated P38 activation is effectively inhibited by a xanthophyll carotenoid, lutein. Diabet. Med. 2022, 39, e14713. [Google Scholar] [CrossRef]
- Das, B.K.; Knott, R.M.; Gadad, P.C. Metformin and asarone inhibit HepG2 cell proliferation in a high glucose environment by regulating AMPK and Akt signaling pathway. Future J. Pharm. Sci. 2021, 7, 43. [Google Scholar] [CrossRef]





| RT | Compound | Molecular Formula | Mol Weight g·mol−1 | Peak Area |
|---|---|---|---|---|
| 8.832 | Benzaldehyde | C7H6O | 106.12 | 19.92 |
| 10.153 | Succinic acid | C5H8O4 | 10.153 | 0.76 |
| 11.120 | Ethyl hydrogen succinate | C6H10O4 | 146.14 | 0.20 |
| 11.933 | Cyclohexane | C6H12 | 84.16 | 0.11 |
| 14.388 | 5-Dimethoxybenzaldehyde | C9H10O3 | 166.17 | 0.98 |
| 14.691 | Isoeugenol | C10H12O2 | 164.204 | 0.41 |
| 15.091 | 2,4-bis (1,1-dimethylethyl | C17H3OSi | 278.5 | 15.89 |
| 15.669 | Penta-fluoropropionic acid | C3HF5O2 | 164.03 | 1.66 |
| 15.944 | 2′,5′-Dimethoxypropiophenone | C11H14O3 | 194.23 | 0.04 |
| 16.722 | Docosane | C22H46 | 310.6 | 0.12 |
| 17.701 | Cyclopentane | C5H10 | 70.13 | 0.32 |
| 18.095 | Myristic acid | C14H28O2 | 228 | 0.80 |
| 18.307 | Oleyl alcohol | C18H36O | 268.5 | 0.03 |
| 18.748 | 3,5-Dimethoxybenzyl alcohol | C9H12O3 | 168.19 | 2.62 |
| 19.463 | 1,2-Benzenedicarboxylic acid | C24H38O4 | 390 | 0.20 |
| 19.658 | Pentadecanoic acid | C15H30O2 | 242.403 | 0.36 |
| 19.846 | Pentatriacontane | C35H72 | 492.9 | 0.02 |
| 20.361 | Citronellal | C10H18O | 154.25 | 0.09 |
| 20.693 | Isotridecanol | C13H28O | 200.36 | 0.10 |
| 21.374 | Dibutyl phthalate | C16H22O4 | 278 | 1.21 |
| 21.394 | Phthalic acid (1,2-Benzenedicarboxylic acid) | C8H6O4 | 166.13 | 1.21 |
| 21.820 | Hexadecanoic acid | C16H32O2 | 256 | 21.55 |
| 22.736 | Oxiranepentanoic acid | C5H10O2 | 102.13 | 0.06 |
| 24.064 | 14b-pregnane | C21H36 | 288.5 | 0.02 |
| 24.207 | Heptadecanoic acid | C17H34O2 | 270.5 | 0.14 |
| 26.530 | Octadecanoic acid | C18H36O2 | 284 | 20.01 |
| 25.792 | 9,12-Octadecadienoic acid | C18H32O2 | 280 | 0.33 |
| 30.449 | Oleic Acid | C18H34O2 | 282.47 | 0.19 |
| RT | Tentative Mass | Exact Mass | Phytoconstituent | Formula | Class | Mass Record |
|---|---|---|---|---|---|---|
| 3.047 | 179.0136 | 178.18700 | 2-Methoxycinnamic acid | C10H10O3 | Cinnamic acid | Mass Bank ID (MBID):PR305807 |
| 3.047 | 291.1891 | 290.27100 | Epicatechin | C15H14O6 | Flavonoid | MBID:PS045605 |
| 3.047 | 453.1309 | 452.13190 | Aspalathin | C21H24O11 | Flavonoid | MBID:BS003543 |
| 3.047 | 463.2752 | 462.07983 | Kaempferol-3-Glucuronide | C21H18O12 | Flavonoid | MBID:PR101022 |
| 3.047 | 417.0264 | 416.4 | Pterocarposide | C21H20O9 | Flavonoid | Pub-Chm CID;01012651 |
| 3.047 | 433.0309 | 432.10565 | Kaempferol-7-O-alpha-L-rhamnoside | C21H20O10 | Coumarins | MBID:PR100942 |
| 3.047 | 434.8582 | 434.12131 | Naringenin-7-O-glucoside | C21H22O10 | Flavonoid | MBID:BS003367 |
| 3.047 | 437.2526 | 436.13696 | Phloridzin | C21H24O10 | Flavonoid | MBID:CE000071 |
| 3.047 | 437.2526 | 436.18860 | Artocaprin | C26H28O6 | Flavonoid | MBID:BS003597 |
| 4.000 | 237.0566 | 236.17763 | Curcumol | C15H24O2 | Sesquiterpenoid | MBID:FIO01064 |
| 4.000 | 257.0337 | 256.25699 | Iso-liquiritigenin | C15H12O4 | Hydroxychalcone | MBID:PR302707 |
| 4.000 | 257.0337 | 256.07355 | Pinocembrin | C15H12O4 | Flavonoid | MBID:BML00869 |
| 4.000 | 257.2227 | 256.30099 | Trans-pterostilbene | C16H16O3 | Stilbenoid | MBID:PR308326 |
| 4.000 | 485.1395 | 284.35550 | Dihydroartemisinin | C15H24O5 | Sesquiterpene | MBID:NGA00138 |
| 4.000 | 485.1395 | 284.06848 | Wogonin | C16H12O5 | Flavonoid | MBID:TY000033 |
| 4.000 | 285.1395 | 284.03207 | Rhein | C15H8O6 | Cassic acid | MBID:BML01013 |
| 4.000 | 485.1395 | 284.06848 | Biochanin A | C16H12O5 | Anthraquinone | MBID:PN000109 |
| 4.000 | 303.1256 | 302.04226 | Quercetin | C15H10O7 | Flavonoid | MBID:PN000111 |
| 4.000 | 337.2532 | 336.12357 | Berberine | C20H18NO | Flavonoid | MBID:KO008886 |
| 4.000 | 336.1189 | 335.17331 | Senecionine | C18H25NO5 | Alkaloid | MBID:NA002260 |
| 4.000 | 352.2504 | 351.16818 | Retrorsine | C18H25NO6 | Alkaloid | MBID:BML82062 |
| 4.374 | 241.0898 | 240.25 | Moscatin | C15H12O3 | Phenol | Pub-Chm CID:194774 |
| 4.374 | 269.1331 | 268.26 | Tectochrysin | C16H12O4 | Flavonoid | Pub-Chm CID:5281954 |
| 4.374 | 268.9441 | 268.26 | Formononetin | C16H12O4 | Isoflavonoid | Pub-Chm CID:5280378 |
| 4.374 | 457.4785 | 456.53900 | Vindoline | C25H32N2O6 | Monoterpene indole alkaloid | MBID:PM000601 |
| 6.519 | 149.0768 | 148.05243 | Citramalic acid | C5H8O5 | Hydroxy-fatty acid | MBID:PR100770 |
| 6.519 | 240.0815 | 239.09464 | 6-Aminoflavanone | C15H13NO2 | Flavonoid | MBID:JP000716 |
| 6.519 | 458.0455 | 457.15842 | Amygdalin | C20H27NO11 | Organooxygen compound | MBID:TY000092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar, M.I.; Rafat, S.; Dev, K.; Abass, S.; Khan, M.U.; Abualsunun, W.A.; Murshid, S.S.; Ahmad, S.; Qureshi, M.I. Heartwood Extract of Pterocarpus marsupium Roxb. Offers Defense against Oxyradicals and Improves Glucose Uptake in HepG2 Cells. Metabolites 2022, 12, 947. https://doi.org/10.3390/metabo12100947
Dar MI, Rafat S, Dev K, Abass S, Khan MU, Abualsunun WA, Murshid SS, Ahmad S, Qureshi MI. Heartwood Extract of Pterocarpus marsupium Roxb. Offers Defense against Oxyradicals and Improves Glucose Uptake in HepG2 Cells. Metabolites. 2022; 12(10):947. https://doi.org/10.3390/metabo12100947
Chicago/Turabian StyleDar, Mohammad Irfan, Sahar Rafat, Kapil Dev, Sageer Abass, Mohammad Umar Khan, Walaa A. Abualsunun, Samar S. Murshid, Sayeed Ahmad, and Mohammad Irfan Qureshi. 2022. "Heartwood Extract of Pterocarpus marsupium Roxb. Offers Defense against Oxyradicals and Improves Glucose Uptake in HepG2 Cells" Metabolites 12, no. 10: 947. https://doi.org/10.3390/metabo12100947
APA StyleDar, M. I., Rafat, S., Dev, K., Abass, S., Khan, M. U., Abualsunun, W. A., Murshid, S. S., Ahmad, S., & Qureshi, M. I. (2022). Heartwood Extract of Pterocarpus marsupium Roxb. Offers Defense against Oxyradicals and Improves Glucose Uptake in HepG2 Cells. Metabolites, 12(10), 947. https://doi.org/10.3390/metabo12100947






