Effects of Acute Moderate Hypoxia versus Normoxia on Metabolic and Cardiac Function and Skeletal Muscle Oxygenation during Endurance Exercise at the Same Heart Rate Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
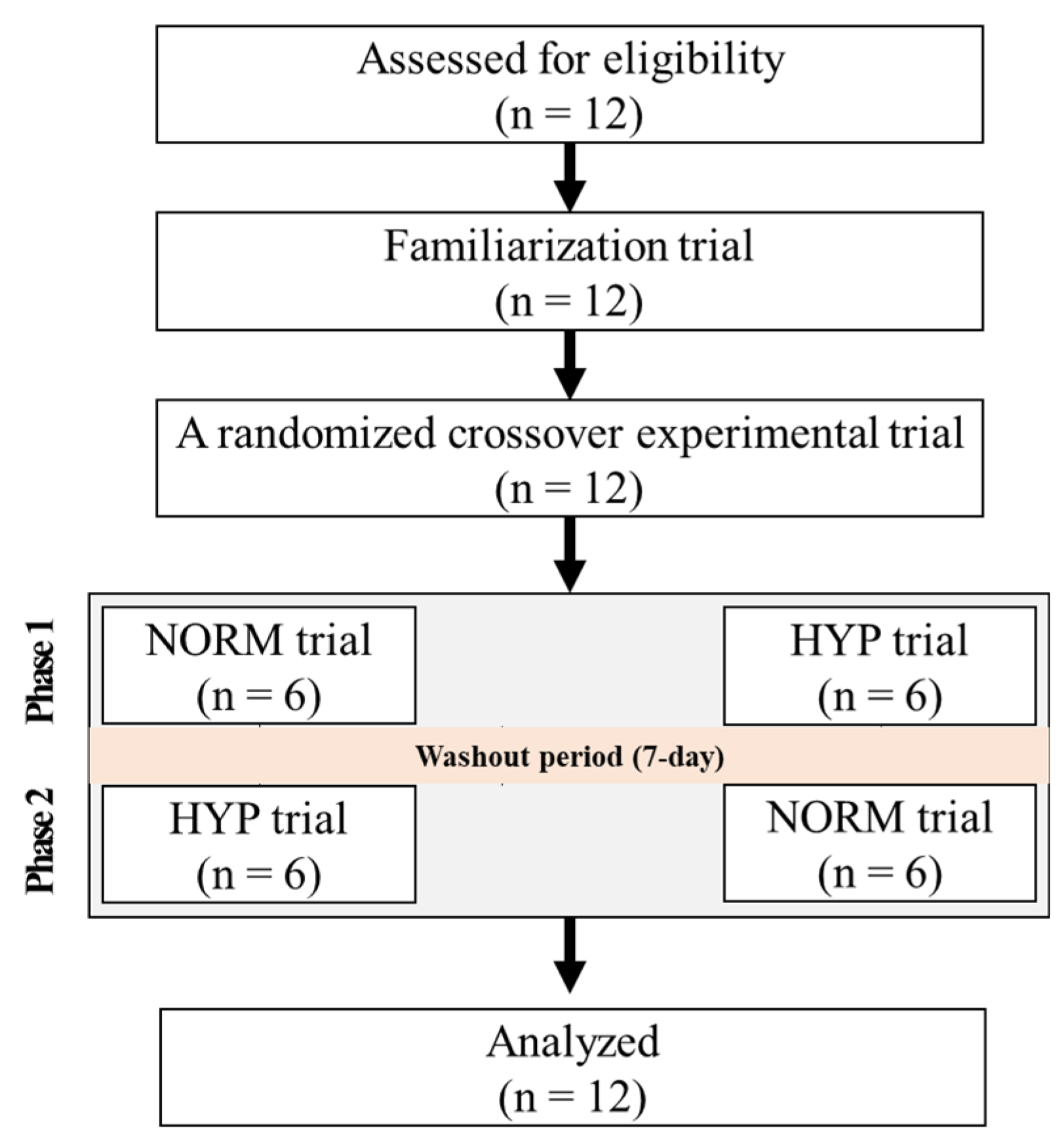
2.2. Study Design
2.3. Measurement
2.4. Statistical Analysis
3. Results
3.1. Exercise Load and RPE
3.2. Metabolic Function
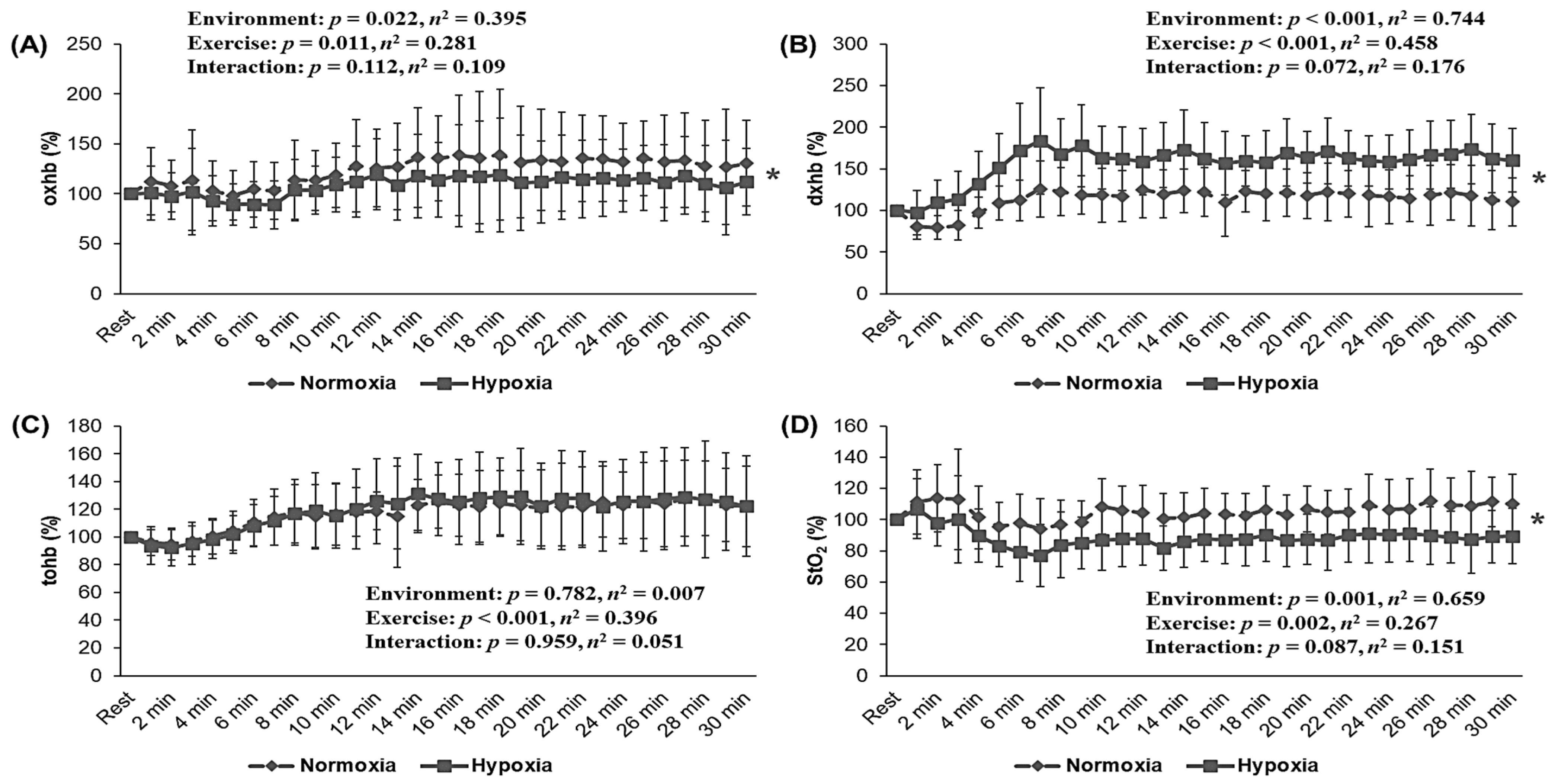
3.3. Skeletal Muscle Oxygenation
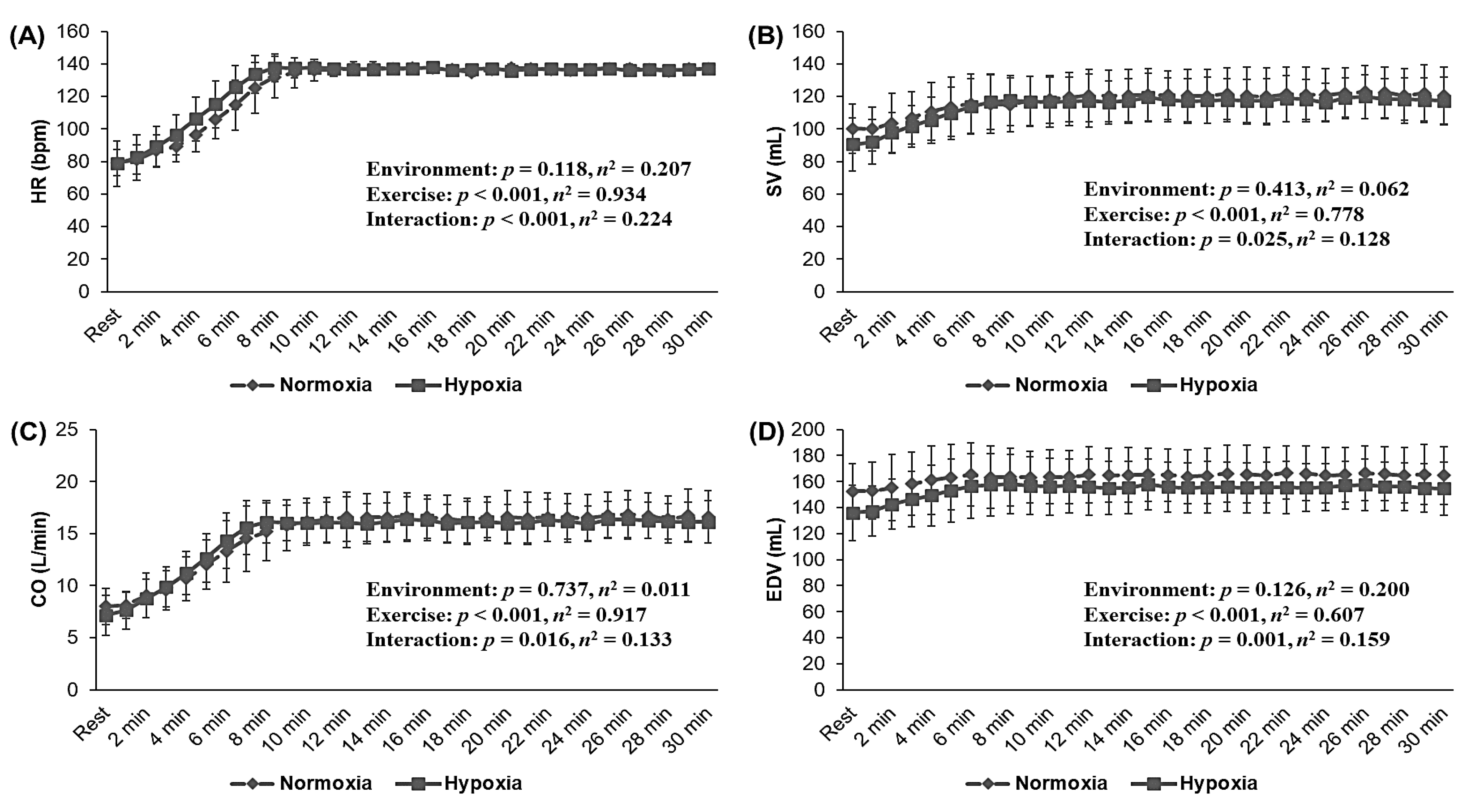
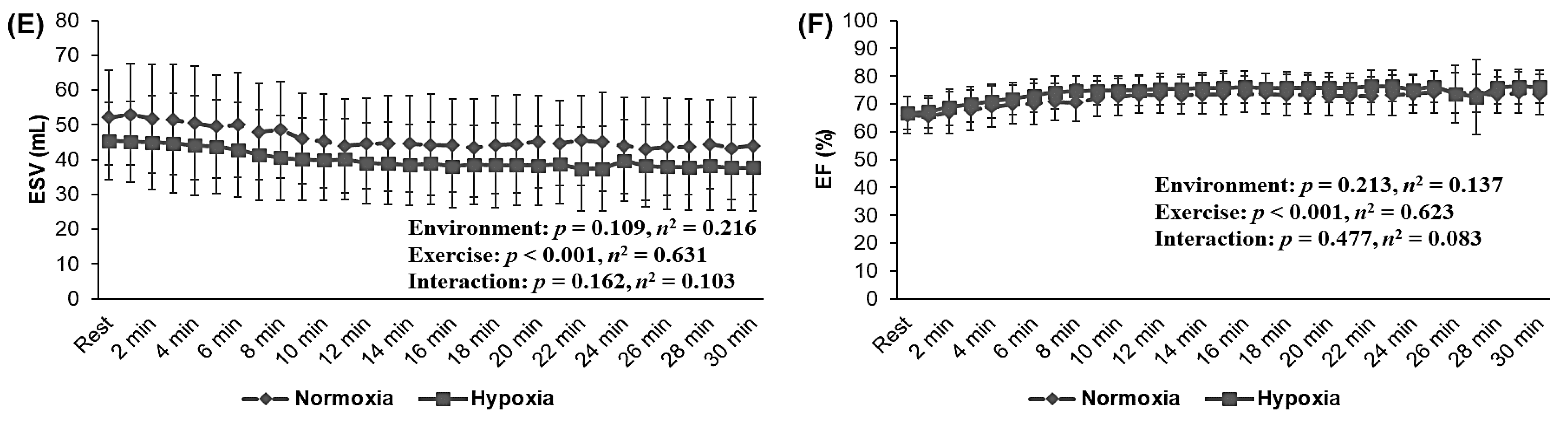
3.4. Cardiac Function
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, W.S.; Kim, S.W.; Kim, J.W.; Park, H.Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia-A Narrative Review. Life 2021, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Hwang, H.; Park, J.; Lee, S.; Lim, K. The effects of altitude/hypoxic training on oxygen delivery capacity of the blood and aerobic exercise capacity in elite athletes—A meta-analysis. J. Exerc. Nutr. Biochem. 2016, 20, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, J.W.; Nam, S.S. Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men. Biology 2022, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.S.; Park, H.Y. Effects of endurance exercise under hypoxia on acid-base and ion balance in healthy males. Phys. Act. Nutr. 2020, 24, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ofner, M.; Wonisch, M.; Frei, M.; Tschakert, G.; Domej, W.; Kröpfl, J.M.; Hofmann, P. Influence of acute normobaric hypoxia on physiological variables and lactate turn point determination in trained men. J. Sports Sci. Med. 2014, 13, 774–781. [Google Scholar] [PubMed]
- Sumi, D.; Kasai, N.; Ito, H.; Goto, K. The Effects of Endurance Exercise in Hypoxia on Acid-Base Balance, Potassium Kinetics, and Exogenous Glucose Oxidation. Front. Physiol. 2019, 10, 504. [Google Scholar] [CrossRef]
- Jung, K.; Seo, J.; Jung, W.S.; Kim, J.; Park, H.Y.; Lim, K. Effects of an Acute Pilates Program under Hypoxic Conditions on Vascular Endothelial Function in Pilates Participants: A Randomized Crossover Trial. Int. J. Environ. Res. Public Health 2020, 17, 2584. [Google Scholar] [CrossRef]
- Park, H.Y.; Nam, S.S.; Tanaka, H.; Lee, D.J. Hemodynamic, Hematological, and Hormonal Responses to Submaximal Exercise in Normobaric Hypoxia in Pubescent Girls. Pediatr. Exerc. Sci. 2016, 28, 417–422. [Google Scholar] [CrossRef]
- Wehrlin, J.P.; Hallén, J. Linear decrease in VO2max and performance with increasing altitude in endurance athletes. Eur. J. Appl. Physiol. 2006, 96, 404–412. [Google Scholar] [CrossRef]
- Yoshiko, A.; Katayama, K.; Ishida, K.; Ando, R.; Koike, T.; Oshida, Y.; Akima, H. Muscle deoxygenation and neuromuscular activation in synergistic muscles during intermittent exercise under hypoxic conditions. Sci. Rep. 2020, 10, 295. [Google Scholar] [CrossRef]
- Martin, D.S.; Levett, D.Z.; Mythen, M.; Grocott, M.P. Changes in skeletal muscle oxygenation during exercise measured by near-infrared spectroscopy on ascent to altitude. Crit. Care 2009, 13 (Suppl. 5), S7. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Imai, T.; Yatsutani, H.; Goto, K. A Combined Hot and Hypoxic Environment during Maximal Cycling Sprints Reduced Muscle Oxygen Saturation: A Pilot Study. J. Sports Sci. Med. 2021, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Ušaj, A.; Mekjavic, I.B.; Kapus, J.; McDonnell, A.C.; Jaki Mekjavic, P.; Debevec, T. Muscle Oxygenation During Hypoxic Exercise in Children and Adults. Front. Physiol. 2019, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Duteil, S.; Wary, C.; Wray, D.W.; Hoff, J.; Carlier, P.G. Human skeletal muscle intracellular oxygenation: The impact of ambient oxygen availability. J. Physiol. 2006, 571, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Vogt, M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001, 204, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.J.; Montgomery, H.E.; Murray, A.J.; Cochlin, L.E.; Codreanu, I.; Hopwood, N.; Johnson, A.W.; Rider, O.J.; Levett, D.Z.; Tyler, D.J.; et al. Cardiac response to hypobaric hypoxia: Persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J. 2011, 25, 792–796. [Google Scholar] [CrossRef]
- Moon, H.W.; Sunoo, S.; Park, H.Y.; Lee, D.J.; Nam, S.S. Effects of various acute hypoxic conditions on metabolic parameters and cardiac function during exercise and recovery. SpringerPlus 2016, 5, 1294. [Google Scholar] [CrossRef]
- Thomson, A.J.; Drummond, G.B.; Waring, W.S.; Webb, D.J.; Maxwell, S.R. Effects of short-term isocapnic hyperoxia and hypoxia on cardiovascular function. J. Appl. Physiol. 2006, 101, 809–816. [Google Scholar] [CrossRef]
- Hsu, A.R.; Barnholt, K.E.; Grundmann, N.K.; Lin, J.H.; McCallum, S.W.; Friedlander, A.L. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J. Appl. Physiol. 2006, 100, 2031–2040. [Google Scholar] [CrossRef]
- Yan, B.; Hu, Y.; Ji, H.; Bao, D. The effect of acute hypoxia on left ventricular function during exercise. Eur. J. Appl. Physiol. 2007, 100, 261–265. [Google Scholar] [CrossRef]
- Mulliri, G.; Magnani, S.; Roberto, S.; Ghiani, G.; Sechi, F.; Fanni, M.; Marini, E.; Stagi, S.; Lai, Y.; Rinaldi, A.; et al. Acute Exercise with Moderate Hypoxia Reduces Arterial Oxygen Saturation and Cerebral Oxygenation without Affecting Hemodynamics in Physically Active Males. Int. J. Environ. Res. Public Health 2022, 19, 4558. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jung, W.S.; Kim, J.; Lim, K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bredin, S.S.; Gledhill, N.; Jamnik, V.K.; Warburton, D.E. PAR-Q+ and ePARmed-X+: New risk stratification and physical activity clearance strategy for physicians and patients alike. Can. Fam. Physician 2013, 59, 273–277. [Google Scholar] [PubMed]
- Girard, O.; Malatesta, D.; Millet, G.P. Walking in Hypoxia: An Efficient Treatment to Lessen Mechanical Constraints and Improve Health in Obese Individuals? Front. Physiol. 2017, 8, 73. [Google Scholar] [CrossRef]
- Adamos, T.; Papanikolaou, Z.; Voutselas, V.; Soulas, D. Effects of hypoxia on interval moderate exercise. Biol. Exerc. 2008, 4, 5–19. [Google Scholar] [CrossRef]
- Hill, N.E.; Stacey, M.J.; Woods, D.R. Energy at high altitude. J. R. Army Med. Corps 2011, 157, 43–48. [Google Scholar] [CrossRef]
- Ferrari, M.; Muthalib, M.; Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011, 369, 4577–4590. [Google Scholar] [CrossRef]
- Wickerson, L.; Mathur, S.; Brooks, D.; Bonetti, L.V.; Singer, L.G.; Granton, J.; Reid, W.D. Skeletal muscle oxygenation and regional blood volume during incremental limb loading in interstitial lung disease. ERJ Open Res. 2020, 6, 83–2019. [Google Scholar] [CrossRef]
- Mason, N. The physiology of high altitude: An introduction to the cardio-respiratory changes occurring on ascent to altitude. Curr. Anaesth. Crit. Care 2000, 11, 34–41. [Google Scholar] [CrossRef][Green Version]
- Fukuda, T.; Maegawa, T.; Matsumoto, A.; Komatsu, Y.; Nakajima, T.; Nagai, R.; Kawahara, T. Effects of acute hypoxia at moderate altitude on stroke volume and cardiac output during exercise. Int. Heart J. 2010, 51, 170–175. [Google Scholar] [CrossRef][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-Y.; Jung, W.-S.; Kim, S.-W.; Seo, J.; Sun, Y.; Choi, J.-H.; Kim, J.; Lim, K. Effects of Acute Moderate Hypoxia versus Normoxia on Metabolic and Cardiac Function and Skeletal Muscle Oxygenation during Endurance Exercise at the Same Heart Rate Level. Metabolites 2022, 12, 975. https://doi.org/10.3390/metabo12100975
Park H-Y, Jung W-S, Kim S-W, Seo J, Sun Y, Choi J-H, Kim J, Lim K. Effects of Acute Moderate Hypoxia versus Normoxia on Metabolic and Cardiac Function and Skeletal Muscle Oxygenation during Endurance Exercise at the Same Heart Rate Level. Metabolites. 2022; 12(10):975. https://doi.org/10.3390/metabo12100975
Chicago/Turabian StylePark, Hun-Young, Won-Sang Jung, Sung-Woo Kim, Jisu Seo, Yerin Sun, Jae-Ho Choi, Jisu Kim, and Kiwon Lim. 2022. "Effects of Acute Moderate Hypoxia versus Normoxia on Metabolic and Cardiac Function and Skeletal Muscle Oxygenation during Endurance Exercise at the Same Heart Rate Level" Metabolites 12, no. 10: 975. https://doi.org/10.3390/metabo12100975
APA StylePark, H.-Y., Jung, W.-S., Kim, S.-W., Seo, J., Sun, Y., Choi, J.-H., Kim, J., & Lim, K. (2022). Effects of Acute Moderate Hypoxia versus Normoxia on Metabolic and Cardiac Function and Skeletal Muscle Oxygenation during Endurance Exercise at the Same Heart Rate Level. Metabolites, 12(10), 975. https://doi.org/10.3390/metabo12100975








