Association of HbA1c with VO2max in Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Selection
2.2. Criteria for Inclusion in the Review
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
Meta-Analysis
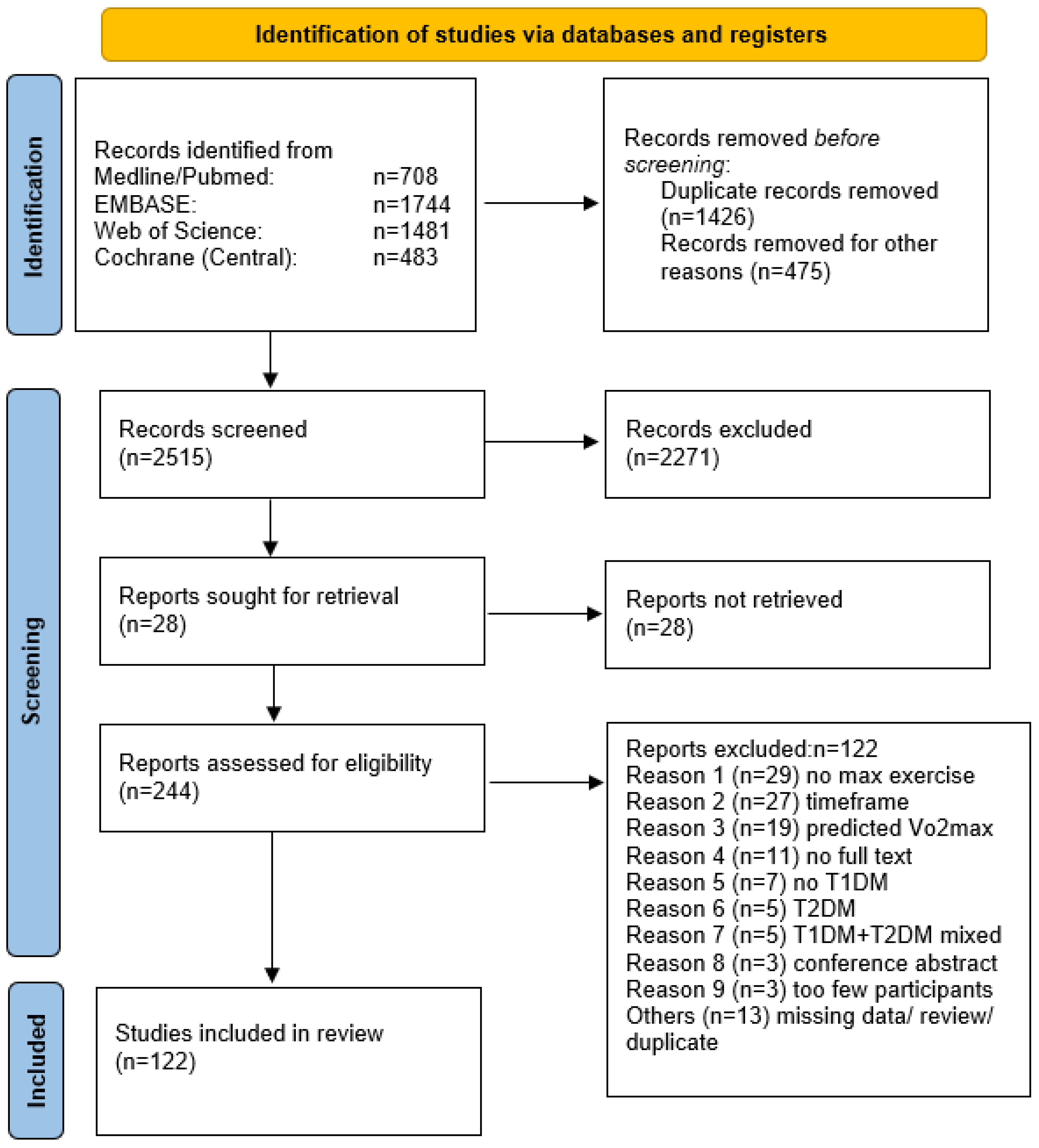
3. Results
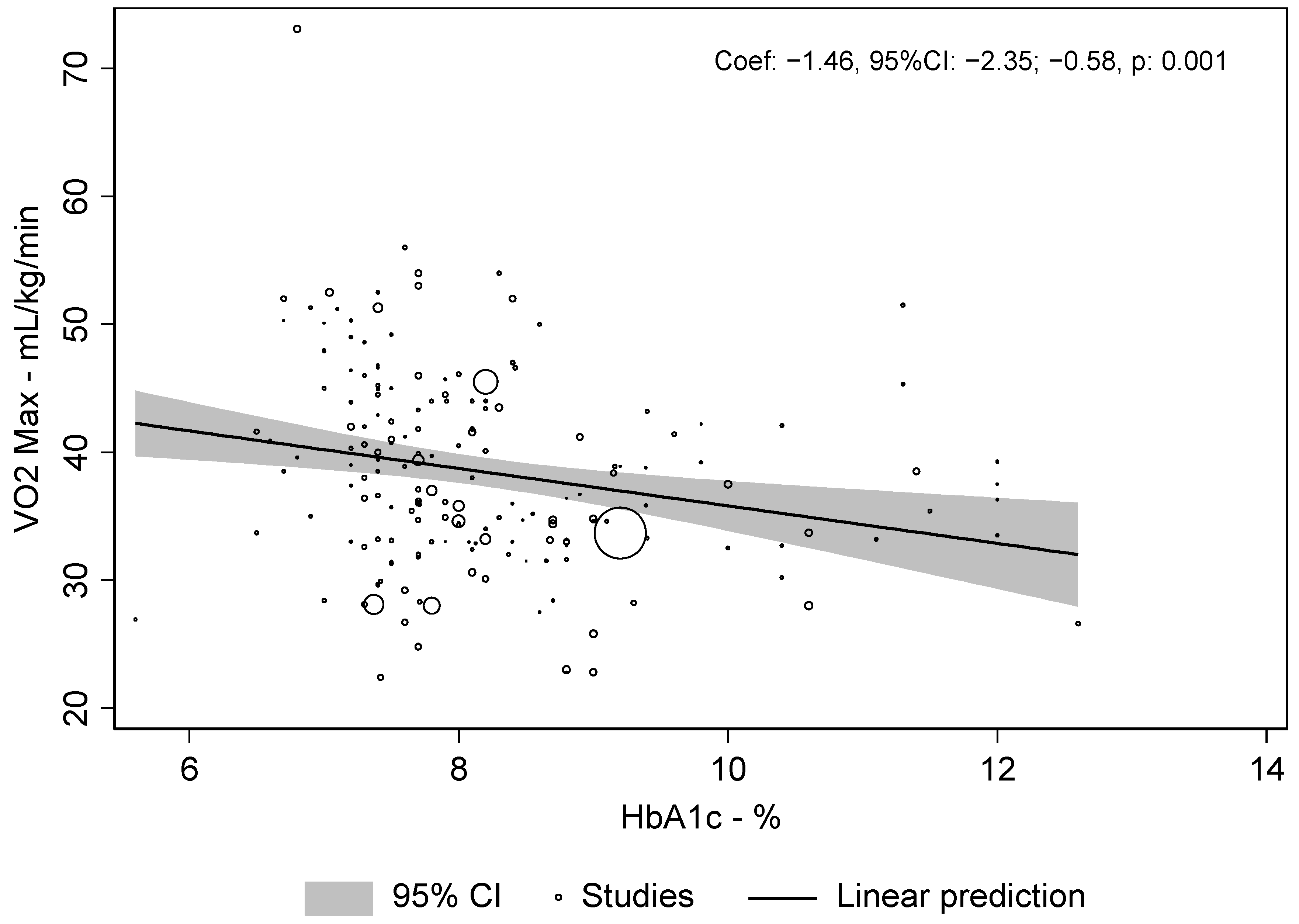
3.1. Primary Endpoint
3.2. Subgroup Analysis
3.3. Multivariate Meta-Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association. Glycemic targets: Standards of medical care in diabetes. Diabetes Care 2019, 42, S61–S70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikkanen-Dolenc, H.; Wadén, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.M.; Saraheimo, M.; Elonen, N.; Hietala, K.; Summanen, P.; Heikki, G.; et al. Frequent physical activity is associated with reduced risk of severe diabetic retinopathy in type 1 diabetes on behalf of the FinnDiane Study Group. Acta Diabetol. 2020, 57, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, T.P.J.; Malin, S.K.; Karstoft, K.; Knudsen, S.H.; Haus, J.M.; Laye, M.J.; Kirwan, J.P. Association Between Cardiorespiratory Fitness and the Determinants of Glycemic Control Across the Entire Glucose Tolerance Continuum. Diabetes Care 2015, 35, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Narendran, P.; Solomon, T.P.; Kennedy, A.; Chimen, M.; Andrews, R.C. The time has come to test the beta cell preserving effects of exercise in patients with new onset type 1 diabetes. Diabetologia 2015, 58, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, M.L.; Farinha, J.B.; McCarthy, O.; West, D.J.; Yardley, J.E.; Bally, L.; Zueger, T.; Stettler, C.; Boff, W.; Reischak-Oliveira, A.; et al. Differences in Physiological Responses to Cardiopulmonary Exercise Testing in Adults With and Without Type 1 Diabetes: A Pooled Analysis. Diabetes Care 2020, dc201496. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Resources for Clinical Exercise Physiology, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009. [Google Scholar]
- Dalleck, L.C.; Tischendorf, J.S. Guidelines for Exercise Testing and Prescription (ACSM). In Encyclopedia of Lifestyle Medicine & Health; Sage: Thousand Oaks, CA, USA, 2012; p. 472. ISBN 9781496339065. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Abraham, M.B.; Davey, R.J.; Cooper, M.N.; Paramalingam, N.; O’Grady, M.J.; Ly, T.T.; Jones, T.W.; Fournier, P.A.; Davis, E.A. Reproducibility of the plasma glucose response to moderate-intensity exercise in adolescents with Type 1 diabetes. Diabet. Med. 2017, 34, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Nilsson, S.; Albertsson-Wikland, K.; Lindblad, B. Hormonal response during physical exercise of different intensities in adolescents with type 1 diabetes and healthy controls. Pediatr. Diabetes 2012, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Al Khalifah, R.A.; Suppère, C.; Haidar, A.; Rabasa-Lhoret, R.; Ladouceur, M.; Legault, L. Association of aerobic fitness level with exercise-induced hypoglycaemia in Type 1 diabetes. Diabet. Med. 2016, 33, 1686–1690. [Google Scholar] [CrossRef] [PubMed]
- Atalay, M.; Laaksonen, D.E.; Niskanen, L.; Uusitupa, M.; Hänninen, O.; Sen, C.K. Altered antioxidant enzyme defences in insulin-dependent diabetic men with increased resting and exercise-induced oxidative stress. Acta Physiol. Scand. 1997, 161, 195–201. [Google Scholar] [CrossRef]
- Austin, A.; Warty, V.; Arslanian, S. The Relationship of Physical Fitness to Lipid and Lipoprotein(a) Levels in Adolescents With IDDM. Diabetes Care 1993, 16, 421–425. [Google Scholar] [CrossRef]
- Bak, J.F.; Jacobsen, U.K.; Jørgensen, F.S.; Pedersen, O. Insulin receptor function and glycogen synthase activity in skeletal muscle biopsies from patients with insulin-dependent diabetes mellitus: Effects of physical training. J. Clin. Endocrinol. Metab. 1989, 69, 158–164. [Google Scholar] [CrossRef]
- Baldi, J.C.; Cassuto, N.A.; Foxx-Lupo, W.T.; Wheatley, C.M.; Snyder, E.M. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med. Sci. Sports Exerc. 2010, 42, 1454–1459. [Google Scholar] [CrossRef]
- Bally, L.; Zueger, T.; Buehler, T.; Dokumaci, A.S.; Speck, C.; Pasi, N.; Ciller, C.; Paganini, D.; Feller, K.; Loher, H.; et al. Metabolic and hormonal response to intermittent high-intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: A randomised crossover study. Diabetologia 2016, 59, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Bally, L.; Zueger, T.; Pasi, N.; Carlos, C.; Paganini, D.; Stettler, C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res. Clin. Pract. 2016, 112, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, E.; Monciotti, C.; Filippone, M.; Santuz, P.; Magagnin, G.; Zanconato, S.; Zacchello, F. Gas exchange during exercise in diabetic children. Pediatr. Pulmonol. 1992, 13, 155–160. [Google Scholar] [CrossRef]
- Benbassat, C.A.; Stern, E.; Kramer, M.; Lebzelter, J.; Blum, I.; Fink, G. Pulmonary function in patients with diabetes mellitus. Am. J. Med. Sci. 2001, 322, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Cree-Green, M.; Baumgartner, A.; Coe, G.; Reyes, Y.G.; Schäfer, M.; Pyle, L.; Regensteiner, J.G.; Reusch, J.E.B.; Nadeau, K.J. Achieving ADA/ISPAD clinical guideline goals is associated with higher insulin sensitivity and cardiopulmonary fitness in adolescents with type 1 diabetes: Results from RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) and Effects of MEtform. Pediatr. Diabetes 2018, 19, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Cree-Green, M.; Baumgartner, A.; Maahs, D.M.; Cherney, D.Z.; Pyle, L.; Regensteiner, J.G.; Reusch, J.E.; Nadeau, K.J. Renal function is associated with peak exercise capacity in adolescents with type 1 diabetes. Diabetes Care 2015, 38, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boff, W.; Da Silva, A.M.; Farinha, J.B.; Rodrigues-Krause, J.; Reischak-Oliveira, A.; Tschiedel, B.; Puñales, M.; Bertoluci, M.C. Superior effects of high-intensity interval vs. moderate-intensity continuous training on endothelial function and cardiorespiratory fitness in patients with type 1 diabetes: A randomized controlled trial. Front. Physiol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bracken, R.M.; Page, R.; Gray, B.; Kilduff, L.P.; West, D.J.; Stephens, J.W.; Bain, S.C. Isomaltulose improves glycemia and maintains run performance in type 1 diabetes. Med. Sci. Sports Exerc. 2012, 44, 800–808. [Google Scholar] [CrossRef]
- Bracken, R.M.; West, D.J.; Stephens, J.W.; Kilduff, L.P.; Luzio, S.; Bain, S.C. Impact of pre-exercise rapid-acting insulin reductions on ketogenesis following running in Type 1 diabetes. Diabet. Med. 2011, 28, 218–222. [Google Scholar] [CrossRef]
- Brazeau, A.S.; Leroux, C.; Mircescu, H.; Rabasa-Lhoret, R. Physical activity level and body composition among adults with Type1 diabetes. Diabet. Med. 2012, 29, 402–408. [Google Scholar] [CrossRef]
- Brazeau, A.S.; Mircescu, H.; Desjardins, K.; Dubé, M.C.; Weisnagel, S.J.; Lavoie, C.; Rabasa-Lhoret, R. The Barriers to Physical Activity in Type 1 Diabetes (BAPAD-1) scale: Predictive validity and reliability. Diabetes Metab. 2012, 38, 164–170. [Google Scholar] [CrossRef]
- Brugnara, L.; Vinaixa, M.; Murillo, S.; Samino, S.; Rodriguez, M.A.; Beltran, A.; Lerin, C.; Davison, G.; Correig, X.; Novials, A. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS ONE 2012, 7, 2–9. [Google Scholar] [CrossRef]
- Bussau, V.A.; Ferreira, L.D.; Jones, T.W.; Fournier, P.A. The 10-s Maximal Sprint. Diabetes Care 2006, 29, 601–606. [Google Scholar] [CrossRef]
- Bussau, V.A.; Ferreira, L.D.; Jones, T.W.; Fournier, P.A. A 10-s sprint performed prior to moderate-intensity exercise prevents early post-exercise fall in glycaemia in individuals with type 1 diabetes. Diabetologia 2007, 50, 1815–1818. [Google Scholar] [CrossRef] [PubMed]
- Campaigne, B.N.; Wallberg-henriksson, H.; Gunnarsson, R. Glucose and Insulin Responses in Relation to Insulin Dose and Caloric Intake 12 h After Acute Physical Exercise in Men With IDDM. Diabetes Care 1987, 10, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Walker, M.; Trenell, M.I.; Jakovljevic, D.G.; Stevenson, E.J.; Bracken, R.M.; Bain, S.C.; West, D.J. Large pre-and postexercise rapid-acting insulin reductions preserve glycemia and prevent early- but not late-onset hypoglycemia in patients with type 1 diabetes. Diabetes Care 2013, 36, 2217–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.D.; Walker, M.; Trenell, M.I.; Luzio, S.; Dunseath, G.; Tuner, D.; Bracken, R.M.; Bain, S.C.; Russell, M.; Stevenson, E.J.; et al. Metabolic implications when employing heavy pre- and post-exercise rapid-acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: A randomised clinical trial. PLoS ONE 2014, 9, e97143. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Walker, M.; Trenell, M.I.; Stevenson, E.J.; Turner, D.; Bracken, R.M.; Shaw, J.A.; West, D.J. A low-glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care 2014, 37, 1845–1853. [Google Scholar] [CrossRef] [Green Version]
- Campbell, M.D.; West, D.J.; Bain, S.C.; Kingsley, M.I.C.; Foley, P.; Kilduff, L.; Turner, D.; Gray, B.; Stephens, J.W.; Bracken, R.M. Simulated games activity vs continuous running exercise: A novel comparison of the glycemic and metabolic responses in T1DM patients. Scand. J. Med. Sci. Sport. 2015, 25, 216–222. [Google Scholar] [CrossRef]
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chokkalingam, K.; Tsintzas, K.; Norton, L.; Jewell, K.; Macdonald, I.A.; Mansell, P.I. Exercise under hyperinsulinaemic conditions increases whole-body glucose disposal without affecting muscle glycogen utilisation in type 1 diabetes. Diabetologia 2007, 50, 414–421. [Google Scholar] [CrossRef] [Green Version]
- de Jesus, Í.C.; Mascarenhas, L.P.G.; de Lima, V.A.; Decimo, J.P.; Nesi-França, S.; Leite, N. Maximal fat oxidation during aerobic exercise in adolescents with type 1 diabetes. Rev. Bras. Med. do Esporte 2019, 25, 299–304. [Google Scholar] [CrossRef] [Green Version]
- De Lima, V.A.; Mascarenhas, L.P.G.; Decimo, J.P.; De Souza, W.C.; Monteiro, A.L.S.; Lahart, I.; França, S.N.; Leite, N. Physical activity levels of adolescents with type 1 diabetes physical activity in T1D. Pediatr. Exerc. Sci. 2017, 29, 213–219. [Google Scholar] [CrossRef]
- D’hooge, R.; Hellinckx, T.; Van Laethem, C.; Stegen, S.; De Schepper, J.; Van Aken, S.; Dewolf, D.; Calders, P. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: A randomized controlled trial. Clin. Rehabil. 2011, 25, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dovc, K.; Macedoni, M.; Bratina, N.; Lepej, D.; Nimri, R.; Atlas, E.; Muller, I.; Kordonouri, O.; Biester, T.; Danne, T.; et al. Closed-loop glucose control in young people with type 1 diabetes during and after unannounced physical activity: A randomised controlled crossover trial. Diabetologia 2017, 60, 2157–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebeling, P.; Tuominen, J.A.; Bourey, R.; Koranyi, L.; Koivisto, V.A. Athletes with IDDM exhibit impaired metabolic control and increased lipid utilization with no increase in insulin sensitivity. Diabetes 1995, 44, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Farinha, J.B.; Ramis, T.R.; Vieira, A.F.; Macedo, R.C.O.; Rodrigues-Krause, J.; Boeno, F.P.; Schroeder, H.T.; Müller, C.H.; Boff, W.; Krause, M.; et al. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J. Diabetes Complicat. 2018, 32, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, M.S.; Michaliszyn, S.F.; Hepworth, J.T. A personalized approach to exercise promotion in adolescents with type 1 diabetes. Pediatr. Diabetes 2010, 11, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, M.S.; Quinn, L.; Rimmer, J.H.; Rich, B.H. Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol. Res. Nurs. 2005, 7, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Fintini, D.; Di Giacinto, B.; Brufani, C.; Cafiero, G.; Patera, P.I.; Turchetta, A.; Giordano, U.; Nobili, V.; Pelliccia, A.; Calzolari, A.; et al. Impaired energy expenditure despite normal cardiovascular capacity in children with type 1 diabetes. Horm. Res. Paediatr. 2012, 78, 1–7. [Google Scholar] [CrossRef]
- Franc, S.; Daoudi, A.; Pochat, A.; Petit, M.H.; Randazzo, C.; Petit, C.; Duclos, M.; Penfornis, A.; Pussard, E.; Not, D.; et al. Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: The DIABRASPORT randomized study. Diabetes, Obes. Metab. 2015, 17, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.L.; Singhvi, A.; Tsalikian, E.; Tansey, M.J.; Janz, K.F. Cross-validation of single-stage treadmill tests for predicting aerobic fitness in adolescents with type I diabetes. Pediatr. Exerc. Sci. 2015, 27, 396–403. [Google Scholar] [CrossRef]
- Fuchsjäger-Mayrl, G.; Pleiner, J.; Wiesinger, G.F.; Sieder, A.E.; Quittan, M.; Nuhr, M.J.; Francesconi, C.; Seit, H.P.; Francesconi, M.; Schmetterer, L.; et al. Exercise training improves vascular endothelial function in patients with type 1 diabetes. Diabetes Care 2002, 25, 1795–1801. [Google Scholar] [CrossRef]
- Giani, E.; Macedoni, M.; Barilli, A.; Petitti, A.; Mameli, C.; Bosetti, A.; Cristiano, A.; Radovanovic, D.; Santus, P.; Zuccotti, G.V. Performance of the Flash Glucose Monitoring System during exercise in youth with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 146, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Goulding, R.P.; Roche, D.M.; Scott, S.N.; Koga, S.; Weston, P.J.; Marwood, S. Limitations to exercise tolerance in type 1 diabetes: The role of pulmonary oxygen uptake kinetics and priming exercise. J. Appl. Physiol. 2020, 128, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.J.; Page, R.; Turner, D.; West, D.J.; Campbell, M.D.; Bracken, R.M. Improved end-stage high intensity performance but similar glycaemic responses after waxy barley starch ingestion compared to dextrose in type 1 diabetes. J. Sports Med. Phys. Fit. 2015, 56, 1392–1400. [Google Scholar]
- Kj, G.; Tw, J.; Pa, F. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005, 28, 1289–1294. [Google Scholar]
- Guelfi, K.J.; Ratnam, N.; Smythe, G.A.; Jones, T.W.; Fournier, P.A. Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E865–E870. [Google Scholar] [CrossRef] [Green Version]
- Gusso, S.; Hofman, P.; Lalande, S.; Cutfield, W.; Robinson, E.; Baldi, J.C. Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia 2008, 51, 1317–1320. [Google Scholar] [CrossRef] [Green Version]
- Gusso, S.; Pinto, T.E.; Baldi, J.C.; Robinson, E.; Cutfield, W.S.; Hofman, P.L. Diastolic function is reduced in adolescents with type 1 diabetes in response to exercise. Diabetes Care 2012, 35, 2089–2094. [Google Scholar] [CrossRef] [Green Version]
- Hägglund, H.; Uusitalo, A.; Peltonen, J.E.; Koponen, A.S.; Aho, J.; Tiinanen, S.; Seppänen, T.; Tulppo, M.; Tikkanen, H.O. Cardiovascular autonomic nervous system function and aerobic capacity in type 1 diabetes. Front. Physiol. 2012, 3, 356. [Google Scholar] [CrossRef] [Green Version]
- Heise, T.; Bain, S.C.; Bracken, R.M.; Zijlstra, E.; Nosek, L.; Stender-Petersen, K.; Rabøl, R.; Rowe, E.; Haahr, H.L. Similar risk of exercise-related hypoglycaemia for insulin degludec to that for insulin glargine in patients with type 1 diabetes: A randomized cross-over trial. Diabetes Obes. Metab. 2016, 18, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Heyman, E.; Daussin, F.; Wieczorek, V.; Caiazzo, R.; Matran, R.; Berthon, P.; Aucouturier, J.; Berthoin, S.; Descatoire, A.; Leclair, E.; et al. Muscle oxygen supply and use in type 1 diabetes, from ambient air to the mitochondrial respiratory chain: Is there a limiting step? Diabetes Care 2020, 43, 209–218. [Google Scholar] [CrossRef]
- Heyman, E.; Delamarche, P.; Berthon, P.; Meeusen, R.; Briard, D.; Vincent, S.; DeKerdanet, M.; Delamarche, A. Alteration in sympathoadrenergic activity at rest and during intense exercise despite normal aerobic fitness in late pubertal adolescent girls with type 1 diabetes. Diabetes Metab. 2007, 33, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, T.; Eichler, E.; Gläser, D.; Schmidt, V.; Gabriel, H.H.W. Platelet activity, reactivity and platelet-leukocyte conjugate formation before and after exhaustive or moderate exercise in patients with IDDM. Platelets 2004, 15, 101–108. [Google Scholar] [CrossRef]
- Jenni, S.; Oetliker, C.; Allemann, S.; Ith, M.; Tappy, L.; Wuerth, S.; Egger, A.; Boesch, C.; Schneiter, P.; Diem, P.; et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus - A prospective single-blinded randomised crossover trial. Diabetologia 2008, 51, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.; Richter, E.A.; Feldt-Rasmussen, B.; Kelbaek, H.; Deckert, T. Impaired aerobic work capacity in insulin dependent diabetics with increased urinary albumin excretion. Br. Med. J. (Clin. Res. Ed). 1988, 296, 1352–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, W.R.; Neto, T.L.B.; Chacra, A.R.; Dib, S.A. Aerobic exercise capacity and pulmonary function in athletes with and without type 1 diabetes. Diabetes Care 2010, 33, 2555–2557. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, W.R.; Lima Gabbay, M.A.; Castro, M.L.; Saraiva, G.L.; Chacra, A.R.; Leite de Barros Neto, T.; Dib, S.A. Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr. Diabetes 2005, 6, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Koponen, A.S.; Peltonen, J.E.; Päivinen, M.K.; Aho, J.M.; Hägglund, H.J.; Uusitalo, A.L.; Lindholm, H.J.; Tikkanen, H.O. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur. J. Appl. Physiol. 2013, 113, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, C.; Malacara, J.M.; Macías-Cervantes, M.H.; Rivera-Cisneros, A.E. Effect of exercise intensity on albuminuria in adolescents with Type1 diabetes mellitus. Diabet. Med. 2012, 29, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, D.E.; Atalay, M.; Niskanen, L.K.; Mustonen, J.; Sen, C.K.; Lakka, T.A.; Uusitupa, M.I.J. Aerobic exercise and the lipid profile in type 1 diabetic men: A randomized controlled trial. Med. Sci. Sports Exerc. 2000, 32, 1541–1548. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Atalay, M.; Niskanen, L.; Uusitupa, M.; Hänninen, O.; Sen, C.K. Increased resting and exercise-induced oxidative stress in young IDDM men. Diabetes Care 1996, 19, 569–574. [Google Scholar] [CrossRef]
- Landt, K.W.; Campaigne, B.N.; James, F.W.; Sperling, M.A. Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care 1985, 8, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Coast, J.R.; Hempleman, S.C.; Baldi, J.C. Type 1 diabetes duration decreases pulmonary diffusing capacity during exercise. Respiration 2016, 91, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Kaplan, V.; Bingisser, R.; Bloch, K.E.; Spinas, G.A. Impact of physical activity on cardiovascular risk factors in IDDM. Diabetes Care 1997, 20, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Matthys, D.; Craen, M.; Wolf, D.D.E.; Walle, J.V.; Verhaaren, H. Reduced decrease of peripheral vascular resistance during exercise in young type I diabetic patients. Diabetes Care 1996, 19, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, O.; Eckstein, M.L.; Scott, S.N.; Fontana, F.Y.; Christiansen, M.P.; Stettler, C.; Fisher, M.; Bode, B.; Riddell, M.C.; Hayes, C.; et al. Glycemic responses to strenuous training in male professional cyclists with type 1 diabetes: A prospective observational study. BMJ Open Diabetes Res. Care 2020, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKewen, M.W.; Rehrer, N.J.; Cox, C.; Mann, J. Glycaemic control, muscle glycogen and exercise performance in IDDM athletes on diets of varying carbohydrate content. Int. J. Sports Med. 1999, 20, 349–353. [Google Scholar] [CrossRef]
- Michaliszyn, S.F.; Shaibi, G.Q.; Quinn, L.; Fritschi, C.; Faulkner, M.S. Physical fitness, dietary intake, and metabolic control in adolescents with type 1 diabetes. Pediatr. Diabetes 2009, 10, 389–394. [Google Scholar] [CrossRef] [Green Version]
- Moser, O.; Eckstein, M.L.; McCarthy, O.; Deere, R.; Bain, S.C.; Haahr, H.L.; Zijlstra, E.; Bracken, R.M. Poor glycaemic control is associated with reduced exercise performance and oxygen economy during cardio-pulmonary exercise testing in people with type 1 diabetes. Diabetol. Metab. Syndr. 2017, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Moser, O.; Eckstein, M.L.; Mueller, A.; Birnbaumer, P.; Aberer, F.; Koehler, G.; Sourij, C.; Kojzar, H.; Pferschy, P.; Dietz, P.; et al. Pre-exercise blood glucose levels determine the amount of orally administered carbohydrates during physical exercise in individuals with type 1 diabetes—A randomized cross-over trial. Nutrients 2019, 11, 1287. [Google Scholar] [CrossRef] [Green Version]
- Moser, O.; Tschakert, G.; Mueller, A.; Groeschl, W.; Eckstein, M.L.; Koehler, G.; Bracken, R.M.; Pieber, T.R.; Hofmann, P. Different Heart Rate Patterns During Cardio-Pulmonary Exercise (CPX) Testing in Individuals With Type 1 Diabetes. Front. Endocrinol. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Moser, O.; Eckstein, M.L.; McCarthy, O.; Deere, R.; Bain, S.C.; Haahr, H.L.; Zijlstra, E.; Heise, T.; Bracken, R.M. Heart rate dynamics during cardio-pulmonary exercise testing are associated with glycemic control in individuals with type 1 diabetes. PLoS ONE 2018, 13, e0194750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MURRAY, F.T.; CAMERON, D.F.; VOGEL, R.B.; THOMAS, R.G.; WYSS, H.U.; ZAUNER, C.W. The Pituitary-Testicular Axis at Rest and During Moderate Exercise in Males with Diabetes Mellitus and Normal Sexual Function. J. Androl. 1988, 9, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, K.J.; Regensteiner, J.G.; Bauer, T.A.; Brown, M.S.; Dorosz, J.L.; Hull, A.; Zeitler, P.; Draznin, B.; Reusch, J.E.B. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J. Clin. Endocrinol. Metab. 2010, 95, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Obeid, J.; Walker, R.G.; Krause, M.P.; Hawke, T.J.; Mcassey, K.; Vandermeulen, J.; Timmons, B.W. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr. Diabetes 2015, 16, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, V.; McBrayer, D.G.; Ramirez, L.C.; Raskin, P.; Hsia, C.C.W. Glycemic control and cardiopulmonary function in patients with insulin- dependent diabetes mellitus. Am. J. Med. 1997, 103, 504–513. [Google Scholar] [CrossRef]
- Peltonen, J.E.; Koponen, A.S.; Pullinen, K.; Hägglund, H.; Aho, J.M.; Kyröläinen, H.; Tikkanen, H.O. Alveolar gas exchange and tissue deoxygenation during exercise in type 1 diabetes patients and healthy controls. Respir. Physiol. Neurobiol. 2012, 181, 267–276. [Google Scholar] [CrossRef]
- Peltoniemi, P.; Yki-Järvinen, H.; Oikonen, V.; Oksanen, A.; Takala, T.O.; Rönnemaa, T.; Erkinjuntti, M.; Knuuti, M.J.; Nuutila, P. Resistance to exercise-induced increase in glucose uptake during hyperinsulinemia in insulin-resistant skeletal muscle of patients with type 1 diabetes. Diabetes 2001, 50, 1371–1377. [Google Scholar] [CrossRef] [Green Version]
- Poortmans, J.R.; Saerens, P.; Edelman, R.; Vertongen, F.; Dorchy, H. Influence of the degree of metabolic control on physical fitness in type 1 diabetic adolescents. Int. J. Sports Med. 1986, 7, 232–235. [Google Scholar] [CrossRef]
- Raguso, C.A.; Coggan, A.R.; Gastaldelli, A.; Sidossis, L.S.; Edward, J.B., III; Wolfe, R.R. Lipid and Carbohydrate Metabolism in IDDM During Moderate and Intense Exercise. Diabetes 1995, 44, 1066–1074. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults With Type 1 Diabetes. Can. J. Diabetes 2019, 43, 406–414.e1. [Google Scholar] [CrossRef]
- Rigla, M.; Sanchez-Quesada, J.L.; Ordonez-Llanos, J.; Prat, T.; Caixas, A.; Jorba, O.; Serra, J.R.; De Leiva, A.; Perez, A. Effect of physical exercise on lipoprotein(a) and low-density lipoprotein modifications in type 1 and type 2 diabetic patients. Metabolism. 2000, 49, 640–647. [Google Scholar] [CrossRef]
- Rigla, M.; Fontcuberta, J.; Mateo, J.; Caixàs, A.; Pou, J.M.; De Leiva, A.; Pérez, A. Physical training decreases plasma thrombomodulin in Type I and Type II diabetic patients. Diabetologia 2001, 44, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, A.P.E.; Tikkanen, H.O.; Koponen, A.S.; Aho, J.M.; Peltonen, J.E. Central and peripheral cardiovascular impairments limit VO2peak in Type 1 diabetes. Med. Sci. Sports Exerc. 2015, 47, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, A.-P.; Tikkanen, H.O.; Koponen, A.S.; Jyrki, M.A.; Peltonen, J.E. One-year unsupervised individualized exercise training intervention enhances cardiorespiratory fitness but not muscle deoxygenation or glycemic control in adults with type 1 diabetes. Appl. Physiol. Nutr. Metab. 2018, 43, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.J.; Burns, A.T.; MacIsaac, R.J.; MacIsaac, A.I.; Prior, D.L.; Gerche, A. La Diagnosis and significance of pulmonary microvascular disease in diabetes. Diabetes Care 2018, 41, 854–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.J.; Burns, A.T.; MacIsaac, R.J.; MacIsaac, A.I.; Prior, D.L.; La Gerche, A. Exercise capacity in diabetes mellitus is predicted by activity status and cardiac size rather than cardiac function: A case control study. Cardiovasc. Diabetol. 2018, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.J.; Barros-Murphy, J.F.; Burns, A.T.; MacIsaac, R.J.; MacIsaac, A.I.; Prior, D.L.; La Gerche, A. Reduced Exercise Capacity in Diabetes Mellitus Is Not Associated with Impaired Deformation or Twist. J. Am. Soc. Echocardiogr. 2020, 33, 481–489. [Google Scholar] [CrossRef]
- Robitaille, M.; Dubé, M.C.; Weisnagel, S.J.; Prud’homme, D.; Massicotte, D.; Péronnet, F.; Lavoie, C. Substrate source utilization during moderate intensity exercise with glucose ingestion in Type 1 diabetic patients. J. Appl. Physiol. 2007, 103, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Roche, D.M.; Edmunds, S.; Cable, T.; Didi, M.; Stratton, G. Skin microvascular reactivity in children and adolescents with type 1 diabetes in relation to levels of physical activity and aerobic fitness. Pediatr. Exerc. Sci. 2008, 20, 426–438. [Google Scholar] [CrossRef] [Green Version]
- Rowland, T.W.; Martha, P.M., Jr.; Reiter, E.O.; Cunningham, L.N. The Influence of Diabetes Mellitus on Cardiovascular Function in Children and Adolescents. Int. J. Sports Med. 1992, 13, 431–435. [Google Scholar] [CrossRef]
- Roy-Fleming, A.; Taleb, N.; Messier, V.; Suppère, C.; Cameli, C.; Elbekri, S.; Smaoui, M.R.; Ladouceur, M.; Legault, L.; Rabasa-Lhoret, R. Timing of insulin basal rate reduction to reduce hypoglycemia during late post-prandial exercise in adults with type 1 diabetes using insulin pump therapy: A randomized crossover trial. Diabetes Metab. 2019, 45, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; Aftab Guy, D.L.; Richardson, M.A.; Ertl, A.C.; Davis, S.N. Effects of Low and Moderate antecedent exercise on counterregulatory responses to subsequent hypoglycemia in type 1 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2004, 290. [Google Scholar] [CrossRef]
- Schneider, S.H.; Khachadurian, A.K.; Amorosa, L.F.; Clemow, L.; Ruderman, N.B. Ten-Year Experience With an Exercise-Based Outpatient Life-Style Modification Program in the Treatment of Diabetes Mellitus. Diabetes Care 1992, 15, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.P.H.; Thijssen, D.H.J.; Noordam, K.; Cranen, M.E.C.; Hopman, M.T.E.; Nijhuis-Van Der Sanden, M.W.G. Exercise training improves physical fitness and vascular function in children with type 1 diabetes. Diabetes, Obes. Metab. 2011, 13, 382–384. [Google Scholar] [CrossRef]
- Shetty, V.B.; Fournier, P.A.; Davey, R.J.; Retterath, A.J.; Paramalingam, N.; Roby, H.C.; Davis, E.A.; Jones, T.W. The time lag prior to the rise in glucose requirements to maintain stable glycaemia during moderate exercise in a fasted insulinaemic state is of short duration and unaffected by the level at which glycaemia is maintained in Type 1 diabetes. Diabet. Med. 2018, 35, 1404–1411. [Google Scholar] [CrossRef]
- Singhvi, A.; Tansey, M.; Janz, K.; Zimmerman, M.; Tsalikian, E. Aerobic Fitness and Glycemic Variability in Adolescents with Type 1 Diabetes. Endocr. Pract. 2014, 20, 566–570. [Google Scholar] [CrossRef]
- Stettler, C.; Jenni, S.; Allemann, S.; Steiner, R.; Hoppeler, H.; Trepp, R.; Christ, E.R.; Zwahlen, M.; Diem, P. Exercise capacity in subjects with type 1 diabetes mellitus in eu- and hyperglycaemia. Diabetes Metab. Res. Rev. 2006, 22, 300–306. [Google Scholar] [CrossRef]
- Stewart, C.J.; Nelson, A.; Campbell, M.D.; Walker, M.; Stevenson, E.J.; Shaw, J.A.; Cummings, S.P.; West, D.J. Gut microbiota of Type 1 diabetes patients with good glycaemic control and high physical fitness is similar to people without diabetes: An observational study. Diabet. Med. 2017, 34, 127–134. [Google Scholar] [CrossRef]
- Tagougui, S.; Leclair, E.; Fontaine, P.; Matran, R.; Marais, G.; Aucouturier, J.; Descatoire, A.; Vambergue, A.; Oussaidene, K.; Baquet, G.; et al. Muscle oxygen supply impairment during exercise in poorly controlled Type 1 diabetes. Med. Sci. Sports Exerc. 2015, 47, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Tagougui, S.; Fontaine, P.; Leclair, E.; Aucouturier, J.; Matran, R.; Oussaidene, K.; Descatoire, A.; Prieur, F.; Mucci, P.; Vambergue, A.; et al. Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care 2015, 38, 858–867. [Google Scholar] [CrossRef] [Green Version]
- Tagougui, S.; Goulet-Gelinas, L.; Taleb, N.; Messier, V.; Suppere, C.; Rabasa-Lhoret, R. Association Between Body Composition and Blood Glucose During Exercise and Recovery in Adolescent and Adult Patients With Type 1 Diabetes. Can. J. Diabetes 2020, 44, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Tonoli, C.; Heyman, E.; Buyse, L.; Roelands, B.; Piacentini, M.F.; Bailey, S.; Pattyn, N.; Berthoin, S.; Meeusen, R. Neurotrophins and cognitive functions in T1D compared with healthy controls: Effects of a high-intensity exercise. Appl. Physiol. Nutr. Metab. 2014, 40, 20–27. [Google Scholar] [CrossRef]
- Trigona, B.; Aggoun, Y.; Maggio, A.; Martin, X.E.; Marchand, L.M.; Beghetti, M.; Farpour-Lambert, N.J. Preclinical noninvasive markers of atherosclerosis in children and adolescents with type 1 diabetes are influenced by physical activity. J. Pediatr. 2010, 157, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.A.; Ebeling, P.; Vuorinen-Markkola, H.; Koivisto, V.A. Post-marathon paradox in IDDM: Unchanged insulin sensitivity in spite of glycogen depletion. Diabet. Med. 1997, 14, 301–308. [Google Scholar] [CrossRef]
- Turinese, I.; Marinelli, P.; Bonini, M.; Rossetti, M.; Statuto, G.; Filardi, T.; Paris, A.; Lenzi, A.; Morano, S.; Palange, P. Metabolic and cardiovascular response to exercise in patients with type 1 diabetes. J. Endocrinol. Invest. 2017, 40, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuttle, K.R.; Marker, J.C.; Dalsky, G.P.; Schwartz, N.S.; Shah, S.D.; Clutter, W.E.; Holloszy, J.O.; Cryer, P.E. Glucagon, not insulin, may play a secondary role in defense against hypoglycemia during exercise. Am. J. Physiol. Endocrinol. Metab. 1988, 254, 713–719. [Google Scholar] [CrossRef]
- Valletta, J.J.; Chipperfield, A.J.; Clough, G.F.; Byrne, C.D. Daily energy expenditure, cardiorespiratory fitness and glycaemic control in people with type 1 diabetes. PLoS ONE 2014, 9, e97534. [Google Scholar] [CrossRef] [Green Version]
- Veves, A.; Saouaf, R.; Donaghue, V.M.; Mullooly, C.A.; Kistler, J.A.; Giurini, J.M.; Horton, E.S.; Fielding, R.A. Aerobic exercise capacity remains normal despite impaired endothelial function in the micro- and macrocirculation of physically active IDDM patients. Diabetes 1997, 46, 1846–1852. [Google Scholar] [CrossRef]
- Waclawovsky, G.; Umpierre, D.; Figueira, F.R.; De Lima, E.S.; Alegretti, A.P.; Schneider, L.; Matte, U.S.; Rodrigues, T.C.; Schaan, B.D. Exercise on progenitor cells in healthy subjects and patients with type 1 diabetes. Med. Sci. Sports Exerc. 2016, 48, 190–199. [Google Scholar] [CrossRef]
- Wallberg-Henriksson, H.; Gunnarsson, R.; Henriksson, J.; DeFronzo, R.; Felig, P.; Ostman, J.; Wahren, J. Increased peripheral insulin sensitivity and muscle mitochondrial enzymes but unchanged blood glucose control in type I diabetics after physical training. Diabetes 1982, 31, 1044–1050. [Google Scholar] [CrossRef]
- Wallberg-Henriksson, H.; Gunnarsson, R.; Rössner, S.; Wahren, J. Long-term physical training in female Type 1 (Insulin-dependent) diabetic patients: Absence of significant effect on glycaemic control and lipoprotein levels. Diabetologia 1986, 1, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanke, T.; Formanek, D.; Auinger, M.; Zwick, H.; Irsigler, K. Pulmonary Gas Exchange and Oxygen Uptake During Exercise in Patients with Type 1 Diabetes Mellitus. Diabet. Med. 1992, 9, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.C.; Peebles, K.C.; Hoye, N.A.; Manning, P.; Sheat, C.; Williams, M.J.A.; Wilkins, G.T.; Wilson, G.A.; Baldi, J.C. Resting heart rate variability and exercise capacity in Type 1 diabetes. Physiol. Rep. 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 2012, 35, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance versus aerobic exercise. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yardley, J.E.; Sigal, R.J.; Kenny, G.P.; Riddell, M.C.; Lovblom, L.E.; Perkins, B.A. Point Accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol. Ther. 2013, 15, 46–49. [Google Scholar] [CrossRef]
- Yardley, J.E.; Iscoe, K.E.; Sigal, R.J.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C. Insulin Pump Therapy is associated with Less Post-Exercise Hyperglycemia than multiple daily injections: An observational study of physically active type 1 diabetes patients. Diabetes Technol. Ther. 2013, 15, 84–88. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; McGaugh, S.; Pooni, R.; Vienneau, T.; Ly, T.; Riddell, M.C. Improved Open-Loop Glucose Control With Basal Insulin Reduction 90 Minutes Before Aerobic Exercise in Patients With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care 2019, 42, 824–831. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; Miadovnik, L.A.; Rowan, C.P.; Gumieniak, R.J.; Jamnik, V.K.; Riddell, M.C. Effects of acute caffeine supplementation on reducing exercise-associated hypoglycaemia in individuals with Type 1 diabetes mellitus. Diabet. Med. 2016, 33, 488–496. [Google Scholar] [CrossRef]
- Zaharieva, D.; Yavelberg, L.; Jamnik, V.; Cinar, A.; Turksoy, K.; Riddell, M.C. The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit-based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technol. Ther. 2017, 19, 370–378. [Google Scholar] [CrossRef]
- Zebrowska, A.; Hall, B.; Kochanska-Dziurowicz, A.; Janikowska, G. The effect of high intensity physical exercise and hypoxia on glycemia, angiogenic biomarkers and cardiorespiratory function in patients with type 1 diabetes. Adv. Clin. Exp. Med. 2018, 27, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.; Nirantharakumar, K.; Chimen, M.; Pang, T.T.; Hemming, K.; Andrews, R.C.; Narendran, P. Does Exercise Improve Glycaemic Control in Type 1 Diabetes? A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e58861. [Google Scholar] [CrossRef]
- Yardley, J.; Mollard, R.; MacIntosh, A.; MacMillan, F.; Wicklow, B.; Berard, L.; Hurd, C.; Marks, S.; McGavock, J. Vigorous intensity exercise for glycemic control in patients with type 1 diabetes. Can. J. Diabetes 2013, 37, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Riddell, M.C.; Eckstein, M.L.; Adolfsson, P.; Rabasa-Lhoret, R.; van den Boom, L.; Gillard, P.; Nørgaard, K.; Oliver, N.S.; Zaharieva, D.P.; et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: Position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020, 63, 2501–2520. [Google Scholar] [CrossRef] [PubMed]
- Bohn, B.; Herbst, A.; Pfeifer, M.; Krakow, D.; Zimny, S.; Kopp, F.; Melmer, A.; Steinacker, J.M.; Holl, R.W. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: A cross-sectional multicenter study of 18,028 patients. Diabetes Care 2015, 38, 1536–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, K.M.; Jaggers, J.R.; Della, L.J.; McKay, T.; Watson, S.; Kozerski, A.E.; Hartson, K.R.; Wintergerst, K.A. Association between Physical Activity and Sport Participation on Hemoglobin A1c Among Children and Adolescents with Type 1 Diabetes. Int. J. Environ. Res. Public Health 2021, 18, 7490. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, J.A.; Zaccardi, F.; Khan, H.; Kurl, S.; Jae, S.Y.; Rauramaa, R. Long-term Change in Cardiorespiratory Fitness and All-Cause Mortality: A Population-Based Follow-up Study. Mayo Clin. Proc. 2016, 91, 1183–1188. [Google Scholar] [CrossRef]
- Strasser, B. Survival of the fittest VO sub 2 sub max a key predictor of longevity. Front. Biosci. 2018, 23, 4657. [Google Scholar] [CrossRef]
- Clements, M.A.; Foster, N.C.; Maahs, D.M.; Schatz, D.A.; Olson, B.A.; Tsalikian, E.; Lee, J.M.; Burt-Solorzano, C.M.; Tamborlane, W.V.; Chen, V.; et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr. Diabetes 2016, 17, 327–336. [Google Scholar] [CrossRef]
- Rodrigues, A.N.; Perez, A.J.; Carletti, L.; Bissoli, N.S.; Abreu, G.R. Maximum oxygen uptake in adolescents as measured by cardiopulmonary exercise testing: A classification proposal. J. Pediatr. 2006, 82, 426–430. [Google Scholar] [CrossRef] [Green Version]
- King, K.M.; McKay, T.; Thrasher, B.J.; Wintergerst, K.A. Maximal Oxygen Uptake, VO2 Max, Testing Effect on Blood Glucose Level in Adolescents with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2022, 19, 5543. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Asmann, Y.W.; Bigelow, M.L.; Short, K.R.; Dhatariya, K.; Coenen-Schimke, J.; Kahl, J.; Mukhopadhyay, D.; Nair, K.S. Effect of Insulin Deprivation on Muscle Mitochondrial ATP Production and Gene Transcript Levels in Type 1 Diabetic Subjects. Diabetes 2007, 56, 2683–2689. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Riddell, M.C.; Hawke, T.J. Effects of type 1 diabetes mellitus on skeletal muscle: Clinical observations and physiological mechanisms. Pediatr. Diabetes 2011, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.S. Pitfalls in Hemoglobin A1c Measurement: When Results may be Misleading. J. Gen. Intern. Med. 2013, 29, 388–394. [Google Scholar] [CrossRef]

| Title | Sex | Age-Set | Type of Exercise | HbA1c ± SD (%) | VO2max/peak ± SD (mL/kg/min) | Sample Size |
|---|---|---|---|---|---|---|
| Abraham, MB 2017 [13] | both (m/w = 4/4) | adolescents | other | 7.8 ± 1 | 39.7 ± 6.10 | 8 |
| Adolfsson, P 2012 (a) [14] | male | adolescents | cycle ergometry | 7.6 ± 0.88 | 56.00 ± 5.50 | 12 |
| Adolfsson, P 2012 (b) | female | adolescents | cycle ergometry | 8.1 ± 0.58 | 44.00 ± 6.68 | 12 |
| Adolfsson, P 2012 (c) | both | adolescents | cycle ergometry | 7.9 ± 3.07 | 49.80 ± 9.90 | 12 |
| Al Khalifah, RA (a) 2016 [15] | both (m/w = 15/8) | adults | cycle ergometry/treadmill | 7.8 ± 0.9 | 44.00 ± 8.80 | 23 |
| Al Khalifah, RA (b) 2016 | both (m/w = 11/10) | adults | cycle ergometry/treadmill | 7.6 ± 0.9 | 26.70 ± 5.00 | 21 |
| Atalay, M 1997 [16] | male | adults | cycle ergometry | 7.3 ± 1.7 | 46.00 ± 6.90 | 9 |
| Austin, A 1993 [17] | both (m/w = 28/31) | adolescents | cycle ergometry | 10.6 ± 2.1 | 33.70 ± 7.00 | 59 |
| Bak, JF 1989 [18] | undefined | adults | cycle ergometry | 7.9 ± 1.4 | 45.70 ± 7.40 | 7 |
| Baldi, JC 2010 [19] | both (m/w = 80%/20%) | adults | cycle ergometry | 7.3 ± 0.8 | 42.00 ± 8.00 | 12 |
| Bally, L (1) 2016 [20] | male | adults | cycle ergometry | 7 ± 0.6 | 47.90 ± 10.20 | 12 |
| Bally, L (2) 2016 [21] | male | adults | cycle ergometry | 7 ± 0.6 | 48.00 ± 11.20 | 10 |
| Baraldi, E 1992 [22] | both (m/w = 17/16) | adolescents | treadmill | 8.9 ± 1.8 | 41.20 ± 5.90 | 33 |
| Benbassat, CA 2001 [23] | both (m/w = 9/7) | adults | cycle ergometry | 8.6 ± 1.8 | 27.50 ± 12.00 | 15 |
| Bjornstad, P 2018 (a) [24] | both (m/w = 48%/52%) | adolescents | cycle ergometry | 9 ± 1.6 | 25.80 ± 4.60 | 27 |
| Bjornstad, P 2018 (b) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.8 ± 1.4 | 33.00 ± 7.80 | 48 |
| Bjornstad, P 2018 (c) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.2 ± 1.4 | 33.20 ± 4.40 | 52 |
| Bjornstad, P 2018 (d) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 9 ± 1.6 | 22.80 ± 4.90 | 27 |
| Bjornstad, P 2018 (e) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.8 ± 1.4 | 23.00 ± 5.80 | 48 |
| Bjornstad, P 2018 (f) | both (m/w = 48%/52%) | adolescents | cycle ergometry | 8.2 ±1.4 | 30.10 ± 8.00 | 52 |
| Bjornstad, P 2015 [25] | both | adolescents | cycle ergometry | 8.5 ± 1.4 | 31.50 ± 6.30 | 69 |
| Boff, W 2019 (a) bl. [26] | both (m/w = 3/6) | adults | cycle ergometry | 8.2 ± 1.3 | 34.00 ± 6.30 | 9 |
| Boff, W 2019 (b) pi. | both (m/w = 3/6) | adults | cycle ergometry | 8.2 ± 1.3 | 40.10 ± 4.30 | 9 |
| Boff, W 2019 (c) bl. | both (m/w = 5/4) | adults | cycle ergometry | 8.4 ± 0.9 | 33.00 ± 8.20 | 9 |
| Boff, W 2019 (d) pi. | both (m/w = 5/4) | adults | cycle ergometry | 8.4 ± 0.9 | 36.00 ± 8.80 | 9 |
| Boff, W 2019 (e) bl. | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 2.3 | 33.20 ± 10.00 | 9 |
| Boff, W 2019 (f) pi. | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 2.3 | 32.70 ± 10.00 | 9 |
| Bracken, RM 2012 [27] | both (m/w = 2/5) | adults | treadmill | 9.16 ± 2.74 | 38.90 ± 4.40 | 7 |
| Bracken, RM 2011 [28] | both (m/w = 6/1) | adults | treadmill | 8.3 ± 0.1 | 43.50 ± 0.90 | 7 |
| Brazeau, AS (1a) 2012 [29] | male | adults | cycle ergometry | 7.71 ± 1.25 | 35.90 ± 10.50 | 22 |
| Brazeau, AS (1b) 2012 | male | adults | cycle ergometry | 7.42 ± 1.25 | 29.90 ± 7.70 | 18 |
| Brazeau, AS (1c) 2012 | female | adults | cycle ergometry | 7.71 ± 1.25 | 28.30 ± 6.20 | 15 |
| Brazeau, AS (1d) 2012 | female | adults | cycle ergometry | 7.42 ± 1.25 | 22.40 ± 5.20 | 20 |
| Brazeau, AS (2a) 2012 [30] | male | adults | cycle ergometry | 7.5 ± 0.9 | 33.10 ± 9.80 | 40 |
| Brazeau, AS (2b) 2012 | female | adults | cycle ergometry | 7.7 ± 1.6 | 24.80 ± 6.30 | 37 |
| Brazeau, AS (2c) 2012 | both | adults | cycle ergometry | 7.6 ± 1.3 | 29.20 ± 9.20 | 77 |
| Brugnara, L 2012 [31] | male | adults | cycle ergometry | 6.9 ± 1 | 35.00 ± 6.50 | 10 |
| Bussau, VA 2006 [32] | male | adults | cycle | 7.4 ± 0.8 | 44.50 ± 4.20 | 7 |
| Bussau, VA 2007 [33] | male | adults | cycle ergometry | 7.4 ± 0.7 | 45.20 ± 5.00 | 7 |
| Campaigne, BN 1987 [34] | male | adults | cycle ergometry | 7.4 ± 0.3 | 36.60 ± 1.60 | 9 |
| Campbell, MD 2013 [35] | male | adults | treadmill | 7.7 ± 0.3 | 53.00 ± 1.00 | 11 |
| Campbell, MD (1) 2014 [36] | male | adults | treadmill | 7.7 ± 0.4 | 54.00 ± 1.00 | 8 |
| Campbell, MD (2) 2014 [37] | male | adults | treadmill | 6.7 ± 0.7 | 52.00 ± 4.00 | 10 |
| Campbell, MD (1) 2015 [38] | both (m/w = 7/2) | adults | treadmill | 8.1 ± 0.2 | 41.80 ± 1.60 | 9 |
| Campbell, MD (2) 2015 [39] | male | adults | treadmill | 6.9 ± 0.2 | 51.30 ± 2.10 | 10 |
| Chokkalingam, K 2007 [40] | male | adults | cycle ergometry | 7.9 ± 0.2 | 44.50 ± 1.20 | 8 |
| de Jesus, IC 2019 [41] | both (m/w = 5/4) | adolescents | cycle ergometry | 9.39 ± 1.25 | 38.79 ± 10.02 | 9 |
| de Lima, VA 2017 [42] | both (m/w = 25/20) | adolescents | cycle ergometry | 9.15 ± 1.61 | 38.38 ± 7.54 | 45 |
| D’hooge, R (a) 2011 bl. [43] | both | adolescents | cycle ergometry | 8.13 ± 1.02 | 32.87 ± 7.83 | 8 |
| D’hooge, R (b) 2011 pi. | both | adolescents | cycle ergometry | 8.08 ± 0.97 | 32.99 ± 9.58 | 8 |
| D’hooge, R (c) 2011 bl. | both | adolescents | cycle ergometry | 8.55 ± 0.82 | 35.19 ± 6.71 | 8 |
| D’hooge, R (d) 2011 pi. | both | adolescents | cycle ergometry | 8.48 ± 0.9 | 34.69 ± 9.24 | 8 |
| Dovc, K (a) 2017 [44] | male | adolescents | cycle ergometry | 7.5 ± 0.5 | 49.2 ± 8.1 | 11 |
| Dovc, K (b) 2017 | female | adolescents | cycle ergometry | 7.9 ± 0.7 | 36.1 ± 4 | 9 |
| Dovc, K (c) 2017 | both | adolescents | cycle ergometry | 7.7 ± 0.6 | 43.3 ± 9.3 | 20 |
| Ebeling, P (a) 1995 [45] | undefined | adults | cycle ergometry | 8.4 ± 0.4 | 52.0 ± 1.0 | 11 |
| Ebeling, P (b) 1995 | undefined | adults | cycle ergometry | 7.2 ± 0.2 | 42.0 ± 1.0 | 12 |
| Farinha, JB (a) 2018 bl. [46] | both (m/w = 5/4) | adults | cycle ergometry | 7.5 ± 1.5 | 31.3 ± 6.0 | 9 |
| Farinha, JB (b) 2018 pi. | both (m/w = 5/4) | adults | cycle ergometry | 7.2 ± 1.1 | 37.4 ± 8.7 | 9 |
| Farinha, JB (c) 2018 bl. | both (m/w = 5/4) | adults | cycle ergometry | 8.1 ± 1.3 | 32.4 ± 6.3 | 9 |
| Farinha, JB (d) 2018 pi. | both (m/w = 5/4) | adults | cycle ergometry | 8 ± 0.8 | 34.3 ± 5.2 | 9 |
| Farinha, JB (e) 2018 bl. | both (m/w = 5/5) | adults | cycle ergometry | 7.5 ± 1 | 31.4 ± 7.1 | 10 |
| Farinha, JB (f) 2018 pi. | both (m/w = 5/5) | adults | cycle ergometry | 7.2 ± 0.7 | 33.0 ± 7.8 | 10 |
| Faulkner, MS (a) 2010 bl. [47] | both (m/w = 9/3) | adolescents | cycle ergometry | 9.4 ± 1.8 | 33.3 ± 6.9 | 12 |
| Faulkner, MS (b) 2010 pi. | both (m/w = 9/3) | adolescents | cycle ergometry | 9.4 ± 2.1 | 35.8 ± 8.8 | 12 |
| Faulkner, MS 2005 [48] | both (m/w = 57/48) | adolescents | cycle ergometry | 8.7 ± 1.6 | 34.4 ± 8.8 | 105 |
| Fintini, D 2012 [49] | both (m/w = 15/20) | children | treadmill | 7.7 ± 0.8 | 36.2 ± 7.4 | 35 |
| Franc, S 2015 [50] | both (m/w = 11/9) | adults | cycle ergometry | 7.9 ± 0.9 | 33.0 ± 10.0 | 20 |
| Francis, SL (a) 2015 [51] | male | adolescents | treadmill | 8.6 ± 0.9 | 50.0 ± 6.5 | 10 |
| Francis, SL (b) 2015 | female | adolescents | treadmill | 8.2 ± 0.9 | 44.0 ± 6.3 | 10 |
| Francis, SL (c) 2015 | both | adolescents | treadmill | 8.4 ± 0.7 | 47.0 ± 6.9 | 20 |
| Fuchsjager-Mayrl, G 2002 (a) bl. [52] | both (m/w = 7/11) | adults | cycle ergometry | 7.3 ± 0.2 | 28.1 ± 1.2 | 18 |
| Fuchsjager-Mayrl, G 2002 (b) pi.(1) | both (m/w = 7/11) | adults | cycle ergometry | 7.7 ± 0.3 | 31.8 ± 2 | 18 |
| Fuchsjager-Mayrl, G 2002 (c) pi.(2) | both | adults | cycle ergometry | 7.5 ± 0.3 | 35.7 ± 2.8 | 15 |
| Fuchsjager-Mayrl, G 2002 (d) pi.(3) | both | adults | cycle ergometry | 7 ± 0.2 | 28.4 ± 1.8 | 13 |
| Fuchsjager-Mayrl, G 2002 (e) bl. | both | adults | cycle ergometry | 7.4 ± 0.4 | 29.6 ± 2.3 | 8 |
| Fuchsjager-Mayrl, G 2002 (f) pi. | both | adults | cycle ergometry | 7.4 ± 0.2 | 29.7 ± 2.4 | 8 |
| Giani, E 2018 [53] | both (m/w = 53%/47%) | adolescents | cycle ergometry | 7.4 ± 1.0 | 33.2 ± 6.2 | 17 |
| Goulding, R 2020 [54] | male | adults | cycle ergometry | 7.3 ± 0.9 | 36.4 ± 4.7 | 17 |
| Gray, BJ 2016 [55] | both (m/w = 2/5) | adults | treadmill | 9.2 ± 0.6 | 38.9 ± 4.4 | 7 |
| Guelfi, KJ 2005 [56] | both (m/w = 4/3) | adults | other | 7.4 ± 1.5 | 39.4 ± 7.4 | 7 |
| Guelfi, KJ 2007 [57] | both (m/w = 5/4) | adults | cycle ergometry | 7.7 ± 0.8 | 41.8 ± 4.6 | 9 |
| Gusso, S 2008 [58] | female | adolescents | cycle ergometry | 8.8 ± 0.3 | 31.6 ± 2 | 12 |
| Gusso, S 2012 [59] | both (m/w = 27/26) | adolescents | cycle ergometry | 8.7 ± 0.2 | 33.1 ± 1.0 | 53 |
| Haagglund, H 2012 [60] | male | adults | cycle ergometry | 7.7 ± 0.9 | 36 ± 4 | 10 |
| Heise, T 2016 [61] | both (m/w = 35/5) | adults | other | 7.7 ± 0.8 | 39.4 ± 3.7 | 40 |
| Heyman, E 2020 [62] | both (m/w = 12/4) | adults | cycle ergometry | 8.3 ± 1.5 | 34.9 ± 7.2 | 16 |
| Heyman, E 2007 [63] | female | adolescents | cycle ergometry | 8.1 ± 1.3 | 30.6 ± 4.0 | 19 |
| Hilberg, T 2004 [64] | male | adults | cycle ergometry | 7.2 ± 0.2 | 49.0 ± 2.2 | 16 |
| Jenni, S 2008 [65] | male | adults | cycle ergometry | 6.7 ± 0.2 | 50.3 ± 4.5 | 7 |
| Jensen, T 1988 (a) [66] | both (m/w = 6/4) | adults | cycle ergometry | 7.5 ± 0.95 | 40.7 ± 9.5 | 10 |
| Jensen, T 1988 (b) | both (m/w = 6/4) | adults | cycle ergometry | 8.7 ± 1.21 | 28.4 ± 8.8 | 10 |
| Jensen, T 1988 (c) | both (m/w = 6/4) | adults | cycle ergometry | 9.3 ± 1.0 | 28.2 ± 4.2 | 10 |
| Komatsu, WR 2010 (a) [67] | undefined | adults | treadmill | 7.5 ± 6.2 | 42.4 ± 5.5 | 15 |
| Komatsu, WR 2010 (b) | undefined | adults | treadmill | 9 ± 1.3 | 34.8 ± 3.3 | 12 |
| Komatsu, WR 2005 [68] | both (m/w = 38/34) | adolescents | treadmill | 8.1 ± 2.2 | 41.6 ± 7.7 | 72 |
| Koponen, AS 2013 [69] | male | adults | cycle ergometry | 7.65 ± 0.8 | 35.4 ± 4.8 | 12 |
| Kornhauser, C 2012 [70] | both (m/w = 5/5) | adolescents | treadmill | 10 ± 1.0 | 37.5 ± 2.7 | 10 |
| Laaksonen, DE 2000 (a) bl. [71] | male | adults | cycle ergometry | 8.2 ± 1.1 | 43.4 ± 8.0 | 20 |
| Laaksonen, DE 2000 (b) pi. | male | adults | cycle ergometry | 8 ± 1.0 | 46.1 ± 6.6 | 20 |
| Laaksonen, DE 1996 [72] | male | adults | cycle ergometry | 7.3 ± 1.7 | 46 ± 6.9 | 9 |
| Landt, KW 1985 (a) bl. [73] | both (m/w = 3/6) | adolescents | cycle ergometry | 12 ± 1.0 | 36.3 ± 3 | 9 |
| Landt, KW 1985 (b) pi. | both (m/w = 3/6) | adolescents | cycle ergometry | 12 ± 1.0 | 39.3 ± 3 | 9 |
| Landt, KW 1985 (c) bl. | both (m/w = 4/2) | adolescents | cycle ergometry | 12 ± 1.0 | 39.2 ± 3.4 | 6 |
| Landt, KW 1985 (d) pi. | both (m/w = 4/2) | adolescents | cycle ergometry | 12 ± 1.0 | 37.5 ± 3.3 | 6 |
| Lee, MJ 2016 [74] | both (m/w = 45.8%/54.2%) | (adolescents)/adults | cycle ergometry | 7.9 ± 1.3 | 34.9 ± 5.8 | 24 |
| Lehmann, R 1997 (a) bl. [75] | both (m/w = 13/7) | adults | cycle ergometry | 7.6 ± 1.0 | 41.2 ± 13.1 | 20 |
| Lehmann, R 1997 (b) pi. | both (m/w = 13/7) | adults | cycle ergometry | 7.5 ± 0.9 | 45.0 ± 13.2 | 20 |
| Matthys, D 1996 [76] | both (m/w = 12/18) | adolescents | cycle ergometry | 10 ± 0.3 | 32.5 ± 2.1 | 30 |
| McCarthy, O 2020 [77] | male | adults | cycle ergometry | 6.8 ± 0.6 | 73.1 ± 3.8 | 16 |
| McKewen, MW 1999 [78] | male | adults | cycle ergometry | 7.2 ± 1.2 | 50.3 ± 7.4 | 7 |
| Michaliszyn, SF 2009 [79] | both (m/w = 60/49) | adolescents | other | 8.7 ± 1.6 | 34.7 ± 8.9 | 109 |
| Moser, O 2017 [80] | both (m/w = 51/13) | adults | cycle ergometry | 7.8 ± 1.0 | 37.0 ± 5.0 | 64 |
| Moser, O 2019 [81] | both (m/w = 5/4) | adults | cycle ergometry | 7.2 ± 0.6 | 39.0 ± 12.0 | 9 |
| Moser, O 2018 (1) [82] | male | adults | cycle ergometry | 7.4 ± 0.6 | 52.5 ± 6.6 | 7 |
| Moser, O 2018 (2) [83] | both (m/w = 51/13) | adults | cycle ergometry | 7.8 ± 1.0 | 37.0 ± 5.0 | 64 |
| Murray, FT 1988 [84] | male | adults | other | 12 ± 0.6 | 33.5 ± 2.6 | 8 |
| Nadeau, KJ 2010 [85] | both (m/w = 6/6) | adolescents | cycle ergometry | 8.65 ± 1.6 | 31.5 ± 7.6 | 12 |
| Nguyen, T 2015 (a) [86] | both (m/w = 5/3) | adolescents | cycle ergometry | 7.4 ± 0.5 | 38.5 ± 5.8 | 8 |
| Nguyen, T 2015 (b) | both (m/w = 5/3) | adolescents | cycle ergometry | 11.1 ± 1.0 | 33.2 ± 5.6 | 8 |
| Niranjan, V 1997 (a) [87] | both (m/w = 7/2) | adults | cycle ergometry | 5.6 ± 0.2 | 26.9 ± 2.6 | 9 |
| Niranjan, V 1997 (b) | both (m/w = 4/5) | adults | cycle ergometry | 8.8 ± 0.5 | 22.8 ± 3.5 | 9 |
| Peltonen, JE 2012 [88] | male | adults | cycle ergometry | 7.7 ± 0.7 | 34.7 ± 4.4 | 10 |
| Peltoniemi, P 2001 [89] | male | adults | cycle ergometry | 7 ± 0.3 | 45.0 ± 2.0 | 12 |
| Poortmans, JR 1986 (a) [90] | male | adolescents | cycle ergometry | 7.3 ± 0.3 | 40.6 ± 1.3 | 9 |
| Poortmans, JR 1986 (b) | male | adolescents | cycle ergometry | 11.4 ± 0.9 | 38.5 ± 1.0 | 8 |
| Raguso, CA 1995 [91] | male | adults | cycle ergometry | 8 ± 0.7 | 40.5 ± 2.0 | 7 |
| Reddy, R 2019 [92] | both (m/w = 4/6) | adults | treadmill | 7.4 ± 1.0 | 46.8 ± 11.6 | 10 |
| Rigla, M 2000 (a) bl. [93] | both (m/w = 7/7) | adults | treadmill | 6.5 ± 0.8 | 33.7 ± 7.0 | 14 |
| Rigla, M 2000 (b) pi. | both (m/w = 7/7) | adults | treadmill | 6.7 ± 1.0 | 38.5 ± 7.7 | 14 |
| Rigla, M 2001 (a) bl. [94] | both (m/w = 7/7) | adults | other | 6.5 ± 0.8 | 33.7 ± 7.0 | 14 |
| Rigla, M 2001 (b) pi. | both (m/w = 7/7) | adults | other | 6.7 ± 1.0 | 38.5 ± 7.7 | 14 |
| Rissanen, APE 2015 [95] | male | adults | cycle ergometry | 7.4 ± 0.9 | 40.0 ± 3.0 | 7 |
| Rissanen, APE 2018 (a) bl. [96] | male | adults | cycle ergometry | 7.3 ± 0.9 | 38.0 ± 4.0 | 8 |
| Rissanen, APE 2018 (b) pi. | male | adults | cycle ergometry | 7.5 ± 1.1 | 41.0 ± 3.0 | 8 |
| Roberts, TJ 2018 (1) [97] | both (m/w = 29/11) | adults | cycle ergometry | 7.7 ± 1.3 | 32.0 ± 10.0 | 40 |
| Roberts, TJ 2018 (2) [98] | both (m/w = 13/7) | adults | cycle ergometry | 8.1 ± 3.9 | 38.0 ± 9.0 | 20 |
| Roberts, TJ 2020 [99] | both (m/w = 24/10) | adults | cycle ergometry | 7.8 ± 1.3 | 33.0 ± 10.0 | 34 |
| Robitaille, M 2007 [100] | both (m/w = 5/3) | adults | cycle ergometer | 7.4 ± 0.4 | 42.9 ± 10.3 | 8 |
| Roche, DM 2008 (a) [101] | male | adolescents | treadmill | 9.4 ± 1.0 | 43.2 ± 7.3 | 15 |
| Roche, DM 2008 (b) | female | adolescents | treadmill | 9.8 ± 1.7 | 39.2 ± 9.0 | 14 |
| Roche, DM 2008 (c) | both | adolescents | treadmill | 9.6 ± 1.4 | 41.4 ± 8.2 | 29 |
| Rowland, TW 1992 [102] | male | adolescents | cycle ergometry | 11.3 ± 3.0 | 51.5 ± 5.8 | 11 |
| Roy-Fleming, A 2019 [103] | both (m/w = 11/11) | adults | cycle ergometry | 7.3 ± 1.0 | 32.6 ± 7.1 | 22 |
| Sandoval, DA 2004 [104] | both (m/w = 14/13) | adults | cycle ergometry | 7.8 ± 0.2 | 28.0 ± 2.0 | 27 |
| Schneider, SH 1992 (a) [105] | both (m/w = 12/4) | adults | cycle ergometry | 12.6 ± 0.8 | 26.6 ± 1.5 | 16 |
| Schneider, SH 1992 (b) | both (m/w = 25/14) | adults | cycle ergometry | 11.5 ± 0.6 | 35.4 ± 1.9 | 39 |
| Seeger, JPH 2011 [106] | both (m/w = 4/5) | children | treadmill | 7.9 ± 0.6 | 44.0 ± 5.9 | 9 |
| Shetty, VB 2018 [107] | both (m/w = 4/4) | adults | cycle ergometry | 8.0 ± 0.7 | 34.5 ± 10.9 | 8 |
| Singhvi, A 2014 [108] | both (m/w = 47%/53%) | adolescents | treadmill | 8.42 ± 0.9 | 46.6 ± 6.8 | 20 |
| Stettler, C 2005 [109] | male | adults | cycle ergometry | 7.4 ± 0.7 | 44.9 ± 8.0 | 8 |
| Stewart, CJ 2017 [110] | male | adults | other | 7.4 ± 0.4 | 51.3 ± 2.2 | 10 |
| Tagougui, S (1a) 2015 [111] | male | adults | cycle ergometry | 6.6 ± 0.7 | 40.9 ± 9.3 | 11 |
| Tagougui, S (1b) 2015 | both (m/w = 7/5) | adults | cycle ergometry | 9.1 ± 0.7 | 34.6 ± 7.2 | 12 |
| Tagougui, S (2a) 2015 [112] | both (m/w = 7/1) | adults | other | 6.8 ± 0.7 | 39.6 ± 8.5 | 8 |
| Tagougui, S (2b) 2015 | both (m/w = 6/4) | adults | other | 9.0 ± 0.7 | 34.6 ± 7.1 | 10 |
| Tagougui, S 2020 [113] | both (m/w = 20/10) | adolescents/adults | treadmill | 7.6 ± 1.0 | 38.9 ± 10.7 | 30 |
| Tonoli, C 2015 [114] | both (m/w = 8/2) | adults | cycle ergometry | 7.0 ± 0.2 | 52.5 ± 2.7 | 10 |
| Trigona, B 2010 [115] | both (m/w = 17/15) | adolescents | treadmill | 8.2 ± 0.2 | 45.5 ± 1.44 | 32 |
| Tuominen, JA 1997 [116] | both (m/w = 6/1) | adults | cycle ergometry | 7.7 ± 0.3 | 46.0 ± 1.0 | 7 |
| Turinese, I 2017 [117] | both (m/w = 13/4) | adults | cycle ergometry | 7.4 ± 0.1 | 28.1 ± 1.3 | 17 |
| Tuttle, KR 1988 [118] | both (m/w = 8/5) | adults | cycle ergometry | 8.8 ± 1.6 | 36.4 ± 5.9 | 13 |
| Valletta, JJ 2014 [119] | both (m/w = 11/12) | adults | treadmill | 7.7 ± 1.3 | 39.9 ± 8.4 | 23 |
| Veves, A 1997 (a) [120] | both (m/w = 20/3) | adults | treadmill | 8.3 ± 1.4 | 54.0 ± 8.1 | 23 |
| Veves, A 1997 (b) | both (m/w = 4/3) | adults | treadmill | 9.8 ± 1.2 | 42.2 ± 11.6 | 7 |
| Veves, A 1997 (c) | both (m/w = 11/7) | adults | treadmill | 8.9 ± 1.5 | 36.7 ± 9.6 | 5 |
| Waclawovsky, G 2016 [121] | male | adults | cycle ergometry | 7.7 ± 0.2 | 37.1 ± 1.4 | 14 |
| Wallberg-Henriksson, H 1982 (a) bl. [122] | male | adults | cycle ergometry | 10.4 ± 0.7 | 42.1 ± 2.1 | 9 |
| Wallberg-Henriksson, H 1982 (b) pi. | male | adults | cycle ergometry | 11.3 ± 0.5 | 45.3 ± 2.2 | 9 |
| Wallberg-Henriksson, H 1986 (a) bl. [123] | female | adults | cycle ergometry | 10.4 ± 0.6 | 30.2 ± 2.1 | 6 |
| Wallberg-Henriksson, H 1986 (b) pi. | female | adults | cycle ergometry | 10.4 ± 0.6 | 32.7 ± 2.1 | 6 |
| Wallberg-Henriksson, H 1986 (c) bl. | female | adults | cycle ergometry | 10.6 ± 0.6 | 28.0 ± 0.8 | 7 |
| Wallberg-Henriksson, H 1986 (d) pi. | female | adults | cycle ergometry | 10.6 ± 0.6 | 28.0 ± 0.8 | 7 |
| Wanke, T 1992 [124] | both (m/w = 31/5) | adults | cycle ergometry | 9.2 ± 2.7 | 33.7 ± 0.7 | 36 |
| West, DJ 2011 (a) [27] | both (m/w = 7/1) | adults | treadmill | 8 ± 0.2 | 35.8 ± 0.6 | 8 |
| West, DJ 2011 (b) | both (m/w = 7/1) | adults | treadmill | 8 ± 0.2 | 34.6 ± 0.5 | 8 |
| Wilson, LC 2017 [125] | both (m/w = 12/11) | adults | cycle ergometry | 8.4 ± 3.7 | 32.0 ± 9.0 | 23 |
| Yardley, JE 2012 [126] | both (m/w = 10/2) | adolescents/adults | treadmill | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (1) [127] | both (m/w = 10/2) | adults | treadmill | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (2) [128] | both (m/w = 10/2) | adults | other | 7.1 ± 1.1 | 51.2 ± 10.8 | 12 |
| Yardley, JE 2013 (3a) [129] | both | adolescents/adults | cycle ergometry/running | 7.2 ± 1.2 | 46.4 ± 10.1 | 9 |
| Yardley, JE 2013 (3b) | both | adolescents/adults | cycle ergometry/running | 7.3 ± 1.1 | 48.6 ± 7.8 | 10 |
| Zaharieva, DP 2019 [130] | both (m/w = 4/13) | adults | treadmill | 6.5 ± 0.5 | 41.6 ± 5.9 | 17 |
| Zaharieva, DP 2016 [131] | both (m/w = 5/8) | adults | treadmill | 7.4 ± 0.8 | 46.6 ± 12.7 | 13 |
| Zaharieva, DP 2017 [132] | both (m/w = 6/6) | adults | treadmill | 7 ± 0.9 | 50.1 ± 13.7 | 12 |
| Zebrowska, A 2018 (a) [133] | undefined | adults | cycle ergometry | 7.2 ± 0.41 | 43.9 ± 7.8 | 14 |
| Zebrowska, A 2018 (b) | undefined | adults | cycle ergometry | 7.2 ± 0.41 | 40.3 ± 7.3 | 14 |
| Coefficient [95% CI] | p Value | |
|---|---|---|
| HbA1c (continuous) | −0.78 [−1.56–−0.003] | 0.049 |
| Gender | ||
| Both | Reference | |
| Female | −2.42 [−5.93–1.09] | 0.176 |
| Male | 9.21 [7.03–11.4] | <0.001 |
| Age | ||
| Other | Reference | |
| Adolescents | −0.51 [−5.36–4.33] | 0.836 |
| Adolescents/Adults | 0.75 [−4.43–5.93] | 0.777 |
| Adults | −2.46 [−7.15–2.23] | 0.304 |
| Exercise type | ||
| Other | Reference | |
| Cycle ergometer | −3.84 [−7.18–−0.5] | 0.024 |
| Treadmill | 4.24 [0.48–8.0] | 0.027 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, M.L.; Aberer, F.; Dobler, F.J.R.; Aziz, F.; Heise, T.; Sourij, H.; Moser, O. Association of HbA1c with VO2max in Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Metabolites 2022, 12, 1017. https://doi.org/10.3390/metabo12111017
Eckstein ML, Aberer F, Dobler FJR, Aziz F, Heise T, Sourij H, Moser O. Association of HbA1c with VO2max in Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Metabolites. 2022; 12(11):1017. https://doi.org/10.3390/metabo12111017
Chicago/Turabian StyleEckstein, Max L., Felix Aberer, Florian J. R. Dobler, Faisal Aziz, Tim Heise, Harald Sourij, and Othmar Moser. 2022. "Association of HbA1c with VO2max in Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis" Metabolites 12, no. 11: 1017. https://doi.org/10.3390/metabo12111017
APA StyleEckstein, M. L., Aberer, F., Dobler, F. J. R., Aziz, F., Heise, T., Sourij, H., & Moser, O. (2022). Association of HbA1c with VO2max in Individuals with Type 1 Diabetes: A Systematic Review and Meta-Analysis. Metabolites, 12(11), 1017. https://doi.org/10.3390/metabo12111017








