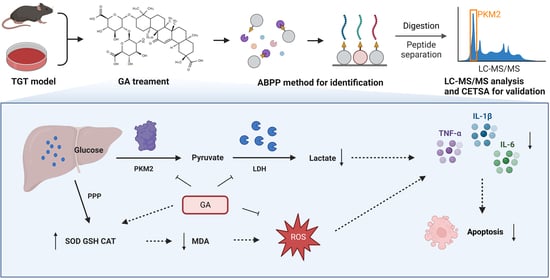
Glycyrrhizic Acid Mitigates Tripterygium-Glycoside-Tablet-Induced Acute Liver Injury via PKM2 Regulated Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. TGT-Induced Acute Liver Injury Mouse Model and Administration
2.3. Cell Culture
2.4. Cytokine Assays
2.5. Oxidative Stress Indicator Detection
2.6. Liver Function Indicator Analysis
2.7. HE and Immunofluorescence Staining
2.8. Apoptosis Assay and Detection of Mitochondrial Membrane Potential
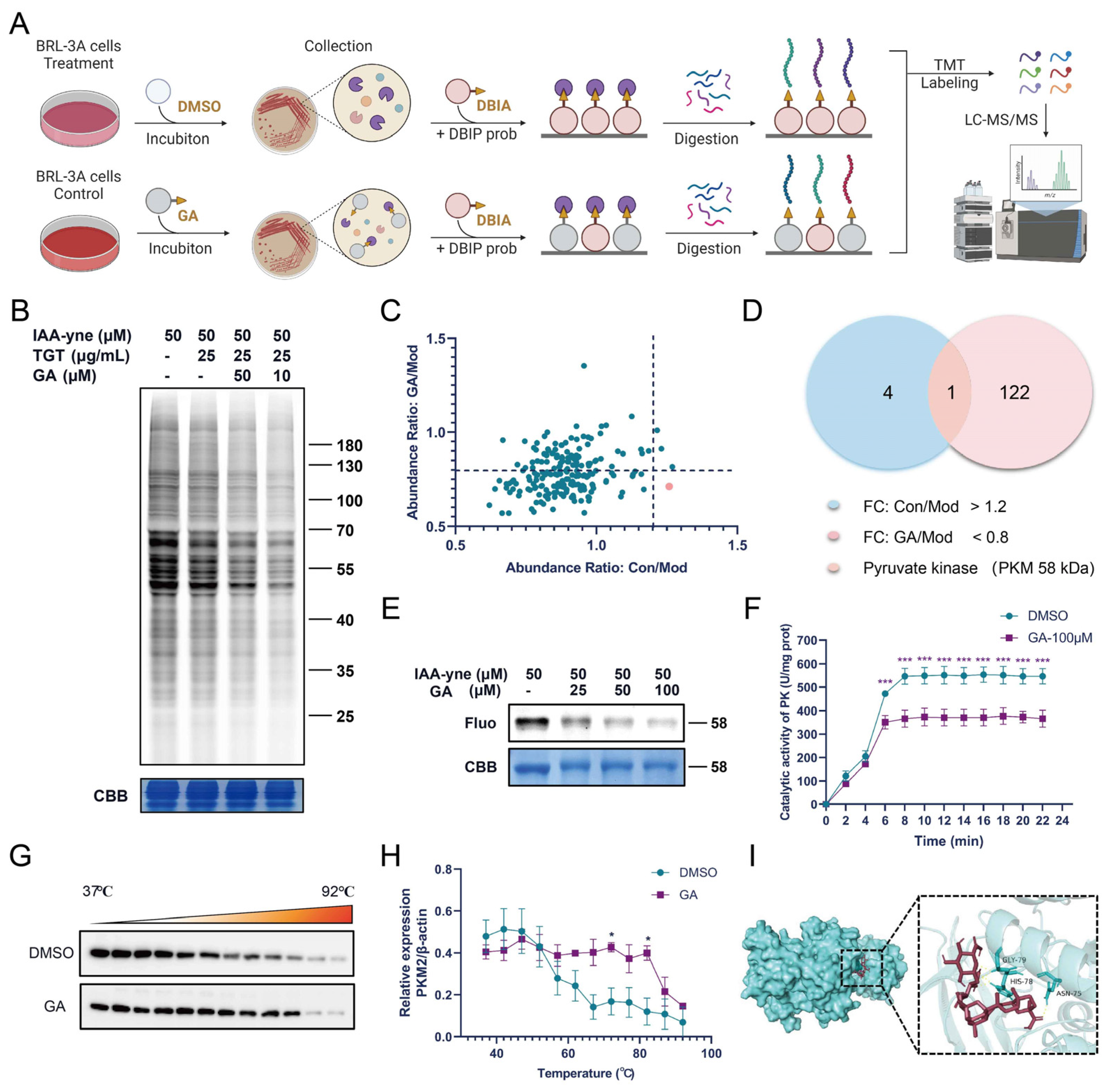
2.9. The Competitive In-Gel Fluorescence Labeling of GA in BRL-3A Cells
2.10. The Streamlined Cysteine-Activity-Based Protein Profiling
2.11. Protein Purifcation
2.12. PKM2 Enzyme Determination
2.13. CETSA Validation
2.14. RNA Interference
2.15. WB Assay
2.16. Statistical Analysis
3. Results
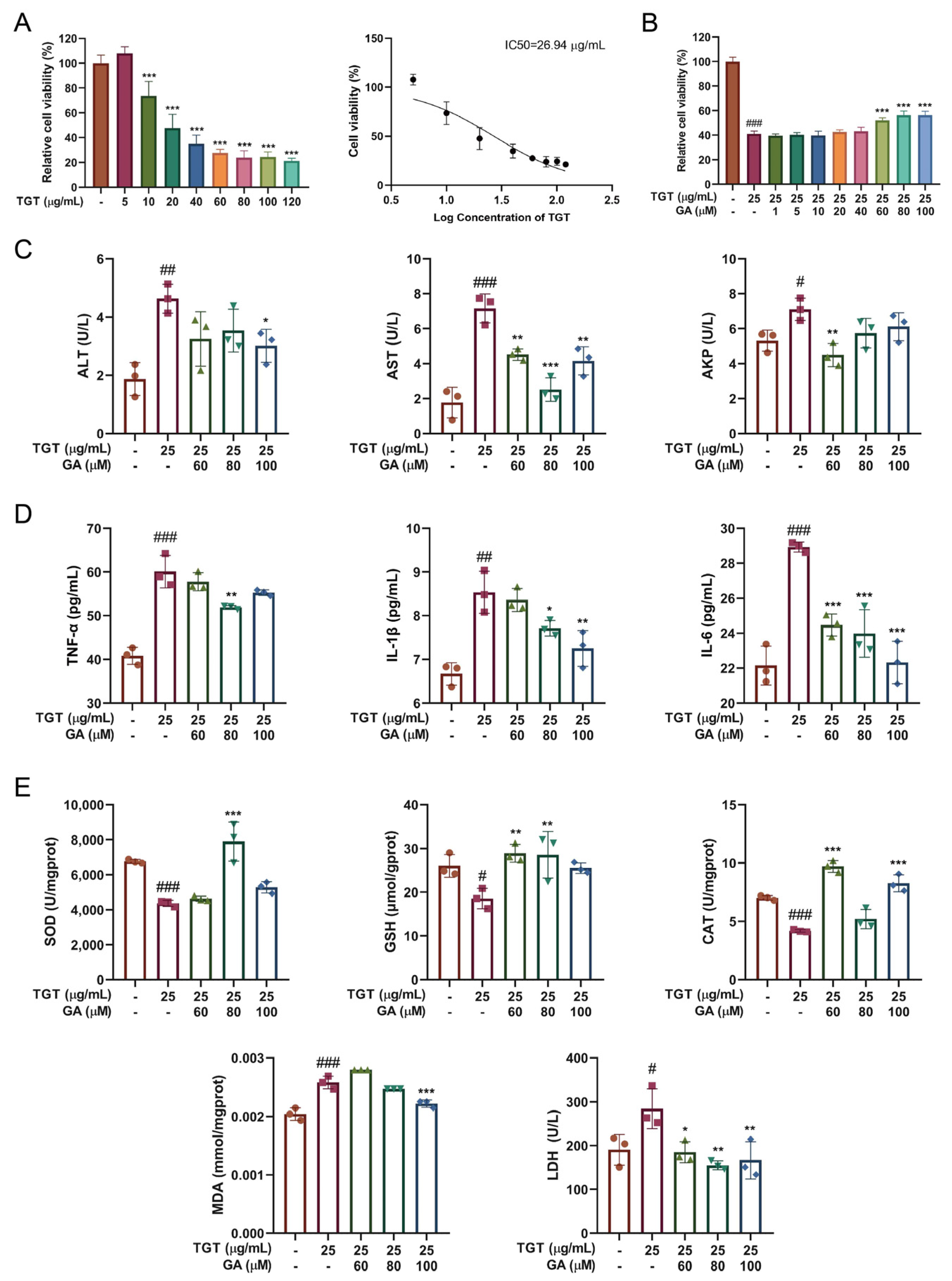
3.1. GA Mitigated Liver Injury by Enhancing Antioxidant Capacity and Inhibiting the Inflammatory Activity in Mice
3.1.1. GA Significantly Altered the Liver Morphology and Organ Index in Mice
3.1.2. GA Significantly Increased the Antioxidant Capacity and Reduced the Level of Inflammatory Cytokines in Mice
3.2. GA Improved Hepatocyte Function by Inhibiting TGT-Induced Inflammation and Oxidative Damage In Vitro
3.2.1. GA Treatment Simultaneously Reduced TGT-Induced Hepatotoxicity In Vitro
3.2.2. GA Effectively Alleviated TGT-Induced Acute Liver Injury In Vitro
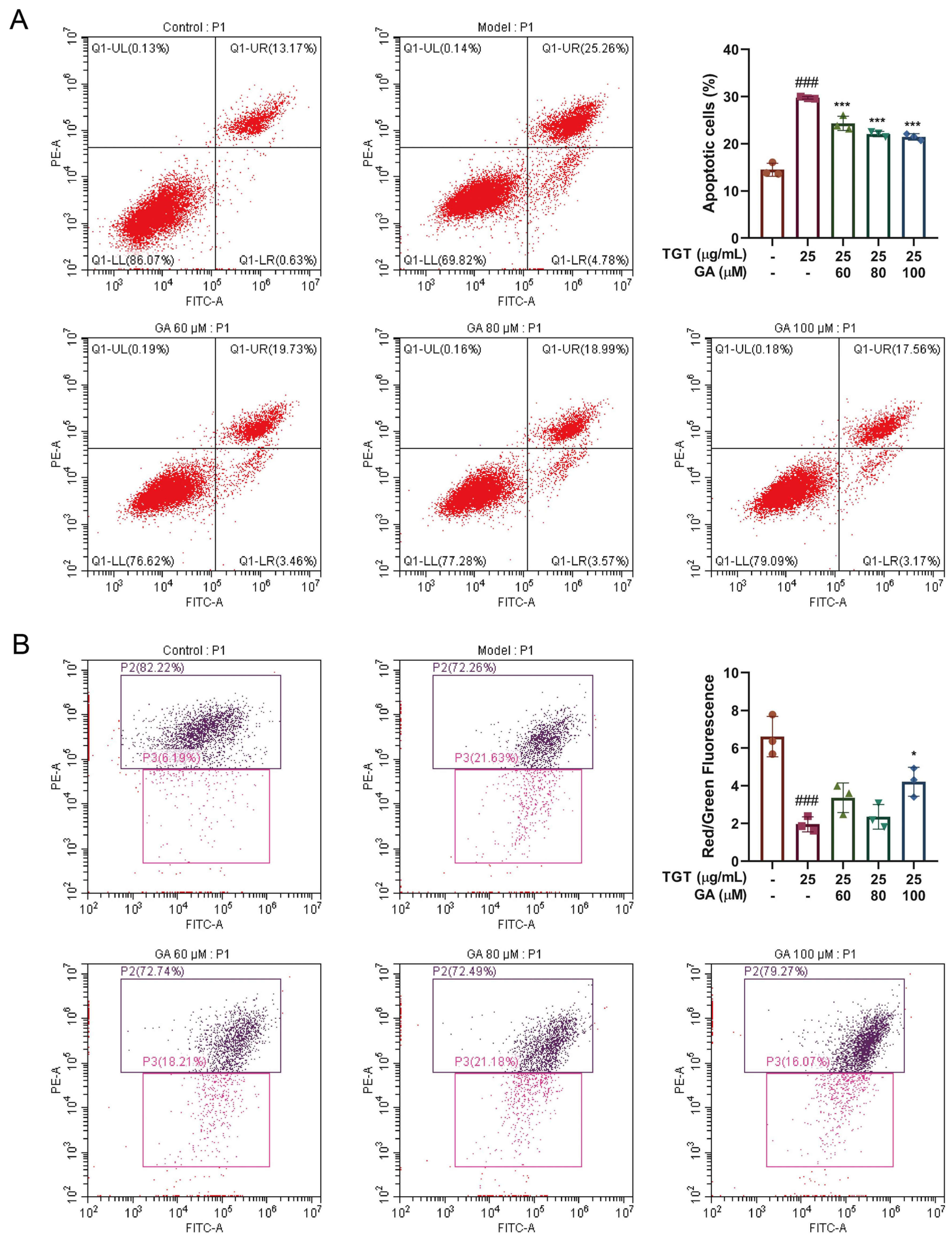
3.3. GA Promoted Proliferation and Reduced Apoptosis of BRL-3A Cells
3.3.1. GA Effectively Reduced TGT-Induced Hepatocyte Apoptosis
3.3.2. GA Reversed the TGT-Induced Increase in Mitochondrial Membrane Potential
3.4. GA Bound Directly to PKM2
3.4.1. Fluorescence Labeling and ABPP Identification of GA Targets
3.4.2. GA Directly Targeted PKM2 in Activated BRL-3A Cells
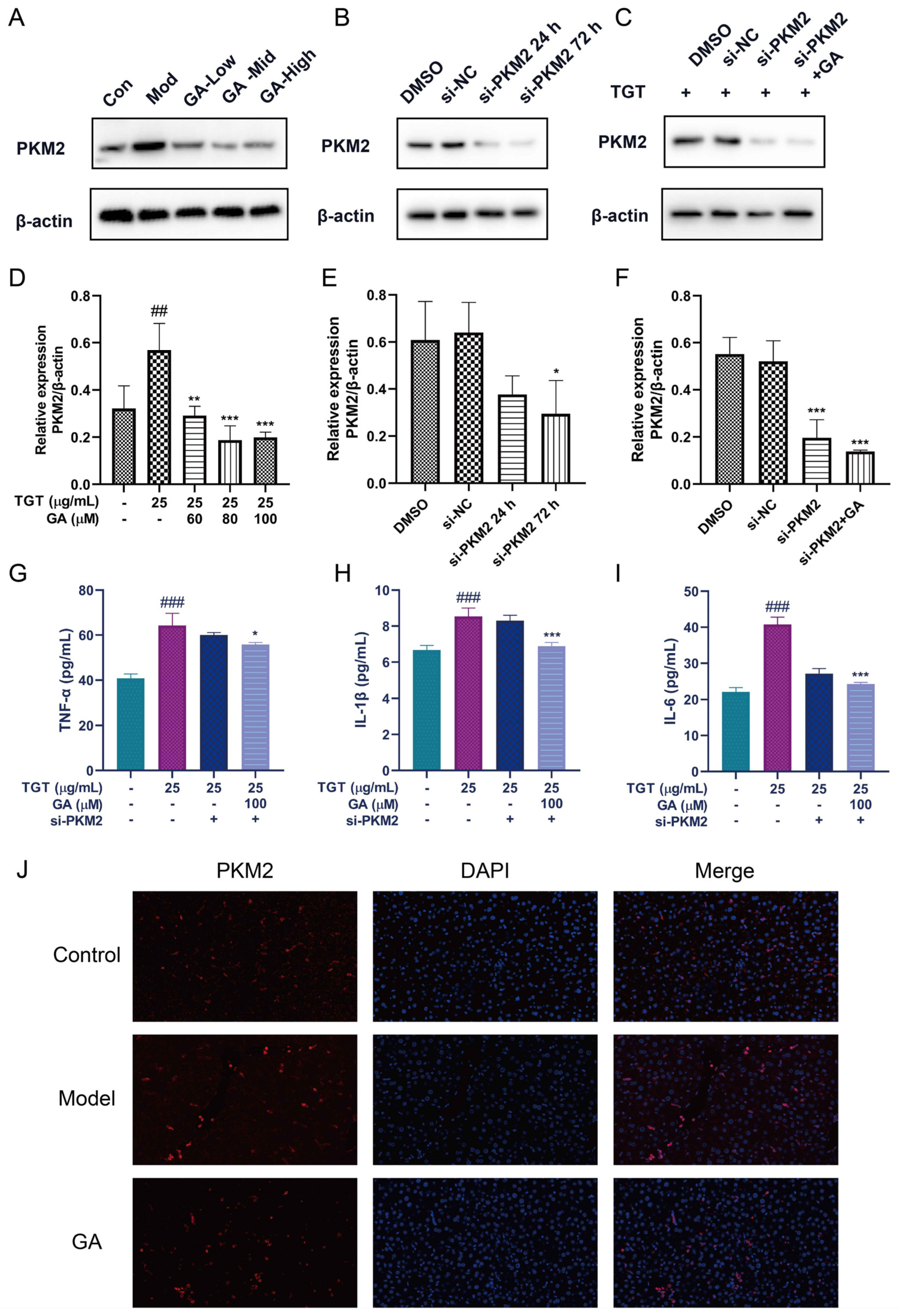
3.5. Silencing of PKM2 Inhibited Inflammation in BRL-3A Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, N.; Zhang, Y.Q.; Jiang, Q.; Liu, W.; Liu, J.; Huang, Q.C.; Wu, K.Y.; Tu, S.H.; Zhou, Z.S.; Chen, W.H.; et al. Clinical practice guideline for Tripterygium glycosides/Tripterygium wilfordii tablets in the treatment of rheumatoid arthritis. Front. Pharmacol. 2021, 11, 608703. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Dai, M.Y.; Bao, L.J.; Zhu, W.F.; Li, F. FXR activation prevents liver injury induced by Tripterygium wilfordii preparations. Xenobiotica 2021, 51, 716–727. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, s104. [Google Scholar] [CrossRef] [PubMed]
- Holt, M.P.; Ju, C. Mechanisms of drug-induced liver injury. AAPS J. 2006, 8, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Huo, Y.; Yin, S.; Hu, H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018, 17, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Labbe, G.; Pessayre, D.; Fromenty, B. Drug-induced liver injury through mitochondrial dysfunction: Mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 2008, 22, 335–353. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.M.; Fan, X.M.; Tan, H.; Zeng, H.; Wang, Y.T.; Chen, P.; Huang, M.; Bi, H.C. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1–p53 signaling pathways. Toxicol. Lett. 2015, 236, 82–89. [Google Scholar] [CrossRef]
- Ye, H.; Nelson, L.J.; Del Moral, M.G.; Martínez-Naves, E.; Cubero, F.J. Dissecting the molecular pathophysiology of drug-induced liver injury. World J. Gastroenterol. 2018, 24, 1373. [Google Scholar] [CrossRef]
- Honma, Y.; Sato-Morita, M.; Katsuki, Y.; Mihara, H.; Baba, R.; Hino, K.; Kawashima, A.; Ariyasu, T.; Harada, M. Trehalose alleviates oxidative stress-mediated liver injury and Mallor-Denk body formation via activating autophagy in mice. Med. Mol. Morphol. 2021, 54, 41–51. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Guo, H.; Sun, J.; Li, D.; Hu, Y.; Yu, X.; Hua, H.; Jing, X.; Chen, F.; Jia, Z.; Xu, J. Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2019, 112, 108704. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; He, L.; Sun, J.; Wenbo, J.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.G.; Zhao, T.T.; Lu, N.; Yang, Y.A.; Zhu, H.L. Research progress of glycyrrhizic acid on antiviral activity. Mini Rev. Med. Chem. 2019, 19, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, Q.; Chen, J.; Xia, F.; Qiu, C.; Li, M.; Zhao, M.; Zhang, Q.; Luo, P.; Lu, T.; et al. Transcriptome and lipid metabolomics-based discovery: Glycyrrhizic acid alleviates tripterygium glycoside tablet-induced acute liver injury by regulating the activities of CYP and the metabolism of phosphoglycerides. Front. Pharmacol. 2022, 12, 822154. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Boschek, C.B.; Hugo, F.; Eigenbrodt, E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005, 15, 300–308. [Google Scholar] [CrossRef]
- Xu, Q.R.; Tu, J.F.; Dou, C.W.; Zhang, J.; Yang, L.; Liu, X.; Lei, K.F.; Liu, Z.K.; Wang, Y.F.; Li, L.J.; et al. HSP90 promotes cell glycolysis, proliferation and inhibits apoptosis by regulating PKM2 abundance via Thr-328 phosphorylation in hepatocellular carcinoma. Mol. Cancer 2017, 16, 178. [Google Scholar] [CrossRef]
- Rao, J.; Wang, H.; Ni, M.; Wang, Z.; Wang, Z.; Wei, S.; Liu, M.; Wang, P.; Qiu, J.; Zhang, L.; et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut 2022, 71, 2539–2550. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Wu, Y.F.; Xu, F.F.; Deng, J.B.; Wu, L.L.; Han, X.D.; Liang, J.; Guo, D.A.; Liu, B. Tripterygium glycoside fraction n2: Alleviation of DSS-induced colitis by modulating immune homeostasis in mice. Phytomed. Int. J. Phytother. Phytopharm. 2019, 58, 152855. [Google Scholar] [CrossRef]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Glycyrrhizic acid and 18β-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomed. Int. J. Phytother. Phytopharm. 2013, 20, 295–302. [Google Scholar] [CrossRef]
- Richard, S.A. Exploring the pivotal immunomodulatory and anti-inflammatory potentials of glycyrrhizic and glycyrrhetinic acids. Mediat. Inflamm. 2021, 2021, 6699560. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, R.; Breinbauer, R. Activity-based protein profiling (ABPP) of oxidoreductases. ChemBioChem 2021, 22, 630–638. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Ding, K.; Li, Z. Applications of activity-based protein profiling (ABPP) and bioimaging in drug discovery. Chemistry 2020, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, J.C.; Pålsson-McDermott, E.M. Pyruvate kinase M2: A potential target for regulating inflammation. Front. Immunol. 2016, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chao, Y.; Xu, W.; Liu, Z.; Wang, H.; Huang, K. Myeloid FBW7 deficiency disrupts redox homeostasis and aggravates dietary-induced insulin resistance. Redox Biol. 2020, 37, 101688. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine; Springer: Kyoto, Japan, 2015; pp. 3–23. [Google Scholar]
- Larsen, F.S.; Wendon, J. Understanding paracetamol-induced liver failure. Intensive Care Med. 2014, 40, 888–890. [Google Scholar] [CrossRef]
- Shirai, T.; Nazarewicz, R.R.; Wallis, B.B.; Yanes, R.E.; Watanabe, R.; Hilhorst, M.; Tian, L.; Harrison, D.G.; Giacomini, J.C.; Assimes, T.L.; et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016, 213, 337–354. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, Y.; Li, Y.; Zhang, L.; Tang, H.; Zhang, J.; Cheng, G.; Zhao, M.; Lu, T.; Zhang, Q.; et al. Glycyrrhizic Acid Mitigates Tripterygium-Glycoside-Tablet-Induced Acute Liver Injury via PKM2 Regulated Oxidative Stress. Metabolites 2022, 12, 1128. https://doi.org/10.3390/metabo12111128
Wang Q, Huang Y, Li Y, Zhang L, Tang H, Zhang J, Cheng G, Zhao M, Lu T, Zhang Q, et al. Glycyrrhizic Acid Mitigates Tripterygium-Glycoside-Tablet-Induced Acute Liver Injury via PKM2 Regulated Oxidative Stress. Metabolites. 2022; 12(11):1128. https://doi.org/10.3390/metabo12111128
Chicago/Turabian StyleWang, Qixin, Yuwen Huang, Yu Li, Luyun Zhang, Huan Tang, Junzhe Zhang, Guangqing Cheng, Minghong Zhao, Tianming Lu, Qian Zhang, and et al. 2022. "Glycyrrhizic Acid Mitigates Tripterygium-Glycoside-Tablet-Induced Acute Liver Injury via PKM2 Regulated Oxidative Stress" Metabolites 12, no. 11: 1128. https://doi.org/10.3390/metabo12111128
APA StyleWang, Q., Huang, Y., Li, Y., Zhang, L., Tang, H., Zhang, J., Cheng, G., Zhao, M., Lu, T., Zhang, Q., Luo, P., Zhu, Y., Xia, F., Zhang, Y., Liu, D., Wang, C., Li, H., Qiu, C., Wang, J., & Guo, Q. (2022). Glycyrrhizic Acid Mitigates Tripterygium-Glycoside-Tablet-Induced Acute Liver Injury via PKM2 Regulated Oxidative Stress. Metabolites, 12(11), 1128. https://doi.org/10.3390/metabo12111128