Palmitic Acid Promotes Lung Metastasis of Melanomas via the TLR4/TRIF-Peli1-pNF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals and Treatment
2.3. Cell Culture and Treatment
2.4. Cell Proliferation Detection
2.5. siRNA Transfection
2.6. Hematoxylin–Eosin (HE) Staining
2.7. Immunofluorescence
2.8. Western Blot Analysis
2.9. Molecular Docking
2.10. Statistical Analysis
3. Results
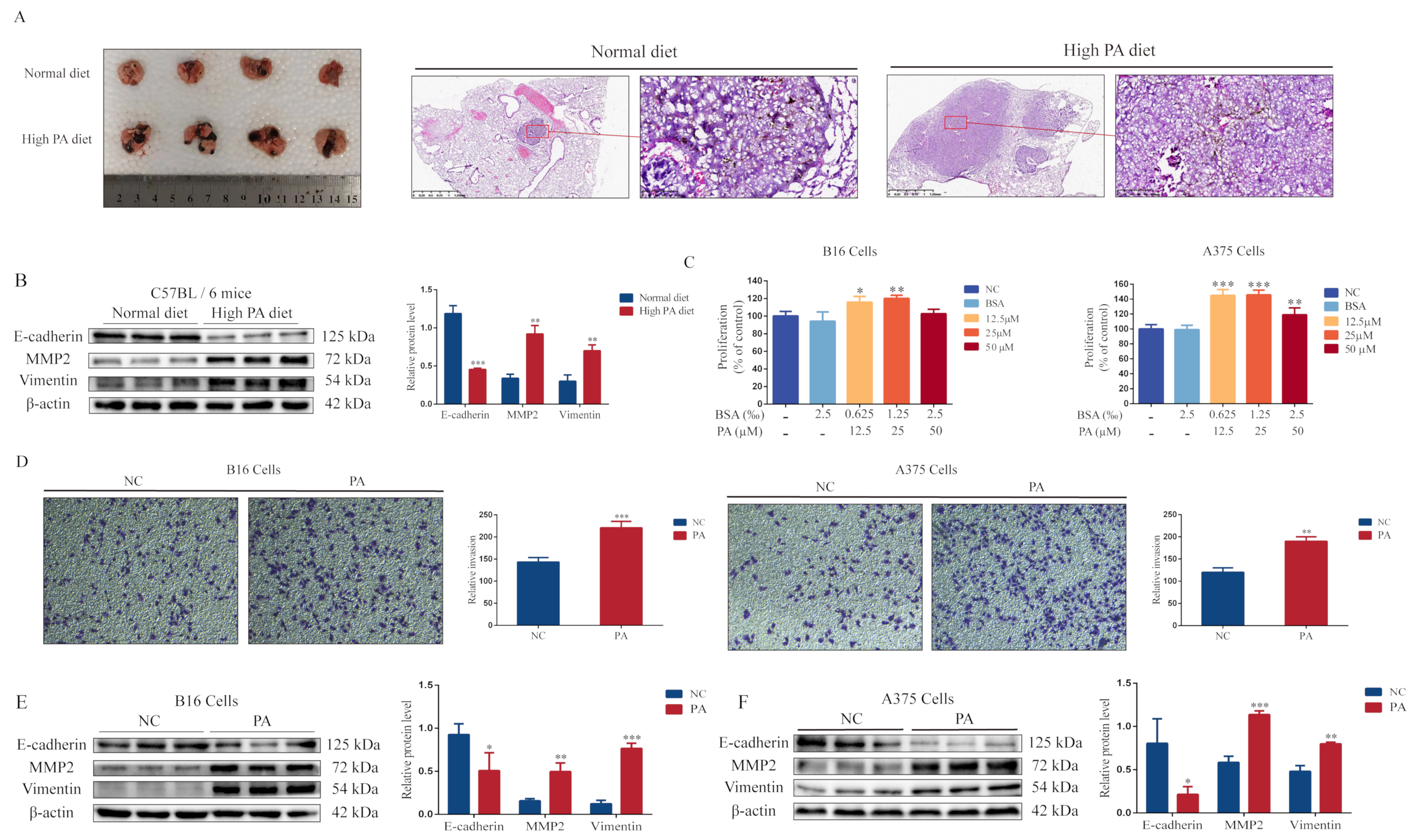
3.1. PA Promotes Melanoma Cell Proliferation, Invasion, and Lung Metastasis
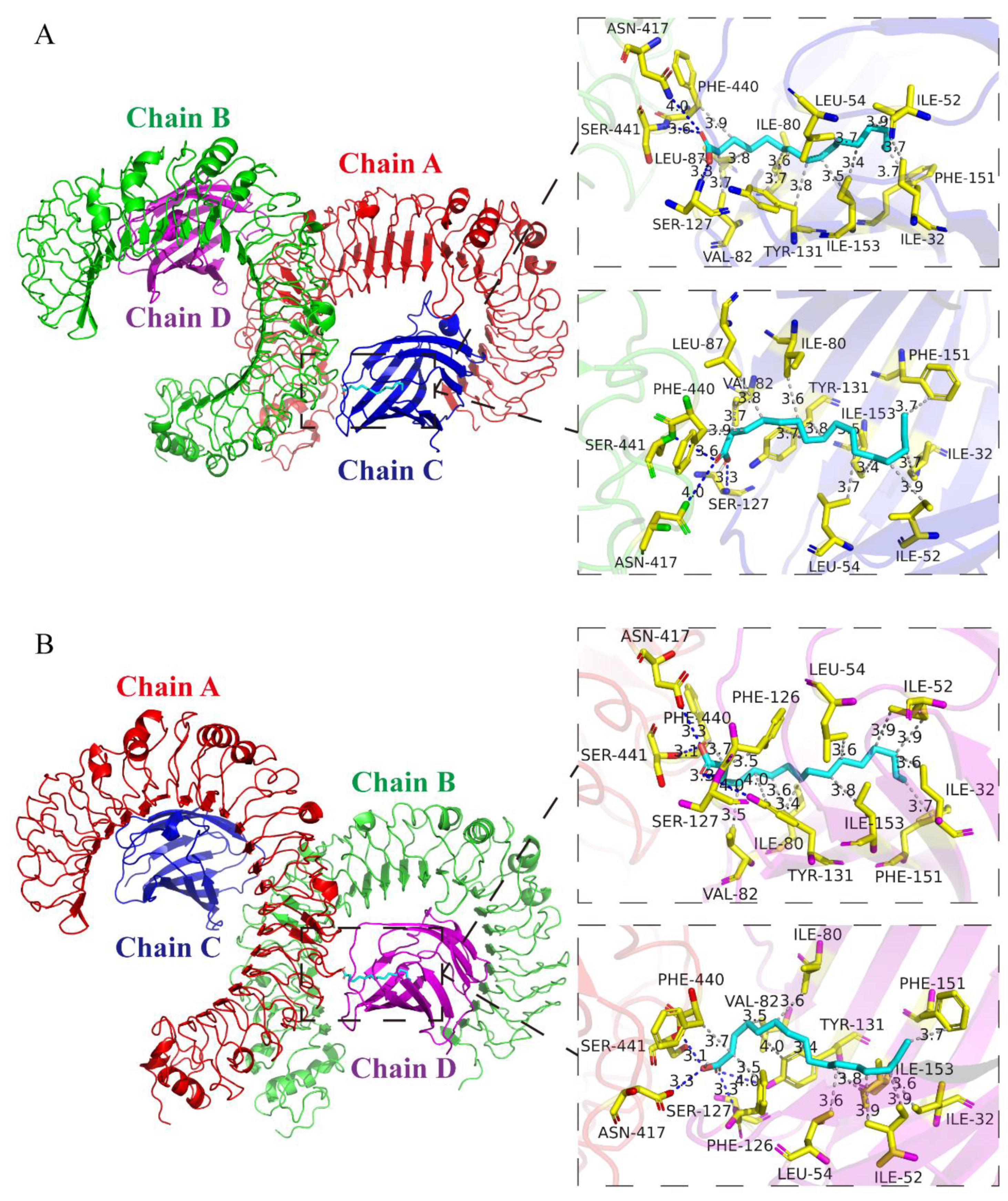
3.2. Analysis of Protein-Binding Model of PA and TLR4
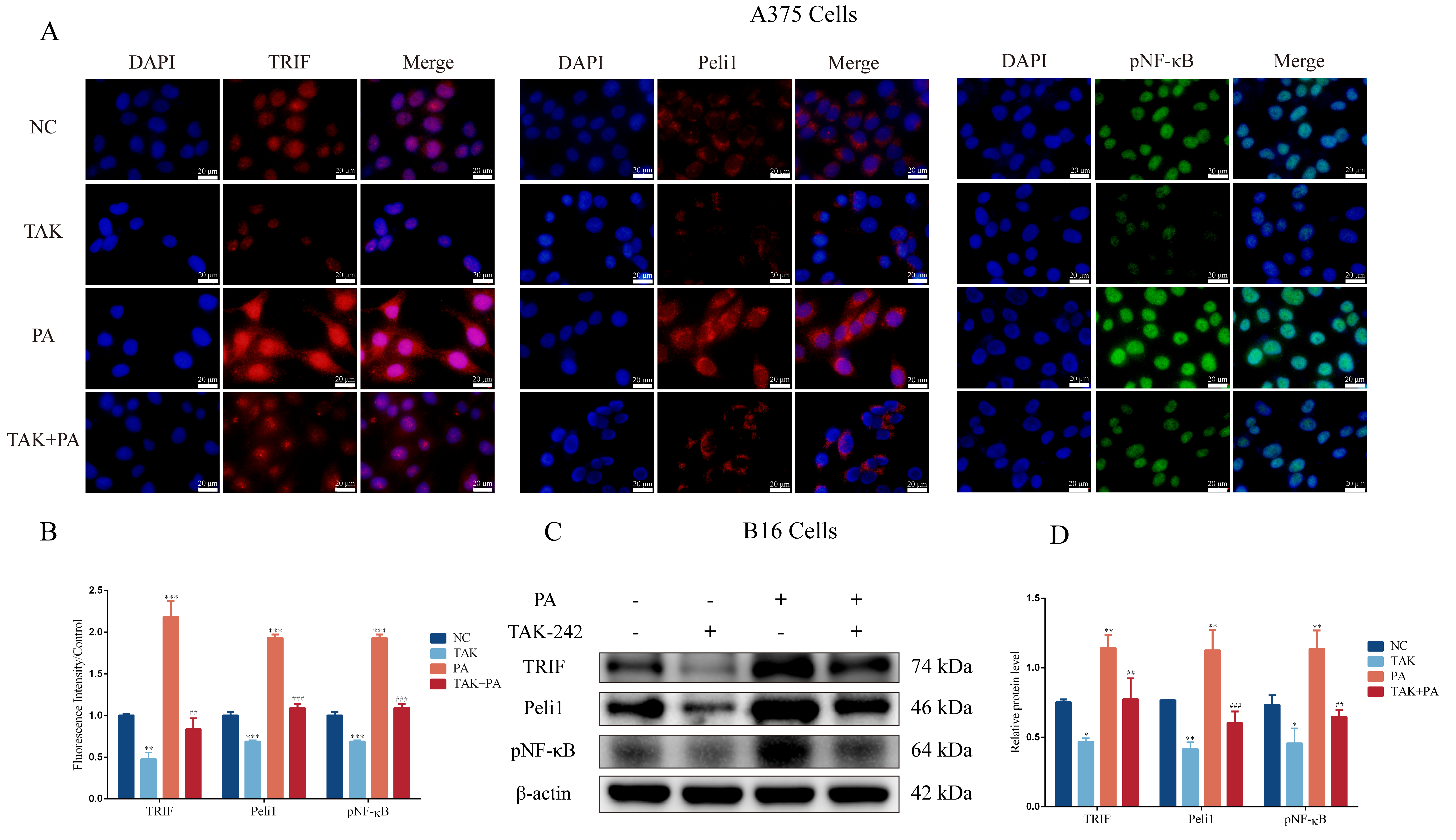
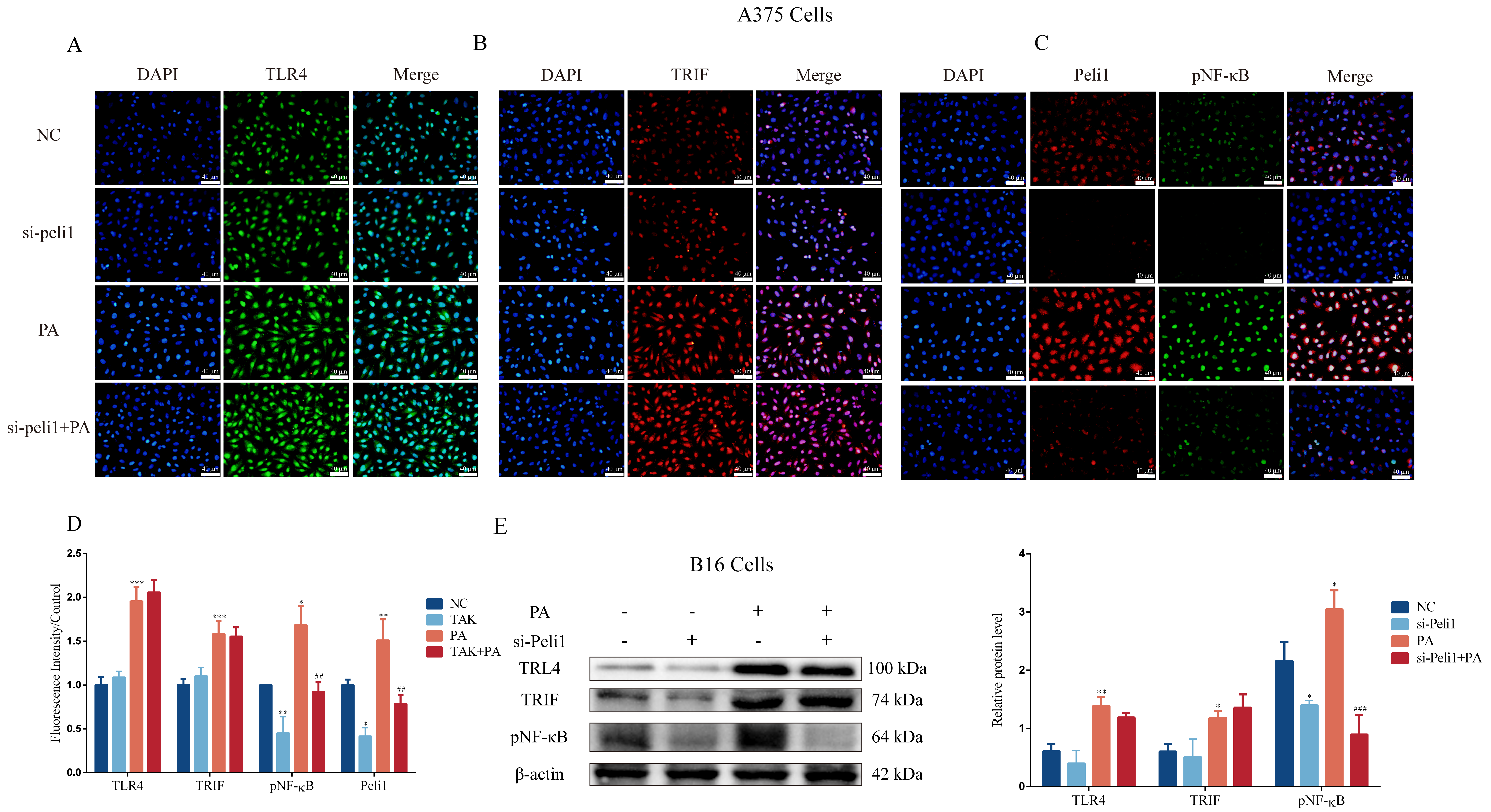
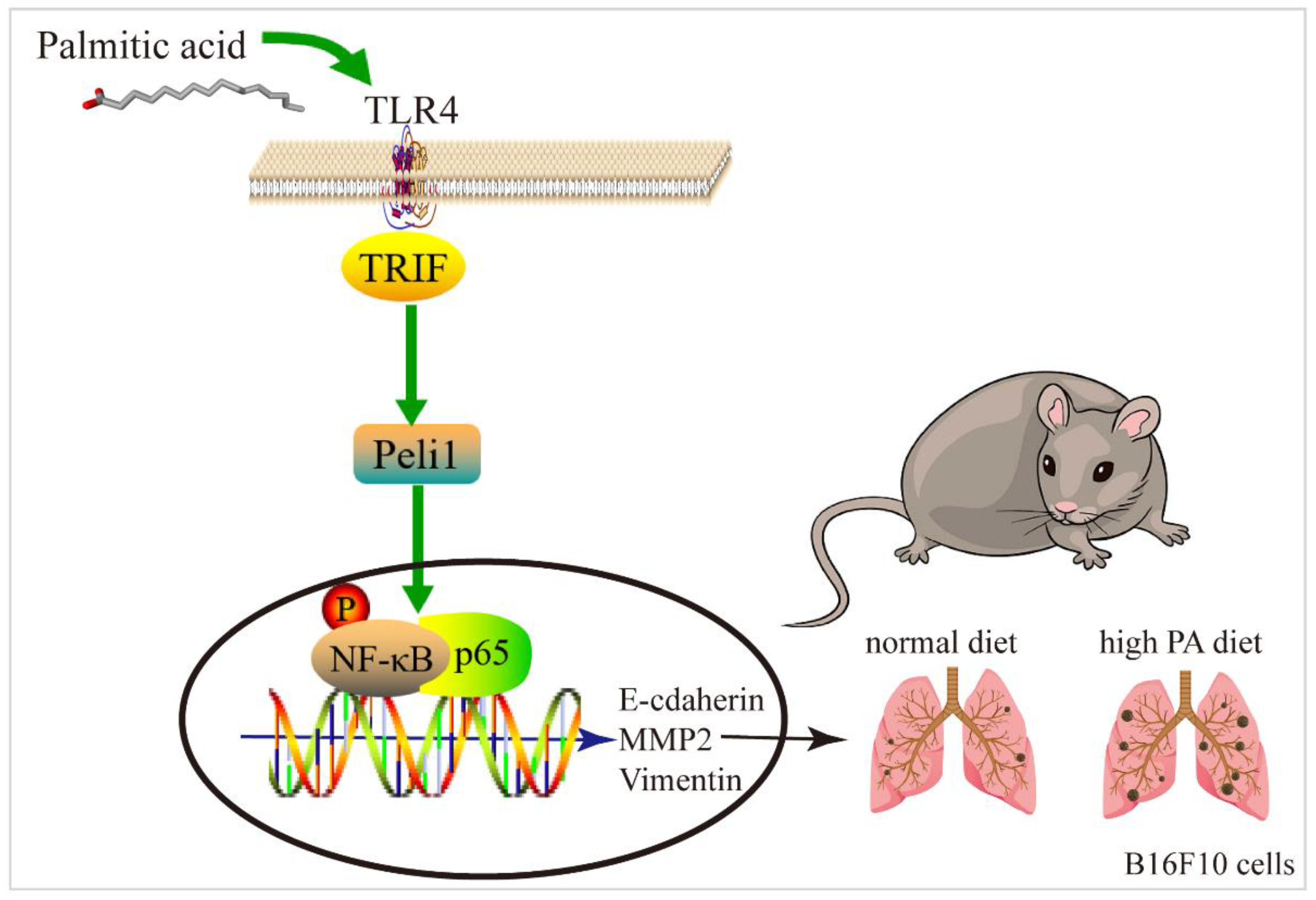
3.3. PA Can Activate the TLR4/TRIF-Peli1-pNF-κB Pathway
3.4. Inhibition of TLR4 Blocks PA-Induced Proliferation and Invasion in Melanoma Cells
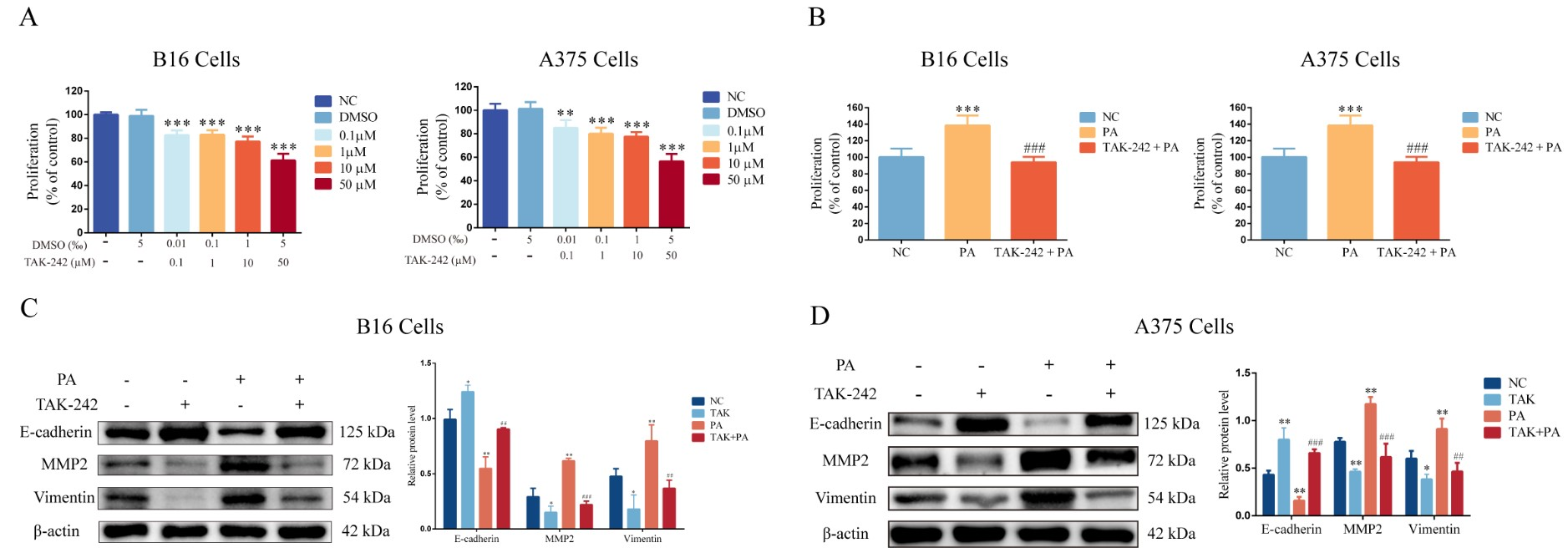
3.5. Inhibition of Peli1 Expression Alleviates the Impact of PA on the Proliferation and Invasion of Melanoma Cells and Peli1-PNF-κB Signaling

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Fu, X.; Qi, Y.; Gao, Q. A study of the clinical characteristics and prognosis of advanced mucosal and cutaneous melanoma in a Chinese population. Immunotherapy 2019, 11, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Hu, X.; Huang, C.; Zhang, W.; Cai, J.; Huang, M.; Gong, R.H.; Chen, M.; Ho, A.H.M.; Su, T.; et al. High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner. Cell Death Dis. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.I.; Hurst, K.E.; Bradshaw, A.; Janakiraman, H.; Wang, C.; Camp, E.R. High-Fat Diet Drives an Aggressive Pancreatic Cancer Phenotype. J. Surg. Res. 2021, 264, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Hu, X.; Gong, R.H.; Huang, C.; Chen, M.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Palmitic acid is an intracellular signaling molecule involved in disease development. Cell. Mol. Life Sci. CMLS 2019, 76, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Shirjang, S.; Mansoori, B.; Solali, S.; Hagh, M.F.; Shamsasenjan, K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell. Immunol. 2017, 315, 1–10. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Rogava, M.; Braun, A.D.; van der Sluis, T.C.; Shridhar, N.; Tüting, T.; Gaffal, E. Tumor cell intrinsic Toll-like receptor 4 signaling promotes melanoma progression and metastatic dissemination. Int. J. Cancer 2022, 150, 142–151. [Google Scholar] [CrossRef]
- Hu, X.; Fatima, S.; Chen, M.; Xu, K.; Huang, C.; Gong, R.H.; Su, T.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Toll-like receptor 4 is a master regulator for colorectal cancer growth under high-fat diet by programming cancer metabolism. Cell Death Dis. 2021, 12, 791. [Google Scholar] [CrossRef]
- Jin, W.; Chang, M.; Sun, S.C. Peli: A family of signal-responsive E3 ubiquitin ligases mediating TLR signaling and T-cell tolerance. Cell. Mol. Immunol. 2012, 9, 113–122. [Google Scholar] [CrossRef]
- Chang, M.; Jin, W.; Sun, S.C. Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat. Immunol. 2009, 10, 1089–1095. [Google Scholar] [CrossRef]
- Zheng, T.; Zhou, Y.; Xu, X.; Qi, X.; Liu, J.; Pu, Y.; Zhang, S.; Gao, X.; Luo, X.; Li, M.; et al. MiR-30c-5p loss-induced PELI1 accumulation regulates cell proliferation and migration via activating PI3K/AKT pathway in papillary thyroid carcinoma. J. Transl. Med. 2022, 20, 20. [Google Scholar] [CrossRef]
- Dai, D.; Zhou, H.; Yin, L.; Ye, F.; Yuan, X.; You, T.; Zhao, X.; Long, W.; Wang, D.; He, X.; et al. PELI1 promotes radiotherapy sensitivity by inhibiting noncanonical NF-κB in esophageal squamous cancer. Mol. Oncol. 2022, 16, 1384–1401. [Google Scholar] [CrossRef]
- Panic, V.; Pearson, S.; Banks, J.; Tippetts, T.S.; Velasco-Silva, J.N.; Lee, S.; Simcox, J.; Geoghegan, G.; Bensard, C.L.; van Ry, T.; et al. Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. eLife 2020, 9, e52558. [Google Scholar] [CrossRef]
- Li, F.; Liang, H.; You, H.; Xiao, J.; Xia, H.; Chen, X.; Huang, M.; Cheng, Z.; Yang, C.; Liu, W.; et al. Targeting HECTD3-IKKα axis inhibits inflammation-related metastasis. Signal Transduct. Target. Ther. 2022, 7, 264. [Google Scholar] [CrossRef]
- Kashani, B.; Zandi, Z.; Bashash, D.; Zaghal, A.; Momeny, M.; Poursani, E.M.; Pourbagheri-Sigaroodi, A.; Mousavi, S.A.; Ghaffari, S.H. Small molecule inhibitor of TLR4 inhibits ovarian cancer cell proliferation: New insight into the anticancer effect of TAK-242 (Resatorvid). Cancer Chemother. Pharmacol. 2020, 85, 47–59. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Fu, X.; Liu, B.; Chao, X.; Chan, C.L.; Cao, H.; Su, T.; Tse, A.K.W.; Fong, W.F.; Yu, Z.L. Subcutaneous adipocytes promote melanoma cell growth by activating the Akt signaling pathway: Role of palmitic acid. J. Biol. Chem. 2014, 289, 30525–30537. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, F.; Zhang, T.; Lv, S.; Zhou, L.; Du, D.; Lin, H.; Guo, F.; Luo, C.; Zhu, S. Structural basis of ketamine action on human NMDA receptors. Nature 2021, 596, 301–305. [Google Scholar] [CrossRef]
- Cano, A.; Pérez-Moreno, M.A.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef]
- Hou, Q.; Han, S.; Yang, L.; Chen, S.; Chen, J.; Ma, N.; Wang, C.; Tang, J.; Chen, X.; Chen, F.; et al. The Interplay of MicroRNA-34a, LGR4, EMT-Associated Factors, and MMP2 in Regulating Uveal Melanoma Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4503–4510. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Z.; Chen, M.; Li, C.; Liu, L.; Li, Y. Silibinin inhibits the migration and invasion of human gastric cancer SGC7901 cells by downregulating MMP-2 and MMP-9 expression via the p38MAPK signaling pathway. Oncol. Lett. 2017, 14, 7577–7582. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. CMLS 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Bakir, B.; Chiarella, A.M.; Pitarresi, J.R.; Rustgi, A.K. EMT, MET, Plasticity, and Tumor Metastasis. Trends Cell Biol. 2020, 30, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, Y.; Kiniwa, Y.; Ogawa, E.; Uchiyama, A.; Ashida, A.; Uhara, H.; Goto, Y.; Okuyama, R. Toll-like receptor 4 signaling promotes the migration of human melanoma cells. Tohoku J. Exp. Med. 2014, 234, 57–65. [Google Scholar] [CrossRef][Green Version]
- Fu, X.Q.; Liu, B.; Wang, Y.P.; Li, J.K.; Zhu, P.L.; Li, T.; Tse, K.W.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; et al. Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression. Cell Death Dis. 2020, 11, 246. [Google Scholar] [CrossRef]
- Dana, N.; Vaseghi, G.; Javanmard, S.H. Activation of PPARγ Inhibits TLR4 Signal Transduction Pathway in Melanoma Cancer In Vitro. Adv. Pharm. Bull. 2020, 10, 458–463. [Google Scholar] [CrossRef]
- Bald, T.; Quast, T.; Landsberg, J.; Rogava, M.; Glodde, N.; Lopez-Ramos, D.; Kohlmeyer, J.; Riesenberg, S.; van den Boorn-Konijnenberg, D.; Hömig-Hölzel, C.; et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014, 507, 109–113. [Google Scholar] [CrossRef]
- Geng, H.; Guo, W.; Feng, L.; Xie, D.; Bi, L.; Wang, Y.; Zhang, T.; Liang, Z.; Yu, D. Diallyl trisulfide inhibited tobacco smoke-mediated bladder EMT and cancer stem cell marker expression via the NF-κB pathway in vivo. J. Int. Med. Res. 2021, 49, 300060521992900. [Google Scholar] [CrossRef]
- An, J.; Guo, Y.; Wang, T.; Pantuck, A.J.; Rettig, M.B. Pomegranate extract inhibits EMT in clear cell renal cell carcinoma in a NF-κB and JNK dependent manner. Asian J. Urol. 2015, 2, 38–45. [Google Scholar] [CrossRef]
- Bocca, G.; Mastoridis, S.; Yeung, T.; James, D.R.C.; Cunningham, C. Visceral-to-subcutaneous fat ratio exhibits strongest association with early post-operative outcomes in patients undergoing surgery for advanced rectal cancer. Int. J. Color. Dis. 2022, 37, 1893–1900. [Google Scholar] [CrossRef]
- Cowen, S.; McLaughlin, S.L.; Hobbs, G.; Coad, J.; Martin, K.H.; Olfert, I.M.; Vona-Davis, L. High-Fat, High-Calorie Diet Enhances Mammary Carcinogenesis and Local Inflammation in MMTV-PyMT Mouse Model of Breast Cancer. Cancers 2015, 7, 1125–1142. [Google Scholar] [CrossRef]
- Agostoni, C.; Moreno, L.; Shamir, R. Palmitic Acid and Health: Introduction. Crit. Rev. Food Sci. Nutr. 2016, 56, 1941–1942. [Google Scholar] [CrossRef]
- Binker-Cosen, M.J.; Richards, D.; Oliver, B.; Gaisano, H.Y.; Binker, M.G.; Cosen-Binker, L.I. Palmitic acid increases invasiveness of pancreatic cancer cells AsPC-1 through TLR4/ROS/NF-κB/MMP-9 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 152–158. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, X.; Xiong, G.; Yun, F.; Feng, Y.; Ni, Q.; Wu, N.; Yang, L.; Yi, Z.; Zhang, Q.; et al. Palmitic Acid Promotes Lung Metastasis of Melanomas via the TLR4/TRIF-Peli1-pNF-κB Pathway. Metabolites 2022, 12, 1132. https://doi.org/10.3390/metabo12111132
Zhang X, Li X, Xiong G, Yun F, Feng Y, Ni Q, Wu N, Yang L, Yi Z, Zhang Q, et al. Palmitic Acid Promotes Lung Metastasis of Melanomas via the TLR4/TRIF-Peli1-pNF-κB Pathway. Metabolites. 2022; 12(11):1132. https://doi.org/10.3390/metabo12111132
Chicago/Turabian StyleZhang, Xuedan, Xiaoyu Li, Guohang Xiong, Fang Yun, Yu Feng, Qinxuan Ni, Na Wu, Lijuan Yang, Zihan Yi, Qiao Zhang, and et al. 2022. "Palmitic Acid Promotes Lung Metastasis of Melanomas via the TLR4/TRIF-Peli1-pNF-κB Pathway" Metabolites 12, no. 11: 1132. https://doi.org/10.3390/metabo12111132
APA StyleZhang, X., Li, X., Xiong, G., Yun, F., Feng, Y., Ni, Q., Wu, N., Yang, L., Yi, Z., Zhang, Q., Yang, Z., Kuang, Y., Sai, B., & Zhu, Y. (2022). Palmitic Acid Promotes Lung Metastasis of Melanomas via the TLR4/TRIF-Peli1-pNF-κB Pathway. Metabolites, 12(11), 1132. https://doi.org/10.3390/metabo12111132




