Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Exclusion Criteria
2.3. Ethical Considerations
2.4. Anthropometric Measurements
2.5. Echocardiographic Examination
2.6. Smoking and Daily Drug Use
2.7. Biochemical Analyses
2.8. Chemicals and Reagents
2.9. Amino Acid Determination
2.10. Asymmetric Dimethylarginine and Symmetric Dimethylarginine Quantification
2.11. Acylcarnitine Determination
2.12. Tryptophan Catabolism Metabolite Determination
2.13. Validation of the Methods
2.14. Statistical Analysis
2.15. Machine Learning Methods
2.16. Logistic Regression
2.17. Random Forest Classifier
2.18. Multiple Neural Networks
2.19. Gradient Boosting
2.20. Support Vector Machine
2.21. Bagging Classifier
3. Results
3.1. General Characteristics of the Participants
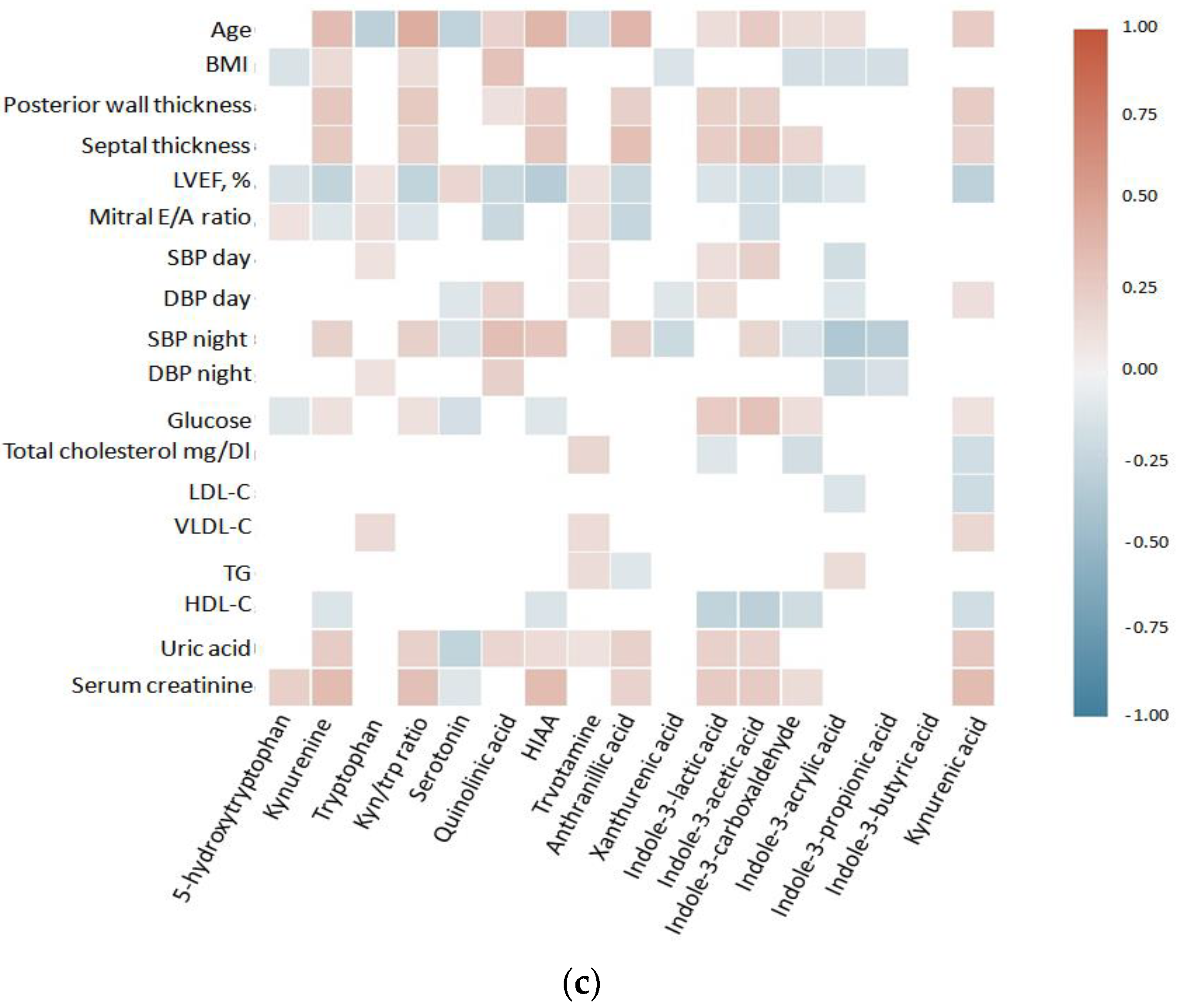
3.2. Heatmap Correlation Matrices
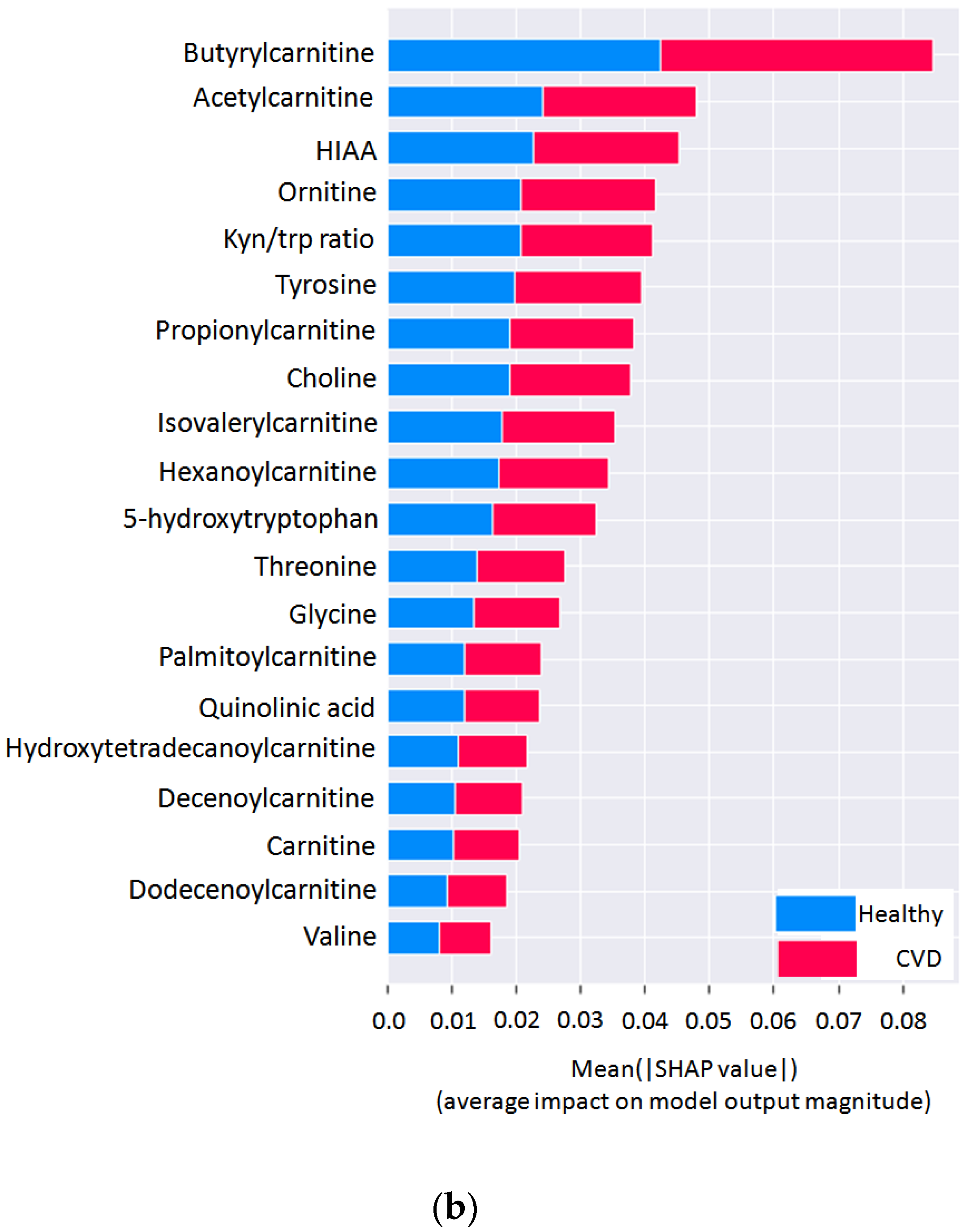
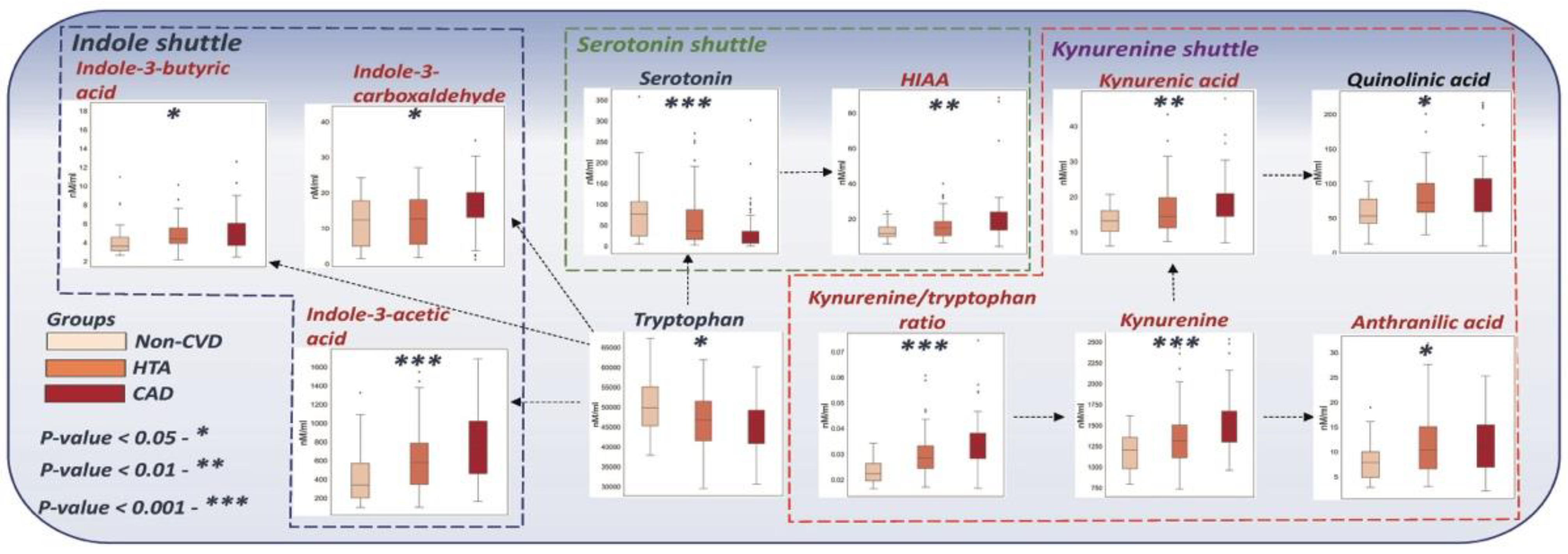
3.3. Discriminated Biomarkers between the Studied Groups
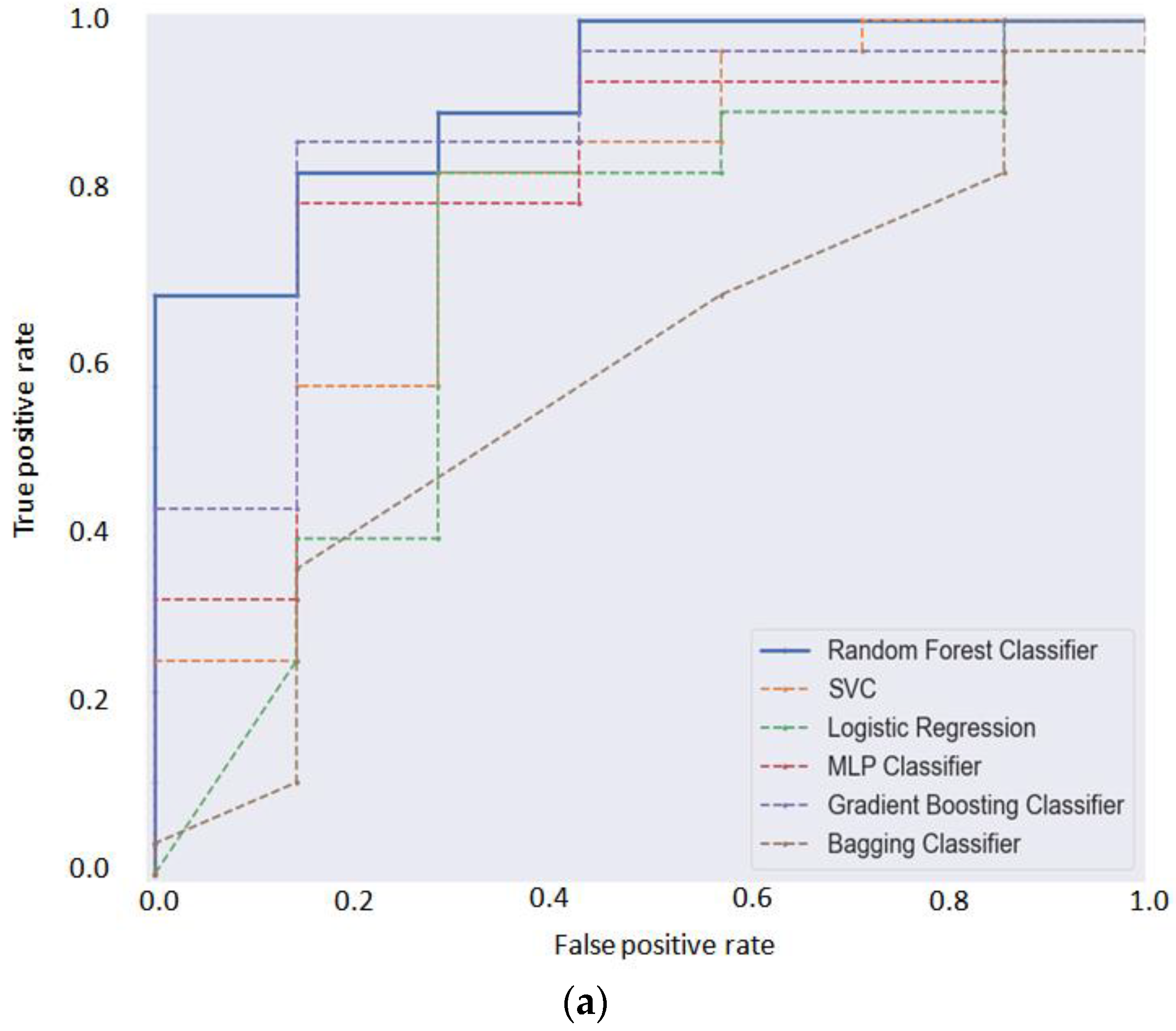
3.4. Application of Machine Learning Modeling for Prediction of CVD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases/ (accessed on 1 August 2022).
- Cannon, C.P. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin. Cornerstone 2007, 8, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Elmariah, S.; Gerszten, R.E.; Dyck, J.R. The emerging role of metabolomics in the diagnosis and prognosis of cardiovascular disease. J. Am. Coll. Cardiol. 2016, 68, 2850–2870. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Castagné, R.; Boulange, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Elliott, P. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur. Heart J. 2019, 40, 2883–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, S.B.; Sharman, J.E.; Adams, M.J.; Edwards, L.M. Metabolomics in hypertension. J. Hypertens. 2014, 32, 1159–1169. [Google Scholar] [CrossRef]
- Tomita, M.; Kami, K. Systems biology, metabolomics, and cancer metabolism. Science 2012, 336, 990–991. [Google Scholar] [CrossRef]
- Markin, P.A.; Brito, A.; Moskaleva, N.; Fodor, M.; Lartsova, E.V.; Shpot, Y.V.; Lerner, Y.V.; Mikhajlov, V.Y.; Potoldykova, N.V.; Enikeev, D.V.; et al. Plasma sarcosine measured by gas chromatography-mass spectrometry distinguishes prostatic intraepithelial neoplasia and prostate cancer from benign prostate hyperplasia. Lab. Med. 2020, 51, 566–573. [Google Scholar] [CrossRef]
- Markin, P.A.; Brito, A.; Moskaleva, N.; Lartsova, E.V.; Shpot, Y.V.; Lerner, Y.V.; Mikhajlov, V.Y.; Potoldykova, N.V.; Enikeev, D.V.; La Frano, M.R.; et al. Plasma metabolomic profile in prostatic intraepithelial neoplasia and prostate cancer and associations with the prostate-specific antigen and the Gleason score. Metabolomics 2020, 16, 74. [Google Scholar] [CrossRef]
- Moskaleva, N.E.; Baranov, P.A.; Mesonzhnik, N.V.; Appolonova, S.A. HPLC–MS/MS method for the simultaneous quantification of desmethylmebeverine acid, mebeverine acid and mebeverine alcohol in human plasma along with its application to a pharmacokinetics study. J. Pharm. Biomed. Anal. 2017, 138, 118–125. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Metabolomics for biomarker discovery: Moving to the clinic. BioMed Res. Int. 2015, 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Lee, S.H.; Shin, M.J.; Hwang, G.S. Alteration in metabolic signature and lipid metabolism in patients with angina pectoris and myocardial infarction. PLoS ONE 2015, 10, e0135228. [Google Scholar] [CrossRef]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.J.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bidulescu, A.; Chambless, L.E.; Siega-Riz, A.M.; Zeisel, S.H.; Heiss, G. Usual choline and betaine dietary intake and incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc. Disord. 2007, 7, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzau, V.; Braunwald, E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: A workshop consensus statement. Am. Heart J. 1991, 121, 1244–1263. [Google Scholar] [CrossRef]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16, S61–S63. [Google Scholar] [CrossRef] [Green Version]
- Barba, I.; Andrés, M.; Garcia-Dorado, D. Metabolomics and Heart Diseases: From Basic to Clinical Approach. Curr. Med. Chem. 2019, 26, 46–59. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Aviram, M. Specific Amino Acids Affect Cardiovascular Diseases and Atherogenesis via Protection against Macrophage Foam Cell Formation: Review Article. Rambam Maimonides Med. J. 2018, 9, e0022. [Google Scholar] [CrossRef] [Green Version]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef]
- Polyzos, K.A.; Ketelhuth, D.F. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie 2015, 35, 128–136. [Google Scholar] [CrossRef]
- Shah, N.R.; Braverman, E.R. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS ONE 2012, 7, e33308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 3, 233–271. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Alan, D.W.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handelsman, Y.; Mechanick, J.I.; Blonde, L.; Grunberger, G.; Bloomgarden, Z.T.; Bray, G.A.; Dagogo-Jack, S.; Davidson, J.A.; Einhorn, D.; Ganda, O.; et al. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: Executive summary. Endocr. Pract. 2011, 17, 287–302. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 2017, 2, 1–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BMI Best Practice Assessment of Elevated Creatinine. Available online: https://bestpractice.bmj.com/topics/en-gb/935 (accessed on 1 January 2021).
- Vapnik, V.N. Statistical Learning Theory, 1st ed.; Wiley-Interscience: New York, NY, USA, 1998; p. 768. [Google Scholar]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferré, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvadó, J.; Liang, L.; Wang, D.D.; Corella, D.; et al. Increases in Plasma Tryptophan Are Inversely Associated with Incident Cardiovascular Disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J. Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Romero-Ibarguengoitia, M.E.; Vadillo-Ortega, F.; Caballero, A.E.; Ibarra-González, I.; Herrera-Rosas, A.; Serratos-Canales, M.F.; López-Alvarenga, J.C. Family history and obesity in youth, their effect on acylcarnitine/aminoacids metabolomics and nonalcoholic fatty liver disease (NAFLD). Structural equation modeling approach. PLoS ONE 2018, 13, e0193138. [Google Scholar]
- Melhem, N.J.; Taleb, S. Tryptophan: From Diet to Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef]
- Wolowczuk, I.; Hennart, B.; Leloire, A.; Bessede, A.; Soichot, M.; Taront, S.; Caiazzo, R.; Raverdy, V.; Pigeyre, M.; ABOS Consortium; et al. Tryptophan metabolism activation by indoleamine 2, 3-dioxygenase in adipose tissue of obese women: An attempt to maintain immune homeostasis and vascular tone. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2012, 303, R135–R143. [Google Scholar] [CrossRef] [Green Version]
- Verheyen, N.; Gaksch, M.G.; Andreas, A.M.; Gruebler, M.G.; Trummer, C.T.; Schwetz, V.S.; Pandis, M.P.; Ablasser, K.A.; Kolesnik, E.K.; Von Lewinski, D.V.L.; et al. P3560 Amino acids kynurenine and quinolinic acid and target organ damage in hypertensive patients—Novel insights from the styrian hypertension study. Eur. Heart J. 2017, 38, P3560. [Google Scholar] [CrossRef]
- Jauhiainen, R.; Vangipurapu, J.; Laakso, A.; Kuulasmaa, T.; Kuusisto, J.; Laakso, M. The Association of 9 Amino Acids with Cardiovascular Events in Finnish Men in a 12-Year Follow-up Study. J. Clin. Endocrinol. Metab. 2021, 106, 3448–3454. [Google Scholar] [CrossRef]
- Pope, A.J.; Druhan, L.; Guzman, J.E.; Forbes, S.P.; Murugesan, V.; Lu, D.; Xia, Y.; Chicoine, L.G.; Parinandi, N.L.; Cardounel, A.J. Role of DDAH-1 in lipid peroxidation product-mediated inhibition of endothelial NO generation. Am. J. Physiol. Cell. Physiol. 2007, 293, 1679–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notsu, Y.; Yano, S.; Shibata, H.; Nagai, A.; Nabika, T. Plasma arginine/ADMA ratio as a sensitive risk marker for atherosclerosis: Shimane CoHRE study. Atherosclerosis 2015, 239, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Böger, R.H.; Sullivan, L.M.; Schwedhelm, E.; Wang, T.J.; Maas, R.; Benjamin, E.J.; Schulze, F.; Xanthakis, V.; Benndorf, R.A.; Vasan, R.S. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation 2009, 119, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Lewis, G.D.; Ericson, U.; Orho-Melander, M.; Hedblad, B.; Engström, G.; Ostling, G.; Clish, C.; Wang, T.J.; Gerszten, R.E.; et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 2013, 34, 1982–1989. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhang, Z.; Kolwicz, S.C., Jr.; Abell, L.; Roe, N.D.; Kim, M.; Zhou, B.; Cao, Y.; Ritterhoff, J.; Gu, H.; et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef] [Green Version]
- Wagenmakers, A.J.; Veerkamp, J.H. Degradation of branched-chain amino acids and their derived 2-oxo acids and fatty acids in human and rat heart and skeletal muscle. Biochem. Med. 1982, 28, 16–31. [Google Scholar] [CrossRef]
- Herman, M.A.; She, P.; Peroni, O.D.; Lynch, C.J.; Kahn, B.B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010, 285, 11348–11356. [Google Scholar] [CrossRef] [Green Version]
- Würtz, P.; Havulinna, A.S.; Soininen, P.; Tynkkynen, T.; Prieto-Merino, D.; Tillin, T.; Ghorbani, A.; Artati, A.; Wang, Q.; Tiainen, M.; et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation 2015, 131, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Strand, E.; Pedersen, E.R.; Svingen, G.F.; Olsen, T.; Bjørndal, B.; Karlsson, T.; Dierkes, J.; Njølstad, P.R.; Mellgren, G.; Tell, G.S.; et al. Serum Acylcarnitines and Risk of Cardiovascular Death and Acute Myocardial Infarction in Patients with Stable Angina Pectoris. J. Am. Heart Assoc. 2017, 6, e003620. [Google Scholar] [CrossRef] [Green Version]
- Kukharenko, A.; Brito, A.; Kozhevnikova, M.V.; Moskaleva, N.; Markin, P.A.; Bochkareva, N.; Korobkova, E.O.; Belenkov, Y.N.; Privalova, E.V.; Larcova, E.V.; et al. Relationship between the plasma acylcarnitine profile and cardiometabolic risk factors in adults diagnosed with cardiovascular diseases. Clin. Chim. Acta 2020, 507, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Deda, O.; Panteris, E.; Meikopoulos, T.; Begou, O.; Mouskeftara, T.; Karagiannidis, E.; Papazoglou, A.S.; Sianos, G.; Theodoridis, G.; Gika, H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules 2022, 12, 354. [Google Scholar] [CrossRef]
- Rizza, S.; Copetti, M.; Rossi, C.; Cianfarani, M.A.; Zucchelli, M.; Luzi, A.; Pecchioli, C.; Porzio, O.; Di Cola, G.; Urbani, A.; et al. Metabolomics Signature Improves the Prediction of Cardiovascular Events in Elderly Subjects. Atherosclerosis 2014, 232, 260–264. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.-F.; Huang, T.; Luo, H.-H.; Chen, J.-X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.-Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients with Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef]
- Newgard, C.B. Metabolomics and metabolic diseases: Where do we stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef]




| Variable | Cut Points | Non-CVD Group (n = 27) | Abnormal % | CVD Group (n = 109) | p Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HT (n = 61) | Abnormal % | CAD (n = 48) | Abnormal % | Non-CVD vs. HT | Non-CVD vs. CAD | HT vs. CAD | ||||
| Gender (% w) | 50 | 52 | 58 | |||||||
| Age, years | 48.5 (43–51) | 62 (55–69) | 65 (59–71) | <0.001 | <0.001 | <0.05 | ||||
| BMI, kg/m2 | >30 | 26.5 (24.7–28.0) | 7 | 31 (29–34) | 55 | 30 (26–34) | 44 | <0.01 | <0.01 | NS |
| Posterior wall thickness, mm (3C) | >10 m | 10 (9–10) | 0 | 11 (11–12) | 77 | 11 (10–13) | 54 | <0.01 | <0.01 | NS |
| >9 w | 8.5 (8–10) | 14 | 10 (10–12) | 81 | 10 (10–12) | 73 | <0.01 | <0.01 | NS | |
| Septal thickness | >10 m | 10 (9–10) | 0 | 11 (10–12) | 70 | 11 (10–13) | 57 | <0.01 | <0.01 | NS |
| >9 w | 8.5 (8–10) | 14 | 10 (9–11) | 71 | 11 (9–12) | 50 | <0.05 | <0.05 | NS | |
| LVEF, % | <50 | 63 (59–66) | 0 | 60 (57–62) | 0 | 57 (50–60) | 14 | NS | <0.001 | <0.001 |
| Diastolic function, Mitral E/A ratio | ≤0.8 | 1.2 (1.1–1.3) | 0 | 0.8 (0.7–1.1) | 50 | 0.7 (0.6–1.0) | 40 | <0.001 | <0.001 | NS |
| SBP day, mmHg | >135 | 123 (120–130) | 3.5 | 135 (123–144) | 31 | 130 (123–141) | 14 | <0.01 | NS | NS |
| SBP night, mmHg | >120 | 103 (99–107) | 3.5 | 121 (111–131) | 34 | 124 (104–134) | 12 | <0.001 | <0.05 | NS |
| DBP day, mmHg | >85 | 77 (73–81) | 10.7 | 84 (74–90) | 33 | 80 (77–88) | 8 | NS | NS | NS |
| DBP night, mmHg | >70 | 69 (67–75) | 25 | 72 (65–82) | 33 | 71 (69–77) | 14 | NS | NS | NS |
| Total cholesterol, mg/dL | >200 | 203 (175–223) | 43 | 210 (165–231) | 55 | 150 (151–220) | 32 | NS | NS | NS |
| LDL-C, mg/dL | >100 | 101 (85–122) | 43 | 124 (93–142) | 56 | 74 (75–140) | 40 | NS | NS | NS |
| VLDL-C, mg/dL | >30 | 21.4 (14–34) | 25 | 22 (18–33) | 25 | 21 (15–27) | 20 | NS | NS | NS |
| HDL-C, mg/dL | <40 m | 63 (59–75) | 0 | 57 (48–62) | 7 | 50 (43–71) | 7 | NS | NS | NS |
| <50 w | 62 (61–82) | 0 | 55 (48–67) | 16 | 55 (41–64) | 23 | NS | NS | NS | |
| TG, mg/dL | >150 | 103 (85–142) | 10.7 | 118 (94–176) | 36 | 104 (77–138) | 16 | NS | NS | NS |
| Plasma glucose, mg/dL | 100–125 | 92 (87–96) | 99 (904–106) | 103 (97–111) | <0.05 | <0.001 | NS | |||
| Serum creatinine, μmol/L | >110 m | 94 (93–101) | 14 | 90 (81–96) | 13 | 95.6 (88.0–109.8) | 18 | NS | NS | NS |
| >90 w | 88 (85–100) | 36 | 93 (84–107) | 48 | 94 (78–111) | 59 | NS | NS | NS | |
| Uric acid, mg/dL | >6.99 m | 7.2 (6.3–7.4) | 43 | 6.99 (6.24–7.65) | 47 | 6.42 (5.54–7.18) | 24 | NS | NS | NS |
| >5.6 w | 5.4 (5.11–5.98) | 36 | 7.1 (5.6–8.0) | 65 | 8.06 (5.95–0.67) | 56 | NS | <0.01 | NS | |
| Smoking (% yes) | 3.5 | 17 | 24 | |||||||
| GFR, CKD-EPI (mL/min/1.73 m2) | 71.9 (65.9–77.9) | 69.7 (66–73.3) | 59.2 (54.4–64.0) | NS | <0.01 | <0.01 | ||||
| ACE inhibitor (% yes) | 3.5 | 53 | 54 | |||||||
| Statins (% yes) | 0 | 41 | 62 | |||||||
| Angiotensin receptor blockers (% yes) | 0 | 24.2 | 8.7 | |||||||
| Calcium channel blocker (% yes) | 0 | 24.2 | 23.9 | |||||||
| Beta-blockers (% yes) | 0 | 33 | 62 | |||||||
| Diuretics (%yes) | 0 | 33.9 | 43.5 | |||||||
| Hypoglycemic Medications (% yes) | 0 | 6.5 | 15.2 | |||||||
| Metabolite | Direction | Raw p-Value | q-Value | AUC (Non-CVD vs. CVD) | AUC (Non-CVD vs. HTA) |
|---|---|---|---|---|---|
| Fischer ratio | Increased | <0.01 | <0.01 | 0.70 | 0.67 |
| Isoleucine | Increased | <0.01 | <0.01 | 0.62 | 0.55 |
| Phenylalanine | Increased | <0.00 | <0.01 | 0.66 | 0.59 |
| ADMA | Increased | 0.002 | <0.01 | 0.71 | 0.67 |
| SDMA | Increased | <0.01 | <0.01 | 0.65 | 0.60 |
| ADMA/Arginine ratio | Increased | 0.011 | 0.02 | 0.72 | 0.71 |
| Ornitine | Increased | 0.013 | 0.02 | 0.64 | 0.61 |
| Glycine | Decreased | 0.017 | 0.02 | 0.68 | 0.73 |
| Leucine | Increased | 0.012 | 0.020 | 0.61 | 0.56 |
| Proline | Increased | 0.014 | 0.022 | 0.65 | 0.61 |
| Alanine | Increased | 0.019 | 0.026 | 0.66 | 0.63 |
| Lysine | Increased | 0.028 | 0.03 | 0.64 | 0.61 |
| Tyrosine | Increased | 0.028 | 0.03 | 0.66 | 0.64 |
| Aor | Decreased | 0.03 | 0.03 | 0.61 | 0.66 |
| Methionine | Increased | 0.04 | 0.04 | 0.54 | 0.51 |
| Metabolite | Direction | Raw p-Value | q-Value | AUC (Non-CVD vs. CVD) | AUC (Non-CVD vs. HTA) |
|---|---|---|---|---|---|
| Adipoylcarnitine | Increased | <0.00001 | <0.001 | 0.61 | 0.51 |
| Carnitine | Increased | <0.0001 | <0.001 | 0.75 | 0.73 |
| Propionylcarnitine | Increased | <0.0001 | <0.001 | 0.77 | 0.75 |
| Butyrylcarnitine | Increased | <0.0001 | <0.001 | 0.74 | 0.70 |
| Isovalerylcarnitine | Increased | <0.0001 | <0.001 | 0.73 | 0.70 |
| Acetylcarnitine | Increased | <0.01 | <0.01 | 0.71 | 0,73 |
| Hexanoylcarnitine | Increased | <0.01 | <0.01 | 0.72 | 0.73 |
| Hydroxyisovalerylcarnitine | Increased | <0.01 | <0.01 | 0.64 | 0.60 |
| Hydroxytetradecanoylcarnitine | Increased | <0.01 | <0.01 | 0.77 | 0.77 |
| Palmitoylcarnitine | Increased | <0.01 | <0.01 | 0.73 | 0.75 |
| Octenoylcarnitine | Increased | 0.019 | 0.026 | 0.67 | 0.69 |
| Oleoylcarnitine | Increased | 0.015 | 0.027 | 0.68 | 0.66 |
| Linoleylcarnitine | Increased | 0.021 | 0.027 | 0.66 | 0.65 |
| Hexadecenoylcarnitine | Increased | 0.028 | 0.033 | 0.68 | 0.68 |
| Decenoylcarnitine | Increased | 0.03 | 0.03 | 0.66 | 0.66 |
| Metabolite | Direction | Raw p-Value | q-Value | AUC (Non-CVD vs. CVD) | AUC (Non-CVD vs. HTA) |
|---|---|---|---|---|---|
| Kynurenine/Tryptophan ratio | Increased | <0.00001 | <0.0001 | 0.77 | 0.72 |
| Serotonin | Decreased | <0.0001 | <0.001 | 0.68 | 0.61 |
| Indole-3-acetic acid | Increased | <0.0001 | <0.001 | 0.73 | 0.68 |
| Kynurenine | Decreased | <0.001 | <0.001 | 0.71 | 0.66 |
| Kynurenic acid | Increased | <0.001 | <0.01 | 0.68 | 0.61 |
| HIAA | Increased | <0.01 | <0.01 | 0.68 | 0.62 |
| Quinolinic acid | Increased | 0.012 | 0.020 | 0.68 | 0.67 |
| Indole-3-carboxaldehyde | Increased | 0.015 | 0.023 | 0.59 | 0.52 |
| Anthranilic acid | Increased | 0.032 | 0.034 | 0.66 | 0.65 |
| Tryptophan | Decreased | 0.032 | 0.034 | 0.65 | 0.62 |
| Indole-3-butyric acid | Increased | 0.049 | 0.049 | 0.65 | 0.64 |
| Accuracy | F1 Score | Cohen’s Kappa | Log_Loss | AUC Score (One-vs.-All) | Matthews Corr Coef | |
|---|---|---|---|---|---|---|
| Random forest classifier | 0.80 | 0.80 | 0.68 | 0.67 | 0.93 | 0.69 |
| MLP classifier | 0.73 | 0.72 | 0.55 | 0.72 | 0.87 | 0.57 |
| Gradient classifier | 0.66 | 0.61 | 0.40 | 0.68 | 0.83 | 0.47 |
| Support vector classifier | 0.76 | 0.75 | 0.60 | 0.61 | 0.88 | 0.61 |
| Algorithm/Metric | TN | FP | FN | TP | Accuracy | AUC-Score | Precision | Recall | F1 |
|---|---|---|---|---|---|---|---|---|---|
| Logistic Regression | 3 | 4 | 5 | 23 | 0.74 | 0.71 | 0.84 | 0.74 | 0.75 |
| Support vector classifier | 0 | 7 | 0 | 28 | 0.80 | 0.78 | 0.80 | 0.8 | 0.71 |
| Random Forest Classifier | 4 | 3 | 0 | 28 | 0.91 | 0.91 | 0.90 | 0.91 | 0.90 |
| MLP Classifier | 4 | 3 | 4 | 24 | 0.8 | 0.81 | 0.88 | 0.8 | 0.8 |
| Gradient Boosting Classifier | 1 | 6 | 1 | 27 | 0.80 | 0.86 | 0.82 | 0.8 | 0.75 |
| BaggingClassifier | 0 | 7 | 0 | 28 | 0.8 | 0.58 | 0.8 | 0.8 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskaleva, N.E.; Shestakova, K.M.; Kukharenko, A.V.; Markin, P.A.; Kozhevnikova, M.V.; Korobkova, E.O.; Brito, A.; Baskhanova, S.N.; Mesonzhnik, N.V.; Belenkov, Y.N.; et al. Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults. Metabolites 2022, 12, 1185. https://doi.org/10.3390/metabo12121185
Moskaleva NE, Shestakova KM, Kukharenko AV, Markin PA, Kozhevnikova MV, Korobkova EO, Brito A, Baskhanova SN, Mesonzhnik NV, Belenkov YN, et al. Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults. Metabolites. 2022; 12(12):1185. https://doi.org/10.3390/metabo12121185
Chicago/Turabian StyleMoskaleva, Natalia E., Ksenia M. Shestakova, Alexey V. Kukharenko, Pavel A. Markin, Maria V. Kozhevnikova, Ekaterina O. Korobkova, Alex Brito, Sabina N. Baskhanova, Natalia V. Mesonzhnik, Yuri N. Belenkov, and et al. 2022. "Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults" Metabolites 12, no. 12: 1185. https://doi.org/10.3390/metabo12121185
APA StyleMoskaleva, N. E., Shestakova, K. M., Kukharenko, A. V., Markin, P. A., Kozhevnikova, M. V., Korobkova, E. O., Brito, A., Baskhanova, S. N., Mesonzhnik, N. V., Belenkov, Y. N., Pyatigorskaya, N. V., Tobolkina, E., Rudaz, S., & Appolonova, S. A. (2022). Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults. Metabolites, 12(12), 1185. https://doi.org/10.3390/metabo12121185







