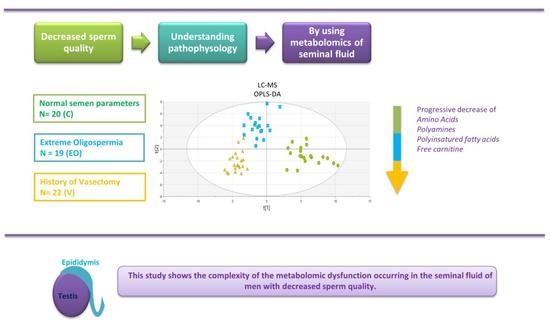
A Metabolomic Profile of Seminal Fluid in Extremely Severe Oligozoopermia Suggesting an Epididymal Involvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval
2.3. Sample Preparation
2.4. Targeted Metabolomics Analysis
2.5. Statistical Analysis
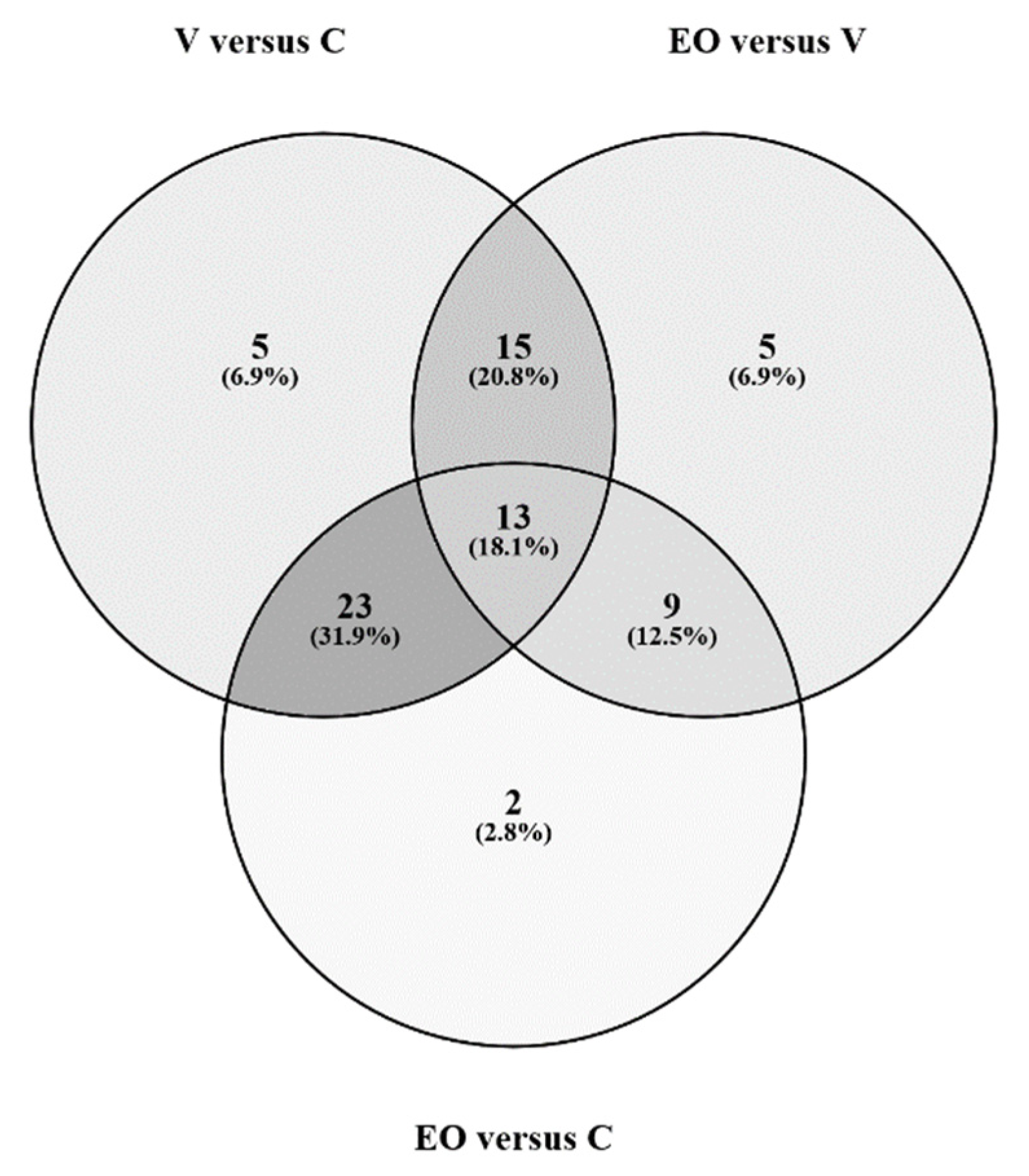
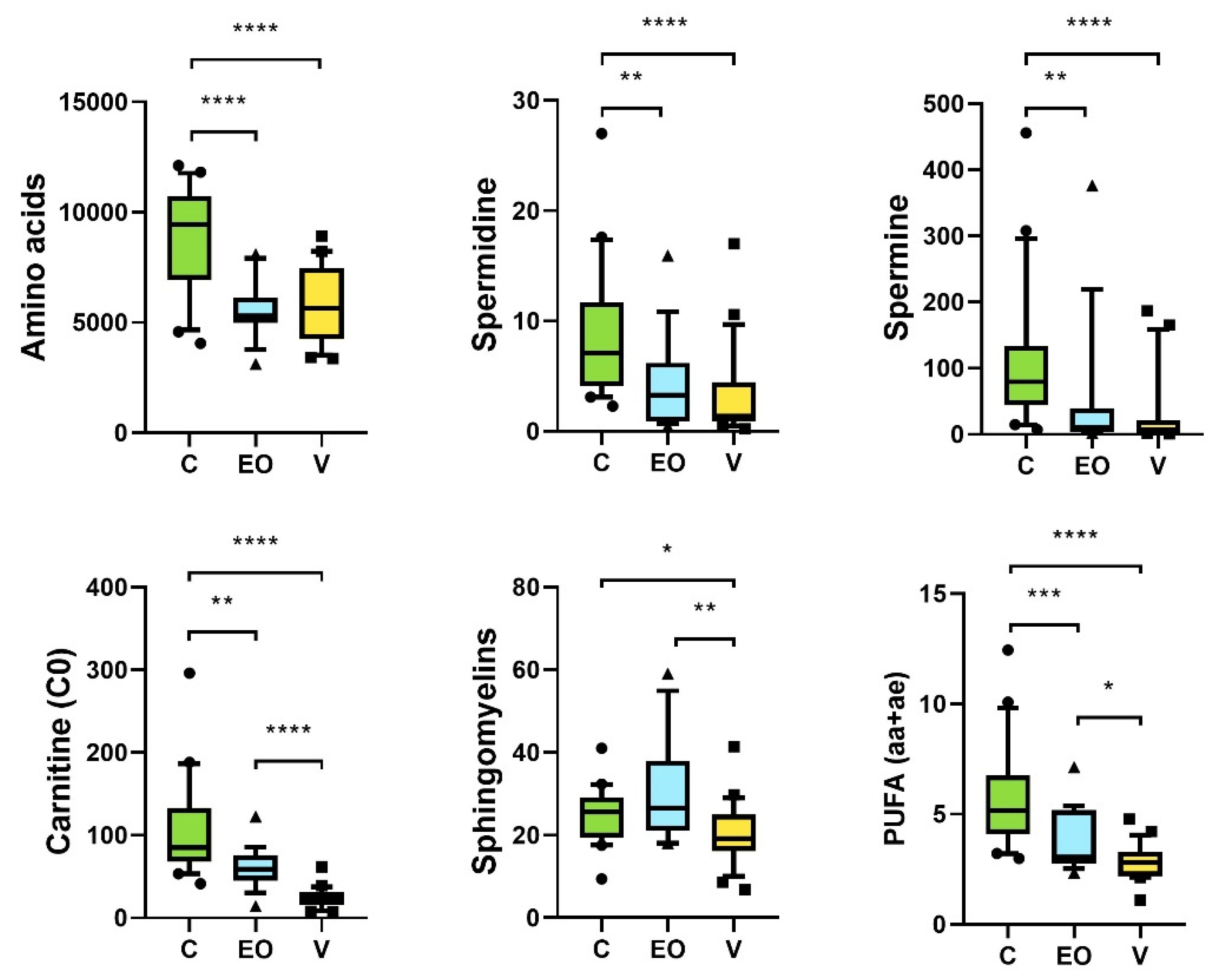
3. Results
3.1. Population Characteristics
3.2. Metabolomics Signature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rolland, M.; Le Moal, J.; Wagner, V.; Royère, D.; De Mouzon, J. Decline in Semen Concentration and Morphology in a Sample of 26 609 Men Close to General Population between 1989 and 2005 in France. Hum. Reprod. 2013, 28, 462–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skakkebæk, N.E.; Lindahl-Jacobsen, R.; Levine, H.; Andersson, A.-M.; Jørgensen, N.; Main, K.M.; Lidegaard, Ø.; Priskorn, L.; Holmboe, S.A.; Bräuner, E.V.; et al. Environmental Factors in Declining Human Fertility. Nat. Rev. Endocrinol. 2022, 18, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Bak, C.W.; Song, S.-H.; Yoon, T.K.; Lim, J.J.; Shin, T.E.; Sung, S. Natural Course of Idiopathic Oligozoospermia: Comparison of Mild, Moderate and Severe Forms: Different Fates of Oligozoospermia. Int. J. Urol. 2010, 17, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, H.; Li, B.; Zheng, Y.; Ye, Y.; Qian, Y.; Xu, C.; Huang, H.; Jin, F. Different Sperm Sources and Parameters Can Influence Intracytoplasmic Sperm Injection Outcomes before Embryo Implantation. J. Zhejiang Univ. Sci. B 2012, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-T.; Luo, C.; Li, Y.; Li, H.; Quan, S.; Deng, Y.-J.; Yang, Y.; Hu, Y.-H.H.; Tan, W.-L.; Chu, Q. Differences and Similarities between Extremely Severe Oligozoospermia and Cryptozoospermia in Intracytoplasmic Sperm Injection. Asian. J. Androl. 2016, 18, 904–907. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003078-7.
- Hauser, R.; Bibi, G.; Yogev, L.; Carmon, A.; Azem, F.; Botchan, A.; Yavetz, H.; Klieman, S.E.; Lehavi, O.; Amit, A.; et al. Virtual Azoospermia and Cryptozoospermia--Fresh/Frozen Testicular or Ejaculate Sperm for Better IVF Outcome? J. Androl. 2011, 32, 484–490. [Google Scholar] [CrossRef]
- Song, S.-H.; Bak, C.W.; Lim, J.J.; Yoon, T.K.; Lee, D.R.; Kwon, S.W. Natural Course of Severe Oligozoospermia in Infertile Male: Influence on Future Fertility Potential. J. Androl. 2010, 31, 536–539. [Google Scholar] [CrossRef]
- Alhathal, N.; Maddirevula, S.; Coskun, S.; Alali, H.; Assoum, M.; Morris, T.; Deek, H.A.; Hamed, S.A.; Alsuhaibani, S.; Mirdawi, A.; et al. A Genomics Approach to Male Infertility. Genet. Med. 2020, 22, 1967–1975. [Google Scholar] [CrossRef]
- Boguenet, M.; Bocca, C.; Bouet, P.; Serri, O.; Chupin, S.; Tessier, L.; Blanchet, O.; El Hachem, H.; Chao de la Barca, J.M.; Reynier, P.; et al. Metabolomic Signature of the Seminal Plasma in Men with Severe Oligoasthenospermia. Andrology 2020, 8, 1859–1866. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania Somnifera on Seminal Plasma Metabolites of Infertile Males: A Proton NMR Study at 800MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
- Gupta, A.; Mahdi, A.A.; Ahmad, M.K.; Shukla, K.K.; Jaiswer, S.P.; Shankhwar, S.N. 1H NMR Spectroscopic Studies on Human Seminal Plasma: A Probative Discriminant Function Analysis Classification Model. J. Pharm. Biomed. Anal. 2011, 54, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Corda, V.; Serrenti, M.; Usai, V.; Santoru, M.L.; Hurt, K.J.; Passaretti, M.; Monni, M.C.; Atzori, L.; Monni, G. Seminal Fluid Metabolomic Markers of Oligozoospermic Infertility in Humans. Metabolites 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wen, C.; Deng, M.; Ping-Li; Zhang, Z.; Zhou, Z.; Wang, X. Metabolic and Transcriptional Changes in Seminal Plasma of Asthenozoospermia Patients. Biomed. Chromatogr. 2020, 34, e4769. [Google Scholar] [CrossRef] [PubMed]
- Mumcu, A.; Karaer, A.; Dogan, B.; Tuncay, G. Metabolomics Analysis of Seminal Plasma in Patients with Idiopathic Oligoasthenoteratozoospermia Using High-resolution NMR Spectroscopy. Andrology 2020, 8, 450–456. [Google Scholar] [CrossRef]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic Profiling Reveals Correlations between Spermiogram Parameters and the Metabolites Present in Human Spermatozoa and Seminal Plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, H.; Wang, Y.; Zhang, Z.; Wu, Q. Comprehensive Metabolic Profiles of Seminal Plasma with Different Forms of Male Infertility and Their Correlation with Sperm Parameters. J. Pharm. Biomed. Anal. 2020, 177, 112888. [Google Scholar] [CrossRef]
- Baskaran, S.; Panner Selvam, M.K.; Agarwal, A. Exosomes of Male Reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [CrossRef]
- Paul, N.; Talluri, T.R.; Nag, P.; Kumaresan, A. Epididymosomes: A Potential Male Fertility Influencer. Andrologia 2021, 53, e14155. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Deng, M.; Lin, F.; Zhou, C.; Chen, Y.; Xuan, L.; Wang, H.; Feng, T.; Hu, L. Determination of 27 Amino Acids’ Levels in Seminal Plasma of Asthenospermia and Oligospermia Patients and Diagnostic Value Analysis. J. Pharm. Biomed. Anal. 2020, 184, 113211. [Google Scholar] [CrossRef]
- Ahmed, H.; Jahan, S.; Khan, A.; Khan, L.; Ullah, H.; Riaz, M.; Ullah, K.; Ullah, F. Supplementation of L-Tryptophan (an Aromatic Amino Acid) in Tris Citric Acid Extender Enhances Post-Thaw Progressive Motility, Plasmalemma, Mitochondrial Membrane Potential, Acrosome, and DNA Integrities, and in Vivo Fertility Rate of Buffalo (Bubalus Bubalis) Bull Spermatozoa. Cryobiology 2020, 92, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Bahadorani, M.; Tavalaee, M.; Abedpoor, N.; Ghaedi, K.; Nazem, M.N.; Nasr-Esfahani, M.H. Effects of Branched-Chain Amino Acid Supplementation and/or Aerobic Exercise on Mouse Sperm Quality and Testosterone Production. Andrologia 2019, 51, e13183. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Wu, D.; Xu, S.-Y.; Li, Q.; Fang, Z.-F.; Che, L.-Q.; Wu, C.-M.; Xu, X.-Y.; Lin, Y. Effect of Dietary Supplementation with Amino Acids on Boar Sperm Quality and Fertility. Anim. Reprod. Sci. 2016, 172, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Koohestanidehaghi, Y.; Torkamanpari, M.; Shirmohamadi, Z.; Lorian, K.; Vatankhah, M. The Effect of Cysteine and Glutamine on Human Sperm Functional Parameters during Vitrification. Andrologia 2021, 53, e13870. [Google Scholar] [CrossRef]
- Hernvann, A.; Gonzales, J.; Troupel, S.; Galli, A. Amino Acid Content of Human Semen in Normal and Infertility Cases. Andrologia 1986, 18, 461–469. [Google Scholar] [CrossRef]
- Li, M.J.; Zhang, Z.M.; Fan, F.; Ma, P.; Wang, Y.; Lu, H.M. Exploring Asthenozoospermia Seminal Plasma Amino Acid Disorder Based on GC-SIM-MS Combined with Chemometrics Methods. Anal. Methods 2019, 11, 2895–2902. [Google Scholar] [CrossRef]
- Zhang, X.; Diao, R.; Zhu, X.; Li, Z.; Cai, Z. Metabolic Characterization of Asthenozoospermia Using Nontargeted Seminal Plasma Metabolomics. Clin. Chim. Acta 2015, 450, 254–261. [Google Scholar] [CrossRef]
- Jayaraman, V.; Ghosh, S.; Sengupta, A.; Srivastava, S.; Sonawat, H.M.; Narayan, P.K. Identification of Biochemical Differences between Different Forms of Male Infertility by Nuclear Magnetic Resonance (NMR) Spectroscopy. J. Assist. Reprod. Genet. 2014, 31, 1195–1204. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, N.; Mani-Varnosfaderani, A.; Minai-Tehrani, A.; Savadi-Shiraz, E.; Sadeghi, M.R.; Gilany, K. Metabolomics Fingerprinting of Seminal Plasma from Unexplained Infertile Men: A Need for Novel Diagnostic Biomarkers. Mol. Reprod. Dev. 2015, 82, 150. [Google Scholar] [CrossRef]
- Qiao, S.; Wu, W.; Chen, M.; Tang, Q.; Xia, Y.; Jia, W.; Wang, X. Seminal Plasma Metabolomics Approach for the Diagnosis of Unexplained Male Infertility. PLoS ONE 2017, 12, e0181115. [Google Scholar] [CrossRef]
- Collodel, G.; Castellini, C.; Lee, J.C.-Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell Longev. 2020, 2020, 7038124. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C.; Hadley, J.; Doman, T. Correlation between Changes in Rat Sperm Membrane Lipids, Protein, and the Membrane Physical State during Epididymal Maturation. J. Androl. 1991, 12, 76–87. [Google Scholar] [PubMed]
- Lenzi, A.; Gandini, L.; Maresca, V.; Rago, R.; Sgrò, P.; Dondero, F.; Picardo, M. Fatty Acid Composition of Spermatozoa and Immature Germ Cells. Mol. Hum. Reprod. 2000, 6, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.P.; Hammerstedt, R.H. Regulation of Membrane Stability and the Acrosome Reaction in Mammalian Sperm. FASEB J. 1997, 11, 670–682. [Google Scholar] [CrossRef]
- Zaneveld, L.J.; De Jonge, C.J.; Anderson, R.A.; Mack, S.R. Human Sperm Capacitation and the Acrosome Reaction. Hum. Reprod. 1991, 6, 1265–1274. [Google Scholar] [CrossRef]
- Zalata, A.A.; Christophe, A.B.; Depuydt, C.E.; Schoonjans, F.; Comhaire, F.H. The Fatty Acid Composition of Phospholipids of Spermatozoa from Infertile Patients. Mol. Hum. Reprod. 1998, 4, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Conquer, J.A.; Martin, J.B.; Tummon, I.; Watson, L.; Tekpetey, F. Fatty Acid Analysis of Blood Serum, Seminal Plasma, and Spermatozoa of Normozoospermic vs. Asthernozoospermic Males. Lipids 1999, 34, 793–799. [Google Scholar] [CrossRef]
- Aksoy, Y.; Aksoy, H.; Altinkaynak, K.; Aydin, H.R.; Ozkan, A. Sperm Fatty Acid Composition in Subfertile Men. Prostaglandins Leukot Essent Fat. Acids 2006, 75, 75–79. [Google Scholar] [CrossRef]
- Tavilani, H.; Doosti, M.; Abdi, K.; Vaisiraygani, A.; Joshaghani, H.R. Decreased Polyunsaturated and Increased Saturated Fatty Acid Concentration in Spermatozoa from Asthenozoospermic Males as Compared with Normozoospermic Males. Andrologia 2006, 38, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Tavilani, H.; Doosti, M.; Nourmohammadi, I.; Mahjub, H.; Vaisiraygani, A.; Salimi, S.; Hosseinipanah, S.M. Lipid Composition of Spermatozoa in Normozoospermic and Asthenozoospermic Males. Prostaglandins Leukot Essent Fat. Acids 2007, 77, 45–50. [Google Scholar] [CrossRef]
- Buffone, M.G.; Doncel, G.F.; Calamera, J.C.; Verstraeten, S.V. Capacitation-Associated Changes in Membrane Fluidity in Asthenozoospermic Human Spermatozoa. Int. J. Androl. 2009, 32, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Huacuja, L.; Delgado, N.M.; Calzada, L.; Wens, A.; Reyes, R.; Pedrón, N.; Rosado, A. Exchange of Lipids between Spermatozoa and Seminal Plasma in Normal and Pathological Human Semen. Arch. Androl. 1981, 7, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Shang, X.; Qi, H.; Li, J.; Ma, B.; An, G.; Zhang, Q. Metabonomic Analysis of Fatty Acids in Seminal Plasma between Healthy and Asthenozoospermic Men Based on Gas Chromatography Mass Spectrometry. Andrologia 2017, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and Seminal Plasma Fatty Acids as Predictors of Cryopreservation Success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Tamessar, C.T.; Trigg, N.A.; Nixon, B.; Skerrett-Byrne, D.A.; Sharkey, D.J.; Robertson, S.A.; Bromfield, E.G.; Schjenken, J.E. Roles of Male Reproductive Tract Extracellular Vesicles in Reproduction. Am. J. Reprod. Immunol. 2021, 85, e13338. [Google Scholar] [CrossRef]
- Tremblay, R.R.; Chapdelaine, P.; Roy, R.; Thabet, M. Correlation between L-Carnitine and Alpha-1, 4-Glucosidase Activity in the Semen of Normal, Infertile and Vasectomized Men. Infertility 1982, 5, 61–70. [Google Scholar] [PubMed]
- Gürbüz, B.; Yalti, S.; FiÇicioğlu, C.; Zehir, K. Relationship between Semen Quality and Seminal Plasma Total Carnitine in Infertile Men. J. Obstet. Gynaecol. 2003, 23, 653–656. [Google Scholar] [CrossRef]
- Sheikh, N.; Goodarzi, M.; Bab Al-Havaejee, H.; Safari, M.; Amiri, I.; Najafi, R.; Hadeie, J. L-Carnitine Level in Seminal Plasma of Fertile and Infertile Men. J. Res. Health Sci. 2007, 7, 43–48. [Google Scholar]
- Pegg, A.E. Functions of Polyamines in Mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, S.; Breitbart, H. Role of Spermine in Mammalian Sperm Capacitation and Acrosome Reaction. Biochem. J. 1991, 278, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Rider, J.E.; Hacker, A.; Mackintosh, C.A.; Pegg, A.E.; Woster, P.M.; Casero, R.A. Spermine and Spermidine Mediate Protection against Oxidative Damage Caused by Hydrogen Peroxide. Amino Acids 2007, 33, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Mann, T. Secretory Function of the Prostate, Seminal Vesicle and Other Male Accessory Organs of Reproduction. J. Reprod. Fertil. 1974, 37, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefèvre, P.L.C.; Palin, M.-F.; Murphy, B.D. Polyamines on the Reproductive Landscape. Endocr. Rev. 2011, 32, 694–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, H.; Rui, H.; Thomassen, Y.; Hald, T.; Purvis, K. Polyamines and Other Accessory Sex Gland Secretions in Human Seminal Plasma 8 Years after Vasectomy. J. Reprod. Fertil. 1989, 87, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Thorup, J.; McLachlan, R.; Cortes, D.; Nation, T.R.; Balic, A.; Southwell, B.R.; Hutson, J.M. What Is New in Cryptorchidism and Hypospadias--a Critical Review on the Testicular Dysgenesis Hypothesis. J. Pediatr. Surg. 2010, 45, 2074–2086. [Google Scholar] [CrossRef]
- Xing, J.-S.; Bai, Z.-M. Is Testicular Dysgenesis Syndrome a Genetic, Endocrine, or Environmental Disease, or an Unexplained Reproductive Disorder? Life Sci. 2018, 194, 120–129. [Google Scholar] [CrossRef]

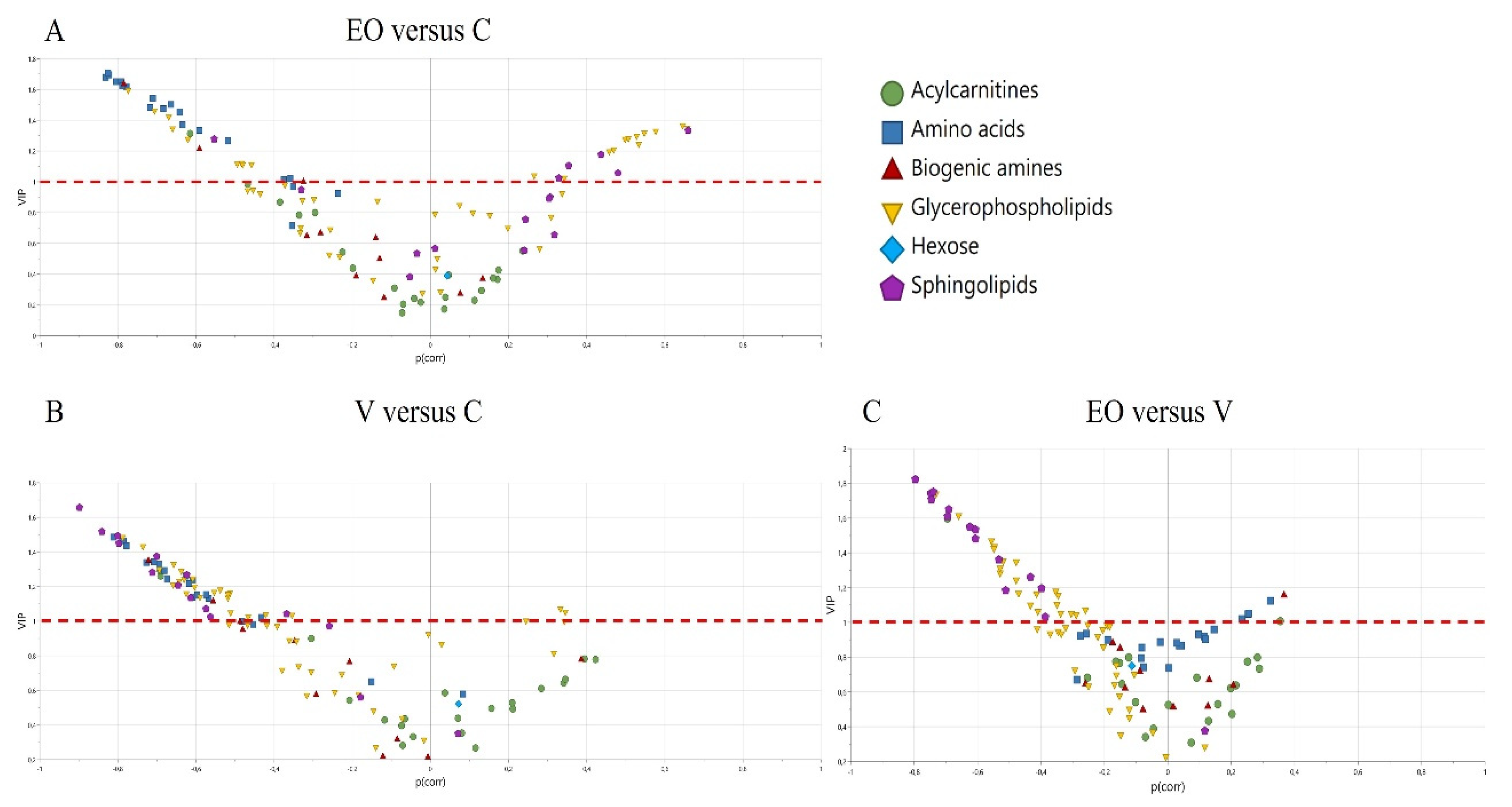
| Comparisons | Q2Y | Q2(cum) | Q2(cum) Permutations Test | p-Value (CV-ANOVA) |
|---|---|---|---|---|
| V vs. C | 0.96 | 0.87 | −0.61 | 5.67084 × 10−14 |
| V vs. EO | 0.94 | 0.76 | −0.62 | 7.38401 × 10−8 |
| EO vs. C | 0.84 | 0.75 | −0.53 | 5.97088 × 10−10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serri, O.; Boguenet, M.; Chao de la Barca, J.M.; Bouet, P.-E.; El Hachem, H.; Blanchet, O.; Reynier, P.; May-Panloup, P. A Metabolomic Profile of Seminal Fluid in Extremely Severe Oligozoopermia Suggesting an Epididymal Involvement. Metabolites 2022, 12, 1266. https://doi.org/10.3390/metabo12121266
Serri O, Boguenet M, Chao de la Barca JM, Bouet P-E, El Hachem H, Blanchet O, Reynier P, May-Panloup P. A Metabolomic Profile of Seminal Fluid in Extremely Severe Oligozoopermia Suggesting an Epididymal Involvement. Metabolites. 2022; 12(12):1266. https://doi.org/10.3390/metabo12121266
Chicago/Turabian StyleSerri, Orianne, Magalie Boguenet, Juan Manuel Chao de la Barca, Pierre-Emmanuel Bouet, Hady El Hachem, Odile Blanchet, Pascal Reynier, and Pascale May-Panloup. 2022. "A Metabolomic Profile of Seminal Fluid in Extremely Severe Oligozoopermia Suggesting an Epididymal Involvement" Metabolites 12, no. 12: 1266. https://doi.org/10.3390/metabo12121266
APA StyleSerri, O., Boguenet, M., Chao de la Barca, J. M., Bouet, P.-E., El Hachem, H., Blanchet, O., Reynier, P., & May-Panloup, P. (2022). A Metabolomic Profile of Seminal Fluid in Extremely Severe Oligozoopermia Suggesting an Epididymal Involvement. Metabolites, 12(12), 1266. https://doi.org/10.3390/metabo12121266