Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity
Abstract
1. Introduction
2. Results
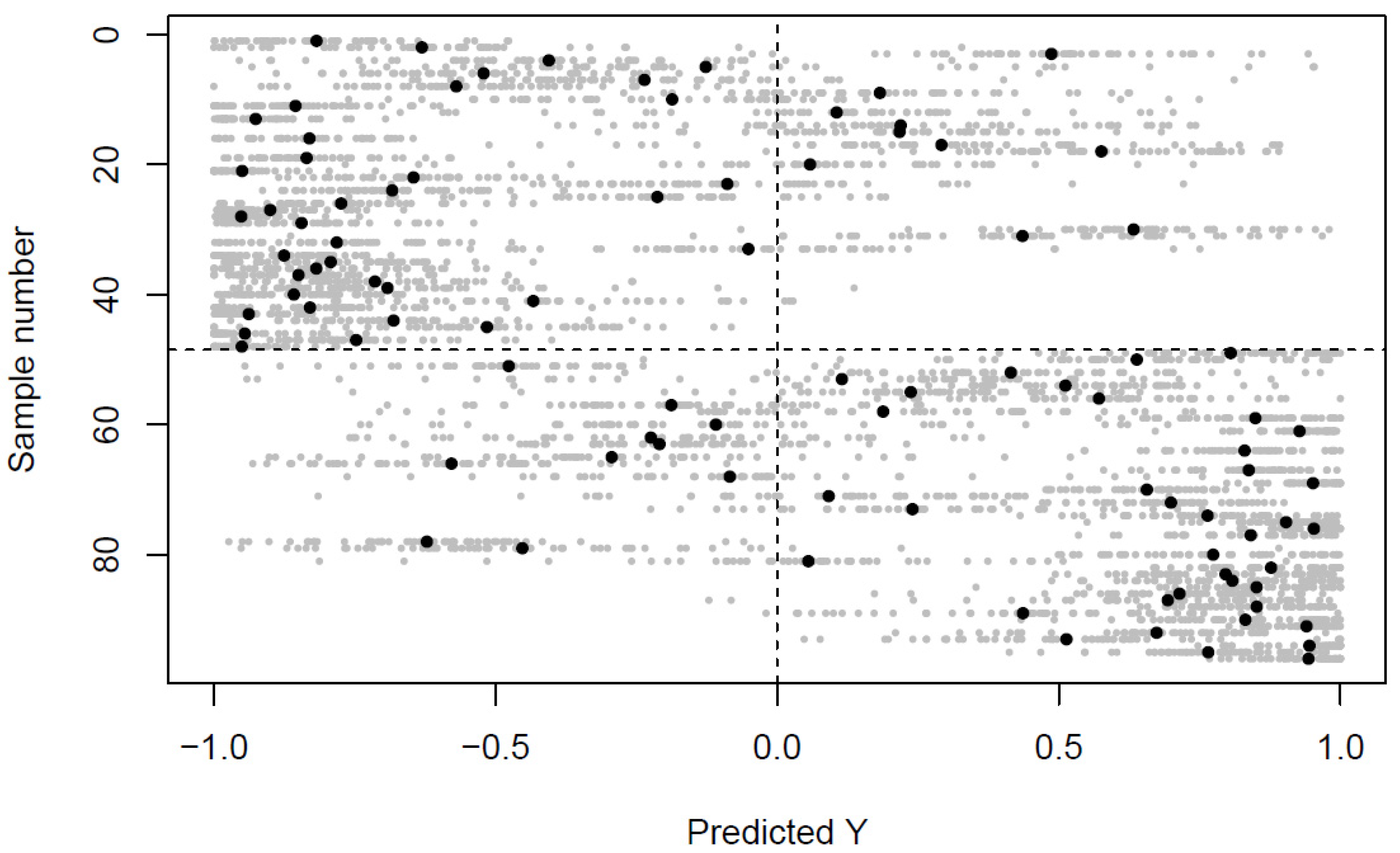
2.1. Differences in the Venous and Arterial Metabolome
2.2. Arterial and Venous Cord Plasma Metabolomes in Relation to Maternal and Infant Traits
2.3. Replication Analysis
3. Discussion
4. Materials and Methods
4.1. Study Subject and Sampling Protocol
4.2. Sample Management
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansson, T. Amino acid transporters in the human placenta. Pediatric Res. 2001, 49, 141. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Sommerfeld, E.; Penn, D.; Sodha, R.J.; Prögler, M.; Novak, M.; Schneider, H. Transfer and metabolism of carnitine and carnitine esters in the in vitro perfused human placenta. Pediatr. Res. 1985, 19, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Moe, A.; Ganapathy, V. Nutrient transport pathways across the epithelium of the placenta. Annu. Rev. Nutr. 1992, 12, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Armstrong, L.; Stenson, B.J. Use of umbilical cord blood gas analysis in the assessment of the newborn. Arch. Dis. Child.-Fetal Neonatal Ed. 2007, 92, F430–F434. [Google Scholar] [CrossRef]
- Walsh, B.H.; Broadhurst, D.I.; Mandal, R.; Wishart, D.S.; Boylan, G.B.; Kenny, L.C.; Murray, D.M. The metabolomic profile of umbilical cord blood in neonatal hypoxic ischaemic encephalopathy. PLoS ONE 2012, 7, e50520. [Google Scholar] [CrossRef]
- Favretto, D.; Cosmi, E.; Ragazzi, E.; Visentin, S.; Tucci, M.; Fais, P.; Cecchetto, G.; Zanardo, V.; Viel, G.; Ferrara, S.D. Cord blood metabolomic profiling in intrauterine growth restriction. Anal. Bioanal. Chem. 2012, 402, 1109–1121. [Google Scholar] [CrossRef]
- Halhali, A.; Tovar, A.R.; Torres, N.; Bourges, H.; Garabedian, M.; Larrea, F. Preeclampsia is associated with low circulating levels of insulin-like growth factor I and 1, 25-dihydroxyvitamin D in maternal and umbilical cord compartments. J. Clin. Endocrinol. Metab. 2000, 85, 1828–1833. [Google Scholar]
- La Torre, D.; Seppänen-Laakso, T.; Larsson, H.E.; Hyötyläinen, T.; Ivarsson, S.A.; Lernmark, Å.; Orešič, M.; Group, D.S. Decreased cord-blood phospholipids in young age–at–onset type 1 diabetes. Diabetes 2013, 62, 3951–3956. [Google Scholar] [CrossRef]
- Sanz-Cortés, M.; Carbajo, R.J.; Crispi, F.; Figueras, F.; Pineda-Lucena, A.; Gratacós, E. Metabolomic profile of umbilical cord blood plasma from early and late intrauterine growth restricted (IUGR) neonates with and without signs of brain vasodilation. PLoS ONE 2013, 8, e80121. [Google Scholar]
- Grosso, L.M.; Triche, E.W.; Belanger, K.; Benowitz, N.L.; Holford, T.R.; Bracken, M.B. Caffeine metabolites in umbilical cord blood, cytochrome P-450 1A2 activity, and intrauterine growth restriction. Am. J. Epidemiol. 2006, 163, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.K.; Chatterjee, N.; Ghosh, G. Umbilical cord blood TSH levels in term neonates: A screening tool for congenital hypothyroidism. Indian Pediatr 2005, 42, 1029–1032. [Google Scholar] [PubMed]
- Alexandre-Gouabau, M.-C.c.; Courant, F.d.r.; Moyon, T.; Küster, A.; Le Gall, G.; Tea, I.; Antignac, J.-P.; Darmaun, D. Maternal and cord blood LC-HRMS metabolomics reveal alterations in energy and polyamine metabolism, and oxidative stress in very-low birth weight infants. J. Proteome Res. 2013, 12, 2764–2778. [Google Scholar] [CrossRef] [PubMed]
- Holm, M.B.; Bastani, N.E.; Holme, A.M.; Zucknick, M.; Jansson, T.; Refsum, H.; Mørkrid, L.; Blomhoff, R.; Henriksen, T.; Michelsen, T.M. Uptake and release of amino acids in the fetal-placental unit in human pregnancies. PLoS ONE 2017, 12, e0185760. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.K.; Hume, R.; Eisenhofer, G.; Watson, J.; Williams, F.L. Arterio-venous differences in cord levels of catecholamines, glucose, lactate and blood gases. J. Perinat. Med. 2016, 44, 695–704. [Google Scholar] [CrossRef][Green Version]
- Hill, M.; Pařízek, A.; Kancheva, R.; Dušková, M.; Velíková, M.; Kříž, L.; Klímková, M.; Pašková, A.; Žižka, Z.; Matucha, P. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J. Steroid Biochem. Mol. Biol. 2010, 121, 594–610. [Google Scholar] [CrossRef]
- LaBarre, J.L.; Puttabyatappa, M.; Song, P.X.; Goodrich, J.M.; Zhou, L.; Rajendiran, T.M.; Soni, T.; Domino, S.E.; Treadwell, M.C.; Dolinoy, D.C. Maternal lipid levels across pregnancy impact the umbilical cord blood lipidome and infant birth weight. Sci. Rep. 2020, 10, 14209. [Google Scholar] [CrossRef]
- Kadakia, R.; Talbot, O.; Kuang, A.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B. Cord blood metabolomics: Association with newborn anthropometrics and c-peptide across ancestries. J. Clin. Endocrinol. Metab. 2019, 104, 4459–4472. [Google Scholar] [CrossRef]
- Ivorra, C.; García-Vicent, C.; Chaves, F.J.; Monleón, D.; Morales, J.M.; Lurbe, E. Metabolomic profiling in blood from umbilical cords of low birth weight newborns. J. Transl. Med. 2012, 10, 142. [Google Scholar] [CrossRef]
- Perng, W.; Rifas-Shiman, S.L.; McCulloch, S.; Chatzi, L.; Mantzoros, C.; Hivert, M.-F.; Oken, E. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism 2017, 76, 11–22. [Google Scholar] [CrossRef]
- Hellmuth, C.; Uhl, O.; Standl, M.; Demmelmair, H.; Heinrich, J.; Koletzko, B.; Thiering, E. Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obes. Facts 2017, 10, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Robinson, O.; Keski-Rahkonen, P.; Chatzi, L.; Kogevinas, M.; Nawrot, T.; Pizzi, C.; Plusquin, M.; Richiardi, L.; Robinot, N.; Sunyer, J. Cord blood metabolic signatures of birth weight: A population-based study. J. Proteome Res. 2018, 17, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. BioSyst. 2015, 11, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, F.; Nishiumi, S.; Miyake, O.; Takeichi, H.; Chitose, M.; Ohtsubo, H.; Ishimori, S.; Ninchoji, T.; Hashimura, Y.; Kaito, H. Metabolomics analysis of umbilical cord blood clarifies changes in saccharides associated with delivery method. Early Hum. Dev. 2013, 89, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Shokry, E.; Marchioro, L.; Uhl, O.; Bermúdez, M.G.; García-Santos, J.A.; Segura, M.T.; Campoy, C.; Koletzko, B. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: Results from the PREOBE cohort study. Acta Diabetol. 2019, 56, 421–430. [Google Scholar] [CrossRef]
- Lowe, W.L.; Bain, J.R.; Nodzenski, M.; Reisetter, A.C.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care 2017, 40, 902–910. [Google Scholar] [CrossRef]
- Ross, A.B.; Barman, M.; Hartvigsson, O.; Lundell, A.-C.; Savolainen, O.; Hesselmar, B.; Wold, A.E.; Sandberg, A.-S. Umbilical cord blood metabolome differs in relation to delivery mode, birth order and sex, maternal diet and possibly future allergy development in rural children. PLoS ONE 2021, 16, e0242978. [Google Scholar] [CrossRef]
- van Velzen, E.J.; Westerhuis, J.A.; van Duynhoven, J.P.; van Dorsten, F.A.; Hoefsloot, H.C.; Jacobs, D.M.; Smit, S.; Draijer, R.; Kroner, C.I.; Smilde, A.K. Multilevel data analysis of a crossover designed human nutritional intervention study. J. Proteome Res. 2008, 7, 4483–4491. [Google Scholar] [CrossRef]
- Shi, L.; Westerhuis, J.A.; Rosén, J.; Landberg, R.; Brunius, C. Variable selection and validation in multivariate modelling. Bioinformatics 2018, 35, 972–980. [Google Scholar] [CrossRef]
- Vahratian, A.; Hoffman, M.K.; Troendle, J.F.; Zhang, J. The impact of parity on course of labor in a contemporary population. Birth 2006, 33, 12–17. [Google Scholar] [CrossRef]
- Qiu, C.; Sorensen, T.K.; Luthy, D.A.; Williams, M.A. A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatr. Perinat. Epidemiol. 2004, 18, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Feuer, E.J.; Miller, B.A.; Freedma, L.S. Breast cancer clusters in the northeast United States: A geographic analysis. Am. J. Epidemiol. 1997, 146, 161–170. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.M.; Bower, C.; Knuiman, M.; Stanley, F.J. Changing risks of stillbirth and neonatal mortality associated with maternal age in Western Australia 1984–2003. Paediatr. Perinat. Epidemiol. 2007, 21, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Xu, X.; Peng, L.; Liu, J.; Guo, Y.; Huo, X. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ. Int. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Bai, J.; Wong, F.W.; Bauman, A.; Mohsin, M. Parity and pregnancy outcomes. Am. J. Obstet. Gynecol. 2002, 186, 274–278. [Google Scholar] [CrossRef]
- Li, W.; Ruan, W.; Lu, Z.; Wang, D. Parity and risk of maternal cardiovascular disease: A dose–response meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 2019, 26, 592–602. [Google Scholar] [CrossRef]
- Barclay, K.; Kolk, M. Parity and mortality: An examination of different explanatory mechanisms using data on biological and adoptive parents. Eur. J. Popul. 2019, 35, 63–85. [Google Scholar] [CrossRef]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-analytical factors that affect metabolite stability in human urine, plasma, and serum: A review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef]
- Barman, M.; Murray, F.; Bernardi, A.I.; Broberg, K.; Bölte, S.; Hesselmar, B.; Jacobsson, B.; Jonsson, K.; Kippler, M.; Rabe, H. Nutritional impact on Immunological maturation during Childhood in relation to the Environment (NICE): A prospective birth cohort in northern Sweden. BMJ Open 2018, 8, e022013. [Google Scholar] [CrossRef]
- Savolainen, O.I.; Sandberg, A.-S.; Ross, A.B. A simultaneous metabolic profiling and quantitative multimetabolite metabolomic method for human plasma using gas-chromatography tandem mass spectrometry. J. Proteome Res. 2016, 15, 259–265. [Google Scholar] [CrossRef]
- Jonsson, P.; Johansson, A.I.; Gullberg, J.; Trygg, J.; Grung, B.; Marklund, S.; Sjöström, M.; Antti, H.; Moritz, T. High-throughput data analysis for detecting and identifying differences between samples in GC/MS-based metabolomic analyses. Anal. Chem. 2005, 77, 5635–5642. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Brunius, C.; Shi, L.; Landberg, R. Large-scale untargeted LC-MS metabolomics data correction using between-batch feature alignment and cluster-based within-batch signal intensity drift correction. Metabolomics 2016, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
| All Samples (n = 48) | Nulliparous (n = 23) | Multiparous (n = 25) | |
|---|---|---|---|
| Gestational length (weeks + days) | 40 + 0 (39 + 2–40 + 6) | 40 + 1 (39 + 3–41 + 1) | 39 + 6 (39 + 2–40 + 3) |
| Birth weight (g) | 3510 (3310–3914) | 3515 (3330–3778) | 3505 (3310–3940) |
| Sex (N female) | 25 (52%) | 14 (61%) | 11 (44%) |
| Parity (N nulliparous) | 23 (48%) | 23 (100%) | 0 (0%) |
| Cesarean section (N yes) | 5 (10%) | 3 (13%) | 2 (8%) |
| Age of mother (years) | 28 (25–32) | 28 (25–30) | 29 (27–34) |
| Maternal BMI (kg/m2) | 23.5 (22–27.1) | 25.4 (23–28.9) | 22.8 (20.8–25.7) |
| Metabolites a | FC b | punadjusted | pFDR | Metabolite Category |
|---|---|---|---|---|
| Higher levels in arterial cord plasma (blood from the fetus) | ||||
| Hypoxanthine | 1.72 | <0.001 | <0.001 | Purine base |
| Hexose | 1.48 | <0.001 | <0.001 | Sugar |
| 177@1917 c | 1.46 | <0.001 | <0.001 | Unknown |
| Hydroxybutyric acid | 1.37 | <0.001 | <0.001 | Glutathione-/fatty acid metabolism |
| Deoxy-hexose | 1.36 | <0.001 | <0.001 | Deoxy-sugar |
| Deoxy-hexose | 1.28 | <0.001 | <0.001 | Deoxy-sugar |
| 72@1988 | 1.20 | <0.001 | <0.001 | Unknown |
| Isoerythritol | 1.17 | <0.001 | <0.001 | Sugar alcohol |
| Higher levels in venous cord plasma (blood from the mother) | ||||
| α-ketoglutaric acid | 0.71 | <0.001 | <0.001 | TCA-cycle |
| Succinic acid | 0.81 | <0.001 | <0.001 | TCA-cycle |
| Glutamic acid | 0.88 | <0.001 | <0.001 | Amino acid |
| Arterial | Venous | |
|---|---|---|
| Gestational length (Q2) | 0.11 | −0.05 |
| Birth weight (Q2) | −0.03 | −0.12 |
| Sex (CR a) | 56% | 46% |
| Parity (CR a) | 62% | 77% b |
| Age of mother (Q2) | −0.00 | −0.07 |
| Maternal BMI (Q2) | −0.22 | −0.10 |
| Metabolites a | FC b | punadjusted | pFDR | Metabolite Category |
|---|---|---|---|---|
| Higher levels in infants with nulliparous mothers | ||||
| 204@1879 c | 2.14 | <0.001 | <0.001 | Unknown |
| 89@1060 c | 1.98 | <0.001 | <0.001 | Unknown |
| Pyruvic acid | 1.95 | <0.001 | <0.001 | Glycolysis |
| Histidine | 1.88 | <0.001 | <0.001 | Amino acid |
| Malic acid | 1.78 | <0.001 | <0.001 | TCA-cycle |
| Glucuronic acid | 1.77 | 0.002 | 0.002 | Carbohydrate conjugate |
| 174@1877 c | 1.70 | <0.001 | <0.001 | Unknown |
| Sarcosine | 1.65 | <0.001 | <0.001 | Amino acid metabolism |
| Oxalic acid | 1.59 | <0.001 | <0.001 | TCA-cycle related |
| Isocitric acid | 1.48 | <0.001 | <0.001 | TCA-cycle |
| 52.05@1106 c | 1.28 | <0.001 | <0.001 | Unknown |
| Nicotinic acid | 1.26 | <0.001 | <0.001 | Vitamin B3 |
| 73@1861 c | 1.20 | 0.003 | 0.003 | Unknown |
| Higher levels in infants with multiparous mothers | ||||
| Aminobutyric acid | 0.84 | 0.13 | 0.13 | Amino acid metabolism |
| Venous | Arterial | |||
|---|---|---|---|---|
| Metabolite | FC a | pFDR | FC a | pFDR |
| N-acetyl mannosamine | 1.16 | 0.109 | 1.16 | 0.041 |
| Isocitric acid | 1.45 | <0.001 | 1.32 | 0.016 |
| Sorbitol | 1.39 | 0.109 | 1.69 | 0.029 |
| Malic acid | 1.79 | <0.001 | 1.22 | 0.060 |
| Lactulose | 1.47 | 0.020 | 1.37 | 0.029 |
| Citric acid | 1.85 | 0.039 | 1.89 | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartvigsson, O.; Barman, M.; Savolainen, O.; Ross, A.B.; Sandin, A.; Jacobsson, B.; Wold, A.E.; Sandberg, A.-S.; Brunius, C. Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity. Metabolites 2022, 12, 175. https://doi.org/10.3390/metabo12020175
Hartvigsson O, Barman M, Savolainen O, Ross AB, Sandin A, Jacobsson B, Wold AE, Sandberg A-S, Brunius C. Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity. Metabolites. 2022; 12(2):175. https://doi.org/10.3390/metabo12020175
Chicago/Turabian StyleHartvigsson, Olle, Malin Barman, Otto Savolainen, Alastair B. Ross, Anna Sandin, Bo Jacobsson, Agnes E. Wold, Ann-Sofie Sandberg, and Carl Brunius. 2022. "Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity" Metabolites 12, no. 2: 175. https://doi.org/10.3390/metabo12020175
APA StyleHartvigsson, O., Barman, M., Savolainen, O., Ross, A. B., Sandin, A., Jacobsson, B., Wold, A. E., Sandberg, A.-S., & Brunius, C. (2022). Differences between Arterial and Venous Umbilical Cord Plasma Metabolome and Association with Parity. Metabolites, 12(2), 175. https://doi.org/10.3390/metabo12020175







