Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds
Abstract
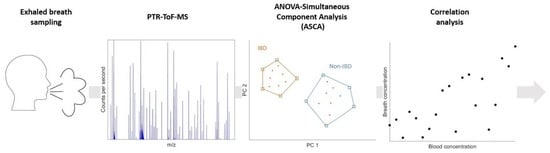
:1. Introduction
2. Results
2.1. Identification of Breath VOCs Affected by Prolonged Exercise and IBD Condition
2.2. Non-Invasive Assessment of Inflammation through Breath Analysis
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Exhaled Breath Sampling Protocol
4.3. PTR-ToF-MS Instrumental Set-Up
4.4. Blood Inflammation Markers
4.5. Data Processing
4.5.1. Spectral Processing
4.5.2. Compound Identification
4.6. Statistical Analysis
4.6.1. ANOVA Simultaneous Component Analysis (ASCA) for Identification of Significant Ions in the Breath VOC Profile
4.6.2. Statistical and Correlation Analysis of Significant VOCs with Plasma Cytokines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podolsky, D.K. Inflammatory Bowel Disease. N. Engl. J. Med. 2010, 325, 928–937. [Google Scholar] [CrossRef]
- Torres, J.; Ellul, P.; Langhorst, J.; Mikocka-Walus, A.; Barreiro-De Acosta, M.; Basnayake, C.; Ding, N.J.S.; Gilardi, D.; Katsanos, K.; Moser, G.; et al. European Crohn’s and Colitis Organisation Topical Review on Complementary Medicine and Psychotherapy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2019, 13, 673–685e. [Google Scholar] [CrossRef] [PubMed]
- Duff, W.; Haskey, N.; Potter, G.; Alcorn, J.; Hunter, P.; Fowler, S. Non-pharmacological therapies for inflammatory bowel disease: Recommendations for self-care and physician guidance. World J. Gastroenterol. 2018, 24, 3055. [Google Scholar] [CrossRef] [PubMed]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Engels, M.; Cross, R.K.; Long, M.D. Exercise in patients with inflammatory bowel diseases: Current perspectives. Clin. Exp. Gastroenterol. 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilski, J.; Brzozowski, B.; Mazur-Bialy, A.; Sliwowski, Z.; Brzozowski, T. The role of physical exercise in inflammatory bowel disease. BioMed Res. Int. 2014, 2014, 429031. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Terink, R.; Bongers, C.C.W.G.; Witkamp, R.F.; Mensink, M.; Eijsvogels, T.M.; Gunnewiek, J.M.T.K.; Hopman, M.T.E. Changes in cytokine levels after prolonged and repeated moderate intensity exercise in middle-aged men and women. Transl. Sport. Med. 2018, 1, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Lamers, C.R.; de Roos, N.M.; Bongers, C.C.W.G.; ten Haaf, D.S.M.; Hartman, Y.A.W.; Witteman, B.J.M.; Hopman, M.T.E. Repeated prolonged moderate-intensity walking exercise does not appear to have harmful effects on inflammatory markers in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 2021, 56, 30–37. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise modifies the gut microbiota with positive health effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Smolinska, A.; Tedjo, D.I.; Blanchet, L.; Bodelier, A.; Pierik, M.J.; Masclee, A.A.M.; Dallinga, J.; Savelkoul, P.H.M.; Jonkers, D.M.A.E.; Penders, J.; et al. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal. Chim. Acta 2018, 1025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Kęsy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Davis, C.; Pleil, J. (Eds.) Breathborne Biomarkers and the Human Volatilome; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Vernia, F.; Valvano, M.; Fabiani, S.; Stefanelli, G.; Longo, S.; Viscido, A.; Latella, G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers 2021, 13, 2361. [Google Scholar] [CrossRef] [PubMed]
- Bodelier, A.G.L.; Smolinska, A.; Baranska, A.; Dallinga, J.W.; Mujagic, Z.; Vanhees, K.; Van Den Heuvel, T.; Masclee, A.A.M.; Jonkers, D.; Pierik, M.J.; et al. Volatile Organic Compounds in Exhaled Air as Novel Marker for Disease Activity in Crohn’s Disease: A Metabolomic Approach. Inflamm. Bowel Dis. 2015, 21, 1776–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, L.C.; Huang, J.; Kumar, S.; Powles, S.T.; Orchard, T.R.; Hanna, G.B.; Williams, H.R.T. Analysis of Exhaled Breath Volatile Organic Compounds in Inflammatory Bowel Disease: A Pilot Study. J. Crohn’s Colitis 2015, 9, 731–737. [Google Scholar] [CrossRef] [Green Version]
- Monasta, L.; Pierobon, C.; Princivalle, A.; Martelossi, S.; Marcuzzi, A.; Pasini, F.; Perbellini, L. Inflammatory bowel disease and patterns of volatile organic compounds in the exhaled breath of children: A case-control study using Ion Molecule Reaction-Mass Spectrometry. PLoS ONE 2017, 12, e0184118. [Google Scholar] [CrossRef]
- Tiele, A.; Wicaksono, A.; Kansara, J.; Arasaradnam, R.P.; Covington, J.A. Breath Analysis Using eNose and Ion Mobility Technology to Diagnose Inflammatory Bowel Disease—A Pilot Study. Biosensors 2019, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Venegas, D.P.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingsgaard, H.; Hauselmann, I.; Schuler, B.; Habib, A.M.; Baggio, L.L.; Meier, D.T.; Eppler, E.; Bouzakri, K.; Wueest, S.; Muller, Y.D.; et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011, 17, 1481–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, B.; Lopes Batista, G.; Bertinetto, C.G.; Meurs, J.; Materić, D.; Bongers, C.C.W.G.; Allard, N.A.E.; Eijsvogels, T.M.H.; Holzinger, R.; Harren, F.J.M.; et al. Exhaled Breath Reflects Prolonged Exercise and Statin Use during a Field Campaign. Metabolites 2021, 11, 192. [Google Scholar] [CrossRef]
- Samudrala, D.; Geurts, B.; Brown, P.A.; Szymańska, E.; Mandon, J.; Jansen, J.; Buydens, L.; Harren, F.J.M.; Cristescu, S.M. Changes in urine headspace composition as an effect of strenuous walking. Metabolomics 2015, 11, 1656–1666. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.H.; Pomare, E.W.; Branch, H.W.J.; Naylor, C.P.E.; MacFarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef] [Green Version]
- Steeghs, M.M.L.; Cristescu, S.M.; Harren, F.J.M. The suitability of Tedlar bags for breath sampling in medical diagnostic research. Physiol. Meas. 2007, 28, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Ellis, A.M.; Mayhew, C.A. Proton Transfer Reaction Mass Spectrometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Holzinger, R. PTRwid: A new widget tool for processing PTR-TOF-MS data. Atmos. Meas. Tech. 2015, 8, 3903–3922. [Google Scholar] [CrossRef] [Green Version]
- Holzinger, R.; Joe Acton, W.F.; Bloss, W.W.; Breitenlechner, M.; Crilley, L.L.; Dusanter, S.; Gonin, M.; Gros, V.; Keutsch, F.F.; Kiendler-Scharr, A.; et al. Validity and limitations of simple reaction kinetics to calculate concentrations of organic compounds from ion counts in PTR-MS. Atmos. Meas. Tech. 2019, 12, 6193–6208. [Google Scholar] [CrossRef] [Green Version]
- Herbig, J.; Müller, M.; Schallhart, S.; Titzmann, T.; Graus, M.; Hansel, A. On-line breath analysis with PTR-TOF. J. Breath Res. 2009, 3, 027004. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the use of Tedlar® bags for breath-gas sampling and analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Smilde, A.K.; Jansen, J.J.; Hoefsloot, H.C.J.; Lamers, R.J.A.N.; van der Greef, J.; Timmerman, M.E. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef]
- Thiel, M.; Féraud, B.; Govaerts, B. ASCA+ and APCA+: Extensions of ASCA and APCA in the analysis of unbalanced multifactorial designs. J. Chemom. 2017, 31, e2895. [Google Scholar] [CrossRef]
- Nueda, M.J.; Conesa, A.; Westerhuis, J.A.; Hoefsloot, H.C.J.; Smilde, A.K.; Talón, M.; Ferrer, A. Discovering gene expression patterns in time course microarray experiments by ANOVA-SCA. Bioinformatics 2007, 23, 1792–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulley, S.B.; Cummings, S.R.; Browner, W.S.; Grady, D.; Newman, T.B. Designing Clinical Research: An Epidemiologic Approach, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]



| m/z | Product Ion Formula | Assigned Compound |
|---|---|---|
| 33.03 | CH4OH+ | Methanol |
| 45.03 | C2H4OH+ | Acetaldehyde |
| 47.05 | C2H6OH+ | Ethanol |
| 59.05 | C3H6OH+ | Acetone |
| 61.03 (43.02, 79.05) | C2H4O2H+ (C2H2OH+, C2H6O3H+) | Acetic acid |
| 63.03 | C2H6SH+ | Dimethyl sulfide |
| 69.07 (41.04) | C5H8H+ (C3H4H+) | Isoprene |
| (71.05) | C4H6OH+ | Butanoic acid |
| 75.04 (57.04, 93.05) | C3H6O2H+ (C3H4OH+, C3H8O3H+) | Propanoic acid |
| Characteristic | IBD (n = 18) | Non-IBD (n = 19) |
|---|---|---|
| Age (years) | 54 ± 11 a | 54 ± 14 a |
| Gender (F/M) | 11/7 | 11/8 |
| Walking distance per day (30/40/50 km) | 5/11/2 | 2/13/4 |
| Body mass index (kg/m2) | 25.7 ± 3.8 a | 26.0 ± 4.5 a |
| IBD medication use (yes/no) | 11/7 | - |
| Smoking status (active smoker/non-smoker/ex-smoker) | 1/9/8 | 0/13/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, B.; Meurs, J.; Lamers, C.R.; Batista, G.L.; Materić, D.; Bertinetto, C.G.; Bongers, C.C.W.G.; Holzinger, R.; Harren, F.J.M.; Jansen, J.J.; et al. Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds. Metabolites 2022, 12, 224. https://doi.org/10.3390/metabo12030224
Henderson B, Meurs J, Lamers CR, Batista GL, Materić D, Bertinetto CG, Bongers CCWG, Holzinger R, Harren FJM, Jansen JJ, et al. Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds. Metabolites. 2022; 12(3):224. https://doi.org/10.3390/metabo12030224
Chicago/Turabian StyleHenderson, Ben, Joris Meurs, Carlijn R. Lamers, Guilherme Lopes Batista, Dušan Materić, Carlo G. Bertinetto, Coen C. W. G. Bongers, Rupert Holzinger, Frans J. M. Harren, Jeroen J. Jansen, and et al. 2022. "Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds" Metabolites 12, no. 3: 224. https://doi.org/10.3390/metabo12030224
APA StyleHenderson, B., Meurs, J., Lamers, C. R., Batista, G. L., Materić, D., Bertinetto, C. G., Bongers, C. C. W. G., Holzinger, R., Harren, F. J. M., Jansen, J. J., Hopman, M. T. E., & Cristescu, S. M. (2022). Non-Invasive Monitoring of Inflammation in Inflammatory Bowel Disease Patients during Prolonged Exercise via Exhaled Breath Volatile Organic Compounds. Metabolites, 12(3), 224. https://doi.org/10.3390/metabo12030224