Taurine: A Maternally Derived Nutrient Linking Mother and Offspring
Abstract
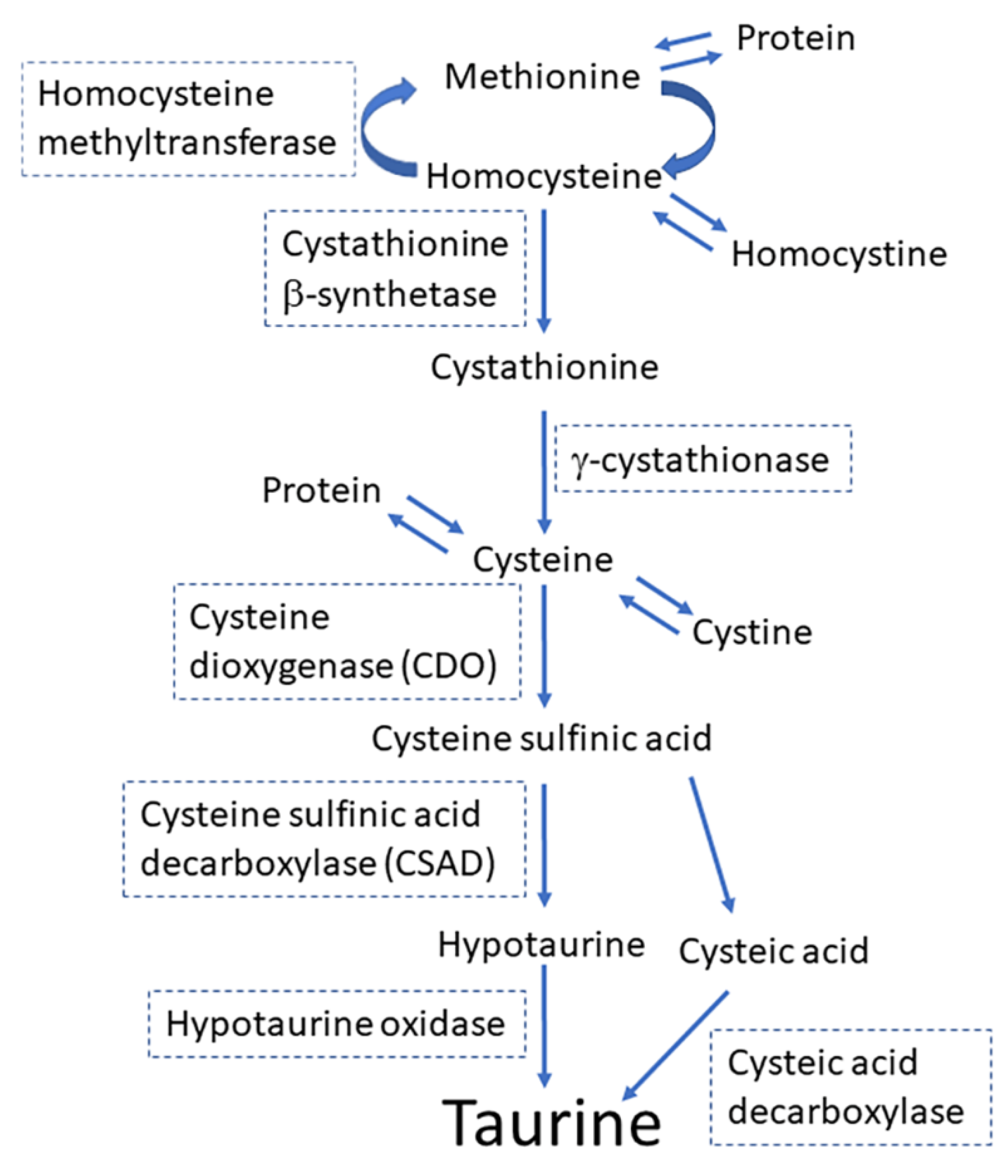
1. Introduction
2. Transport of Taurine from Mothers to Offspring
2.1. Cloning of a Taurine Transporter and Its Function
2.2. Mechanisms Regulating the Activity of the Taurine Transporter
2.3. Transfer of Taurine to the Fetus via the Placenta
2.3.1. Machinery for Taurine Transport in the Placenta
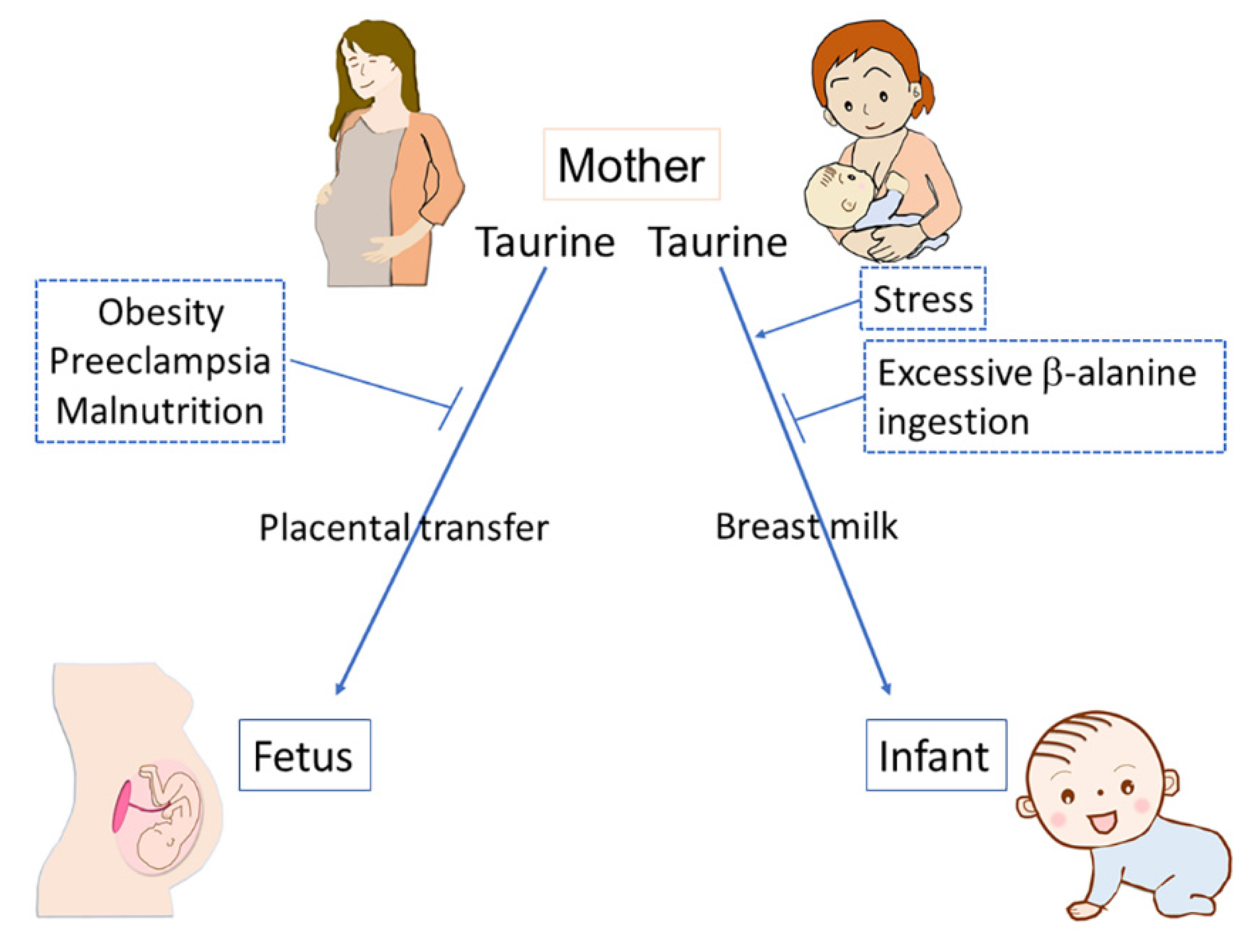
2.3.2. Environmental Factors Affecting Placental Taurine Transport
2.4. Transfer of Taurine to Offspring during Postnatal Care
2.4.1. Transfer of Taurine to Offspring via Breast Milk
2.4.2. Taurine in Infant Formula
2.4.3. Environmental Factors Affecting Taurine Transfer during Postnatal Period via Breast Milk
3. Biological Functions of Taurine during Development
3.1. Developmental Outcomes of Taurine-Depletion
3.2. Possible Biological Mechanisms Underlying the Function of Taurine during Development
3.2.1. Taurine as Agonist for Receptors
3.2.2. Other Mechanisms Related to Various Physiological Functions of Taurine during Development
3.2.3. Function of Taurine as a Factor to Shape the Gut Microbiota
3.3. Possible Influence of Limited Levels of Taurine during Development on Disease Risk in Adults
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Broad, K.D.; Curley, J.P.; Keverne, E.B. Mother-infant bonding and the evolution of mammalian social relationships. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 2199–2214. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Nagasawa, M.; Kikusui, T. Developmental consequences and biological significance of mother-infant bonding. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Daglar, G.; Nur, N. Level of mother-baby bonding and influencing factors during pregnancy and postpartum period. Psychiatr Danub. 2018, 30, 433–440. [Google Scholar] [CrossRef]
- Wu, J.Y.; Prentice, H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010, 17 (Suppl. S1), S1. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S. Functions of Maternally-Derived Taurine in Fetal and Neonatal Brain Development. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 17–25. [Google Scholar] [CrossRef]
- Linne, M.L.; Jalonen, T.O.; Saransaari, P.; Oja, S.S. Taurine-induced single-channel currents in cultured rat cerebellar granule cells. Adv. Exp. Med. Biol. 1996, 403, 455–462. [Google Scholar] [PubMed]
- Ye, G.; Tse, A.C.; Yung, W. Taurine inhibits rat substantia nigra pars reticulata neurons by activation of GABA- and glycine-linked chloride conductance. Brain Res. 1997, 749, 175–179. [Google Scholar] [CrossRef]
- Sturman, J.A.; Hayes, K.C. The Biology of Taurine in Nutrition and Development. Adv. Nutr. Res. 1980, 231–299. [Google Scholar] [CrossRef]
- Lambert, I.H.; Kristensen, D.M.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine--from organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A.; Rassin, D.K.; Gaull, G.E. Taurine in development. Life Sci. 1977, 21, 1–22. [Google Scholar] [CrossRef]
- Sturman, J.A. Origin of taurine in developing rat brain. Brain Res. 1981, 254, 111–128. [Google Scholar] [CrossRef]
- Hayes, K.C.; Carey, R.E.; Schmidt, S.Y. Retinal degeneration associated with taurine deficiency in the cat. Science 1975, 188, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A.; Moretz, R.C.; French, J.H.; Wisniewski, H.M. Taurine deficiency in the developing cat: Persistence of the cerebellar external granule cell layer. J. Neurosci. Res. 1985, 13, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Kulanthaivel, P.; Cool, D.R.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Transport of taurine and its regulation by protein kinase C in the JAR human placental choriocarcinoma cell line. Biochem. J. 1991, 277 Pt 1, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Shennan, D.B.; McNeillie, S.A.; Curran, D.E. Stimulation of taurine efflux from human placental tissue by a hypoosmotic challenge. Exp. Physiol. 1993, 78, 843–846. [Google Scholar] [CrossRef]
- Uchida, S.; Kwon, H.M.; Yamauchi, A.; Preston, A.S.; Marumo, F.; Handler, J.S. Molecular cloning of the cDNA for an MDCK cell Na(+)- and Cl(-)-dependent taurine transporter that is regulated by hypertonicity. Proc. Natl. Acad. Sci. USA 1992, 89, 8230–8234. [Google Scholar] [CrossRef]
- Liu, Q.R.; Lopez-Corcuera, B.; Nelson, H.; Mandiyan, S.; Nelson, N. Cloning and expression of a cDNA encoding the transporter of taurine and beta-alanine in mouse brain. Proc. Natl. Acad. Sci. USA 1992, 89, 12145–12149. [Google Scholar] [CrossRef]
- Smith, K.E.; Borden, L.A.; Wang, C.H.; Hartig, P.R.; Branchek, T.A.; Weinshank, R.L. Cloning and expression of a high affinity taurine transporter from rat brain. Mol. Pharm. 1992, 42, 563–569. [Google Scholar]
- Ramamoorthy, S.; Leibach, F.H.; Mahesh, V.B.; Han, H.; Yang-Feng, T.; Blakely, R.D.; Ganapathy, V. Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem. J. 1994, 300 Pt 3, 893–900. [Google Scholar] [CrossRef]
- Jayanthi, L.D.; Ramamoorthy, S.; Mahesh, V.B.; Leibach, F.H.; Ganapathy, V. Substrate-specific regulation of the taurine transporter in human placental choriocarcinoma cells (JAR). Biochim. Biophys. Acta 1995, 1235, 351–360. [Google Scholar] [CrossRef]
- Satsu, H.; Watanabe, H.; Arai, S.; Shimizu, M. Characterization and regulation of taurine transport in Caco-2, human intestinal cells. J. Biochem. 1997, 121, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Ola, M.S.; Prasad, P.D.; El-Sherbeny, A.; Ganapathy, V.; Smith, S.B. Regulation of taurine transporter expression by NO in cultured human retinal pigment epithelial cells. Am. J. Physiol. Cell Physiol. 2001, 281, C1825–C1836. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chesney, R.W. Regulation of taurine transporter gene (TauT) by WT1. FEBS Lett. 2003, 540, 71–76. [Google Scholar] [CrossRef]
- Takasaki, M.; Satsu, H.; Shimizu, M. Physiological significance of the taurine transporter and taurine biosynthetic enzymes in 3T3-L1 adipocytes. Biofactors 2004, 21, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, E.; Pop-Busui, R.; Towns, R.; Thomas, T.P.; Hosaka, Y.; Nakamura, J.; Greene, D.A.; Killen, P.D.; Schroeder, J.; Larkin, D.D.; et al. Regulation of the human taurine transporter by oxidative stress in retinal pigment epithelial cells stably transformed to overexpress aldose reductase. Antioxid. Redox Signal. 2005, 7, 1530–1542. [Google Scholar] [CrossRef]
- Uozumi, Y.; Ito, T.; Hoshino, Y.; Mohri, T.; Maeda, M.; Takahashi, K.; Fujio, Y.; Azuma, J. Myogenic differentiation induces taurine transporter in association with taurine-mediated cytoprotection in skeletal muscles. Biochem. J. 2006, 394, 699–706. [Google Scholar] [CrossRef]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: Implications for pathogenesis of diabetic neuropathy. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E620–E628. [Google Scholar] [CrossRef]
- Lambert, I.H.; Hansen, D.B. Regulation of taurine transport systems by protein kinase CK2 in mammalian cells. Cell. Physiol. Biochem. 2011, 28, 1099–1110. [Google Scholar] [CrossRef]
- Loo, D.D.F.; Hirsch, J.R.; Sarkar, H.K.; Wright, E.M. Regulation of the mouse retinal taurine transporter (TAUT) by protein kinases in Xenopus oocytes. FEBS Lett. 1996, 392, 250–254. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kang, Y.S. Regulation of taurine transport at the blood-placental barrier by calcium ion, PKC activator and oxidative stress conditions. J. Biomed. Sci. 2010, 17 (Suppl. S1). [Google Scholar] [CrossRef]
- Kang, Y.S.; Ohtsuki, S.; Takanaga, H.; Tomi, M.; Hosoya, K.; Terasaki, T. Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-alpha, taurine and hypertonicity. J. Neurochem. 2002, 83, 1188–1195. [Google Scholar] [CrossRef]
- Mochizuki, T.; Satsu, H.; Nakano, T.; Shimizu, M. Regulation of the human taurine transporter by TNF-alpha and an anti-inflammatory function of taurine in human intestinal Caco-2 cells. Biofactors 2004, 21, 141–144. [Google Scholar] [CrossRef]
- Mochizuki, T.; Satsu, H.; Shimizu, M. Signaling pathways involved in tumor necrosis factor alpha-induced upregulation of the taurine transporter in Caco-2 cells. FEBS Lett. 2005, 579, 3069–3074. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Manabe, M.; Shimizu, M. Activation of Ca2+/calmodulin-dependent protein kinase II is involved in hyperosmotic induction of the human taurine transporter. FEBS Lett. 2004, 569, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.B.; Friis, M.B.; Hoffmann, E.K.; Lambert, I.H. Downregulation of the taurine transporter TauT during hypo-osmotic stress in NIH3T3 mouse fibroblasts. J. Membr. Biol. 2012, 245, 77–87. [Google Scholar] [CrossRef][Green Version]
- Park, S.H.; Lee, H.; Park, T. Cortisol and IGF-1 synergistically up-regulate taurine transport by the rat skeletal muscle cell line, L6. Biofactors 2004, 21, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A.; Rassin, D.K.; Gaull, G.E. Taurine in developing rat brain: Maternal-fetal transfer of [35S] taurine and its fate in the neonate. J. Neurochem. 1977, 28, 31–39. [Google Scholar] [CrossRef]
- Desforges, M.; Parsons, L.; Westwood, M.; Sibley, C.P.; Greenwood, S.L. Taurine transport in human placental trophoblast is important for regulation of cell differentiation and survival. Cell Death Dis. 2013, 4, e559. [Google Scholar] [CrossRef]
- Hibbard, J.U.; Pridjian, G.; Whitington, P.F.; Moawad, A.H. Taurine transport in the in vitro perfused human placenta. Pediatric Res. 1990, 27, 80–84. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Balkovetz, D.F.; Leibach, F.H.; Mahesh, V.B.; Ganapathy, V. Na+ + Cl− -gradient-driven, high-affinity, uphill transport of taurine in human placental brush-border membrane vesicles. FEBS Lett. 1988, 231, 263–267. [Google Scholar] [CrossRef]
- Lager, S.; Powell, T.L. Regulation of nutrient transport across the placenta. J. Pregnancy 2012, 2012, 179827. [Google Scholar] [CrossRef] [PubMed]
- Norberg, S.; Powell, T.L.; Jansson, T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatric Res. 1998, 44, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kusinski, L.C.; Jones, C.J.; Baker, P.N.; Sibley, C.P.; Glazier, J.D. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta 2010, 31, 53–59. [Google Scholar] [CrossRef]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Haussinger, D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2005, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Powell, T.L.; Jansson, T. Human placental taurine transporter in uncomplicated and IUGR pregnancies: Cellular localization, protein expression, and regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R886–R893. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, A.M.; Desforges, M.; Mills, T.A.; Glazier, J.D.; Wareing, M.; Mynett, K.; Sibley, C.P.; Greenwood, S.L. Maternal obesity is associated with a reduction in placental taurine transporter activity. Int. J. Obes. 2015, 39, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Ditchfield, A.; Hirst, C.R.; Pegorie, C.; Martyn-Smith, K.; Sibley, C.P.; Greenwood, S.L. Reduced placental taurine transporter (TauT) activity in pregnancies complicated by pre-eclampsia and maternal obesity. Adv. Exp. Med. Biol. 2013, 776, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, J.V.; Rosario, F.J.; Nijland, M.J.; McDonald, T.J.; Wu, G.; Kanai, Y.; Powell, T.L.; Nathanielsz, P.W.; Jansson, T. Down-regulation of placental mTOR, insulin/IGF-I signaling, and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J. 2014, 28, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Gemmel, M.; Powers, R.W. Nitric oxide signaling in pregnancy and preeclampsia. Nitric Oxide 2020, 95, 55–62. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Mudd, L.M. Preeclampsia and diabetes. Curr. Diab. Rep. 2015, 15, 9. [Google Scholar] [CrossRef]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Wang, G.; Hong, X.; Mallow, E.B.; Walker, S.O.; Pearson, C.; Heffner, L.; Zuckerman, B.; Wang, X. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am. J. Obstet. Gynecol. 2013, 209, 438.e1–438.e12. [Google Scholar] [CrossRef]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The role of maternal obesity in the risk of neuropsychiatric disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Malchow, B.; Hasan, A.; Falkai, P. The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 2014, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- WHO. Breastfeeding Policy Brief. 2014. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.7 (accessed on 1 March 2022).
- Sturman, J.A.; Rassin, D.K.; Gaull, G.E. Taurine in developing rat brain: Transfer of [35S] taurine to pups via the milk. Pediatric Res. 1977, 11, 28–33. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.; Wang, Z.; Liu, Y.; Yu, Y.; Gao, X. Taurine Promotes Milk Synthesis via the GPR87-PI3K-SETD1A Signaling in BMECs. J. Agric. Food Chem. 2019, 67, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Boutinaud, M.; Guinard-Flament, J.; HélèneJammes. The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef]
- Jonas, W.; Woodside, B. Physiological mechanisms, behavioral and psychological factors influencing the transfer of milk from mothers to their young. Horm. Behav. 2016, 77, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Aleman, G.; Lopez, A.; Ordaz, G.; Torres, N.; Tovar, A.R. Changes in messenger RNA abundance of amino acid transporters in rat mammary gland during pregnancy, lactation, and weaning. Metabolism 2009, 58, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, D.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol. Genom. 2007, 28, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ueki, I.; Stipanuk, M.H. Enzymes of the taurine biosynthetic pathway are expressed in rat mammary gland. J. Nutr. 2007, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Ikemura, R.; Chang, K.T.; Suzuki, M.; Nishihara, M.; Takahashi, M. Expression of cysteine sulfinate decarboxylase mRNA in rat mammary gland. J. Vet. Med. Sci. 2000, 62, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.M.; Rho, J.Y.; Suzuki, M.; Nishihara, M.; Takahashi, M. Effect of taurine in rat milk on the growth of offspring. J. Vet. Med. Sci. 2000, 62, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Gaull, G.; Sturman, J.A.; Raiha, N.C. Development of mammalian sulfur metabolism: Absence of cystathionase in human fetal tissues. Pediatric Res. 1972, 6, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, S.H.; Anderson, G.H. The development of cystathionase activity during the first year of life. Pediatric Res. 1982, 16, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W.; Helms, R.A.; Christensen, M.; Budreau, A.M.; Han, X.; Sturman, J.A. The role of taurine in infant nutrition. Adv. Exp. Med. Biol. 1998, 442, 463–476. [Google Scholar] [CrossRef]
- Ferreira, I.M. Quantification of non-protein nitrogen components of infant formulae and follow-up milks: Comparison with cows’ and human milk. Br. J. Nutr. 2003, 90, 127–133. [Google Scholar] [CrossRef]
- Almeida, C.C.; Mendonca Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive Compounds in Infant Formula and Their Effects on Infant Nutrition and Health: A Systematic Literature Review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef] [PubMed]
- Nishigawa, T.; Nagamachi, S.; Chowdhury, V.S.; Yasuo, S.; Furuse, M. Taurine and beta-alanine intraperitoneal injection in lactating mice modifies the growth and behavior of offspring. Biochem. Biophys. Res. Commun. 2018, 495, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Nishigawa, T.; Nagamachi, S.; Ikeda, H.; Chowdhury, V.S.; Furuse, M. Restraint stress in lactating mice alters the levels of sulfur-containing amino acids in milk. J. Vet. Med. Sci. 2018, 80, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A.; Gargano, A.D.; Messing, J.M.; Imaki, H. Feline maternal taurine deficiency: Effect on mother and offspring. J. Nutr. 1986, 116, 655–667. [Google Scholar] [CrossRef]
- Neuringer, M.; Sturman, J. Visual acuity loss in rhesus monkey infants fed a taurine-free human infant formula. J. Neurosci. Res. 1987, 18, 597–601. [Google Scholar] [CrossRef]
- Hayes, K.C.; Stephan, Z.F.; Sturman, J.A. Growth depression in taurine-depleted infant monkeys. J. Nutr. 1980, 110, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Heller-Stilb, B.; van Roeyen, C.; Rascher, K.; Hartwig, H.G.; Huth, A.; Seeliger, M.W.; Warskulat, U.; Haussinger, D. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2002, 16, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kimura, Y.; Uozumi, Y.; Takai, M.; Muraoka, S.; Matsuda, T.; Ueki, K.; Yoshiyama, M.; Ikawa, M.; Okabe, M.; et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J. Mol. Cell. Cardiol. 2008, 44, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, U.; Flogel, U.; Jacoby, C.; Hartwig, H.G.; Thewissen, M.; Merx, M.W.; Molojavyi, A.; Heller-Stilb, B.; Schrader, J.; Haussinger, D. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004, 18, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Oishi, S.; Takai, M.; Kimura, Y.; Uozumi, Y.; Fujio, Y.; Schaffer, S.W.; Azuma, J. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J. Biomed. Sci. 2010, 17 (Suppl. S1), S20. [Google Scholar] [CrossRef]
- Ito, T.; Yoshikawa, N.; Inui, T.; Miyazaki, N.; Schaffer, S.W.; Azuma, J. Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS ONE 2014, 9, e107409. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Diaz, P.; Miranda-Contreras, L.; Mendoza-Briceno, R.V.; Pena-Contreras, Z.; Palacios-Pru, E. Prenatal and postnatal contents of amino acid neurotransmitters in mouse parietal cortex. Dev. Neurosci. 2003, 25, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, R.J. Taurine in the central nervous system and the mammalian actions of taurine. Prog. Neurobiol. 1989, 32, 471–533. [Google Scholar] [CrossRef]
- Kilb, W.; Fukuda, A. Taurine as an Essential Neuromodulator during Perinatal Cortical Development. Front. Cell. Neurosci. 2017, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Kletke, O.; Gisselmann, G.; May, A.; Hatt, H.; Sergeeva, O.A. Partial agonism of taurine at gamma-containing native and recombinant GABAA receptors. PLoS ONE 2013, 8, e61733. [Google Scholar] [CrossRef] [PubMed]
- Kontro, P.; Oja, S.S. Interactions of taurine with GABAB binding sites in mouse brain. Neuropharmacology 1990, 29, 243–247. [Google Scholar] [CrossRef]
- Smith, S.S.; Li, J. GABAB receptor stimulation by baclofen and taurine enhances excitatory amino acid induced phosphatidylinositol turnover in neonatal rat cerebellum. Neurosci. Lett. 1991, 132, 59–64. [Google Scholar] [CrossRef]
- Gotz, M.; Huttner, W.B. The cell biology of neurogenesis. Nat. Reviews. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Okano, H.; Temple, S. Cell types to order: Temporal specification of CNS stem cells. Curr. Opin. Neurobiol. 2009, 19, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Kawaguchi, D.; Kawaguchi, A.; Gotoh, Y. Mechanisms that regulate the number of neurons during mouse neocortical development. Curr. Opin. Neurobiol. 2010, 20, 22–28. [Google Scholar] [CrossRef]
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.; Davis, M.B.; Kriegstein, A.R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef]
- Tochitani, S.; Sakata-Haga, H.; Fukui, Y. Embryonic exposure to ethanol disturbs regulation of mitotic spindle orientation via GABA(A) receptors in neural progenitors in ventricular zone of developing neocortex. Neurosci. Lett. 2010, 472, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Furukawa, T.; Bando, R.; Kondo, S.; Ito, T.; Matsushima, Y.; Kojima, T.; Matsuzaki, H.; Fukuda, A. GABAA Receptors and Maternally Derived Taurine Regulate the Temporal Specification of Progenitors of Excitatory Glutamatergic Neurons in the Mouse Developing Cortex. Cereb. Cortex 2021, 31, 4554–4575. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Kondo, S. Immunoreactivity for GABA, GAD65, GAD67 and Bestrophin-1 in the meninges and the choroid plexus: Implications for non-neuronal sources for GABA in the developing mouse brain. PLoS ONE 2013, 8, e56901. [Google Scholar] [CrossRef] [PubMed]
- Palackal, T.; Moretz, R.; Wisniewski, H.; Sturman, J. Abnormal visual cortex development in the kitten associated with maternal dietary taurine deprivation. J. Neurosci. Res. 1986, 15, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Behar, T.N.; Smith, S.V.; Kennedy, R.T.; McKenzie, J.M.; Maric, I.; Barker, J.L. GABA(B) receptors mediate motility signals for migrating embryonic cortical cells. Cereb. Cortex 2001, 11, 744–753. [Google Scholar] [CrossRef]
- Furukawa, T.; Yamada, J.; Akita, T.; Matsushima, Y.; Yanagawa, Y.; Fukuda, A. Roles of taurine-mediated tonic GABAA receptor activation in the radial migration of neurons in the fetal mouse cerebral cortex. Front. Cell. Neurosci. 2014, 8, 88. [Google Scholar] [CrossRef]
- Nimmervoll, B.; Denter, D.G.; Sava, I.; Kilb, W.; Luhmann, H.J. Glycine receptors influence radial migration in the embryonic mouse neocortex. Neuroreport 2011, 22, 509–513. [Google Scholar] [CrossRef]
- Kilb, W.; Ikeda, M.; Uchida, K.; Okabe, A.; Fukuda, A.; Luhmann, H.J. Depolarizing glycine responses in Cajal-Retzius cells of neonatal rat cerebral cortex. Neuroscience 2002, 112, 299–307. [Google Scholar] [CrossRef]
- Kirmse, K.; Dvorzhak, A.; Henneberger, C.; Grantyn, R.; Kirischuk, S. Cajal Retzius cells in the mouse neocortex receive two types of pre- and postsynaptically distinct GABAergic inputs. J. Physiol. 2007, 585, 881–895. [Google Scholar] [CrossRef]
- Qian, T.; Chen, R.; Nakamura, M.; Furukawa, T.; Kumada, T.; Akita, T.; Kilb, W.; Luhmann, H.J.; Nakahara, D.; Fukuda, A. Activity-dependent endogenous taurine release facilitates excitatory neurotransmission in the neocortical marginal zone of neonatal rats. Front. Cell. Neurosci. 2014, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.C.; Liu, X.; Kriegstein, A.R. Nonsynaptic Glycine Receptor Activation during Early Neocortical Development. Neuron 1998, 20, 43–53. [Google Scholar] [CrossRef]
- Lehtinen, M.K.; Walsh, C.A. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011, 27, 653–679. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Zappaterra, M.W.; Chen, X.; Yang, Y.J.; Hill, A.D.; Lun, M.; Maynard, T.; Gonzalez, D.; Kim, S.; Ye, P.; et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011, 69, 893–905. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Chang, K.J. Taurine protects the liver against lipid peroxidation and membrane disintegration during rat hepatocarcinogenesis. Adv. Exp. Med. Biol. 1998, 442, 105–112. [Google Scholar] [CrossRef]
- Lambert, I.H. Regulation of the cellular content of the organic osmolyte taurine in mammalian cells. Neurochem. Res. 2004, 29, 27–63. [Google Scholar] [CrossRef]
- El Idrissi, A. Taurine increases mitochondrial buffering of calcium: Role in neuroprotection. Amino Acids 2008, 34, 321–328. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, T.; Wada, T.; Saigo, K.; Watanabe, K. Taurine as a constituent of mitochondrial tRNAs: New insights into the functions of taurine and human mitochondrial diseases. EMBO J. 2002, 21, 6581–6589. [Google Scholar] [CrossRef]
- Asano, K.; Suzuki, T.; Saito, A.; Wei, F.Y.; Ikeuchi, Y.; Numata, T.; Tanaka, R.; Yamane, Y.; Yamamoto, T.; Goto, T.; et al. Metabolic and chemical regulation of tRNA modification associated with taurine deficiency and human disease. Nucleic. Acids Res. 2018, 46, 1565–1583. [Google Scholar] [CrossRef]
- Fakruddin, M.; Wei, F.Y.; Suzuki, T.; Asano, K.; Kaieda, T.; Omori, A.; Izumi, R.; Fujimura, A.; Kaitsuka, T.; Miyata, K.; et al. Defective Mitochondrial tRNA Taurine Modification Activates Global Proteostress and Leads to Mitochondrial Disease. Cell Rep. 2018, 22, 482–496. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.W.; Azuma, J.; Mozaffari, M. Role of antioxidant activity of taurine in diabetes. Can. J. Physiol. Pharmacol. 2009, 87, 91–99. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; D’Angelantonio, M.; D’Erme, M.; Pecci, L.; Fontana, M. The Interaction of Hypotaurine and Other Sulfinates with Reactive Oxygen and Nitrogen Species: A Survey of Reaction Mechanisms. Adv. Exp. Med. Biol. 2017, 975 Pt 1, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Ohmori, H.; Maeda, S. Taurine supplementation attenuates delayed increase in exercise-induced arterial stiffness. Appl. Physiol. Nutr. Metab. 2016, 41, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Taranukhin, A.G.; Taranukhina, E.Y.; Saransaari, P.; Djatchkova, I.M.; Pelto-Huikko, M.; Oja, S.S. Taurine reduces caspase-8 and caspase-9 expression induced by ischemia in the mouse hypothalamic nuclei. Amino Acids 2008, 34, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Ra, S.G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supplementation on delayed onset muscle soreness and muscle damage in high-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. [Google Scholar] [CrossRef]
- Tochitani, S. Vertical transmission of gut microbiota: Points of action of environmental factors influencing brain development. Neurosci. Res. 2021, 168, 83–94. [Google Scholar] [CrossRef]
- Ratsika, A.; Codagnone, M.C.; O’Mahony, S.; Stanton, C.; Cryan, J.F. Priming for Life: Early Life Nutrition and the Microbiota-Gut-Brain Axis. Nutrients 2021, 13, 426. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Ikeno, T.; Ito, T.; Sakurai, A.; Yamauchi, T.; Matsuzaki, H. Administration of Non-Absorbable Antibiotics to Pregnant Mice to Perturb the Maternal Gut Microbiota Is Associated with Alterations in Offspring Behavior. PLoS ONE 2016, 11, e0138293. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid. Res. 2010, 51, 226–246. [Google Scholar] [CrossRef]
- Selwyn, F.P.; Csanaky, I.L.; Zhang, Y.; Klaassen, C.D. Importance of Large Intestine in Regulating Bile Acids and Glucagon-Like Peptide-1 in Germ-Free Mice. Drug Metab. Dispos. 2015, 43, 1544–1556. [Google Scholar] [CrossRef]
- Barnum, C.J.; Pace, T.W.; Hu, F.; Neigh, G.N.; Tansey, M.G. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J. Neuroinflammation 2012, 9, 9. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sasaki, S.I.; Toyoda, A.; Wei, F.Y.; Shirai, M.; Morishita, Y.; Ikegami, T.; Tomizawa, K.; Honda, A. Impaired bile acid metabolism with defectives of mitochondrial-tRNA taurine modification and bile acid taurine conjugation in the taurine depleted cats. Sci. Rep. 2020, 10, 4915. [Google Scholar] [CrossRef]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627. [Google Scholar] [CrossRef]
- Collard, J.M.; Sansonetti, P.; Papon, N. Taurine Makes Our Microbiota Stronger. Trends Endocrinol. Metab. 2021, 32, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in Infancy and Death from Ischaemic Heart Disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Masztalerz-Kozubek, D.; Zielinska-Pukos, M.A.; Hamulka, J. Maternal Diet, Nutritional Status, and Birth-Related Factors Influencing Offspring’s Bone Mineral Density: A Narrative Review of Observational, Cohort, and Randomized Controlled Trials. Nutrients 2021, 13, 2302. [Google Scholar] [CrossRef]
- Armengaud, J.B.; Yzydorczyk, C.; Siddeek, B.; Peyter, A.C.; Simeoni, U. Intrauterine growth restriction: Clinical consequences on health and disease at adulthood. Reprod. Toxicol. 2021, 99, 168–176. [Google Scholar] [CrossRef]
- Nam, H.K.; Lee, K.H. Small for gestational age and obesity: Epidemiology and general risks. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 9–13. [Google Scholar] [CrossRef]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS ONE 2019, 14, e0218437. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tochitani, S. Taurine: A Maternally Derived Nutrient Linking Mother and Offspring. Metabolites 2022, 12, 228. https://doi.org/10.3390/metabo12030228
Tochitani S. Taurine: A Maternally Derived Nutrient Linking Mother and Offspring. Metabolites. 2022; 12(3):228. https://doi.org/10.3390/metabo12030228
Chicago/Turabian StyleTochitani, Shiro. 2022. "Taurine: A Maternally Derived Nutrient Linking Mother and Offspring" Metabolites 12, no. 3: 228. https://doi.org/10.3390/metabo12030228
APA StyleTochitani, S. (2022). Taurine: A Maternally Derived Nutrient Linking Mother and Offspring. Metabolites, 12(3), 228. https://doi.org/10.3390/metabo12030228





