Relationship between Urinary Calcium Excretion and Lower Urinary Tract Symptoms
Abstract
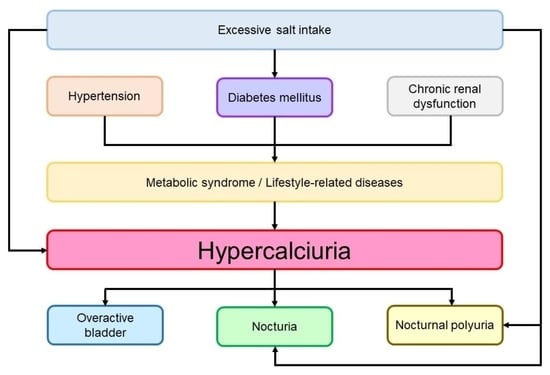
:1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Relationship between Urinary Calcium Excretion and LUTS
2.3. Relationship between Urinary Calcium and Frequency Volume Chart
2.4. Relationship between Urinary Calcium Excretion and Severity of LUTS
2.5. Predictive Marker for OAB Using Univariate and Multivariate Analysis
2.6. Relationship between Urinary Calcium Excretion and Lower Urinary Symptoms Based on Propensity Score Matching
2.7. Relationship between the Presence Rate of Various Urinary Parameters and Salt Intake and Urinary Calcium Excretion
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Evaluation of Vital Signs
4.3. Evaluation of Daily Salt Intake and Urinary Calcium Volume
4.4. Evaluation of LUTS
4.5. Propensity Score Matching
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parekh, D.J.; Pope JC, I.V.; Adams, M.C.; Brock, J.W. The Role of Hypercalciuria in a Subgroup of Dysfunctional Voiding Syndromes of Childhood. J. Urol. 2000, 164, 1008–1010. [Google Scholar] [CrossRef]
- Yousefichaijan, P.; Rafiei, M.; Aziminejad, A.; Pakniyat, A. The Prevalence of Hypercalciuria in Girl Kids with over Active Bladder. J. Ren. Inj. Prev. 2015, 4, 117–119. [Google Scholar] [PubMed]
- Raes, A.; Dossche, L.; Hertegonne, N.; Nuytemans, L.; Hoebeke, P.; Van Laecke, E.; Donckerwolcke, R.; Walle, J.V. Hypercalciuria Is Related to Osmolar Excretion in Children with Nocturnal Enuresis. J. Urol. 2010, 183, 297–301. [Google Scholar] [CrossRef]
- Young, E.W.; Morris, C.D.; McCarron, D.A. Urinary Calcium Excretion in Essential Hypertension. J. Lab. Clin. Med. 1992, 120, 624–632. [Google Scholar] [PubMed]
- Matkovic, V.; Ilich, J.Z.; Andon, M.B.; Hsieh, L.C.; Tzagournis, M.A.; Lagger, B.J.; Goel, P.K. Urinary Calcium, Sodium, and Bone Mass of Young Females. Am. J. Clin. Nutr. 1995, 62, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zeng, Y.; Tao, L.; Liu, S.; Ni, Z.; Huang, Q.; Wang, Q. Meta-analysis of hypertension and osteoporotic fracture risk in women and men. Osteoporos. Int. 2017, 28, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; Meilahn, E.; Zmuda, J.M.; Cauley, J.A. High blood pressure and bone-mineral loss in elderly white women: A prospective study. Lancet 1999, 354, 971–975. [Google Scholar] [CrossRef]
- Tamma, G.; Di Mise, A.; Ranieri, M.; Svelto, M.; Pisot, R.; Bilancio, G.; Cavallo, P.; De Santo, N.G.; Cirillo, M.; Valenti, G. A Decrease in Aquaporin 2 Excretion Is Associated with Bed Rest Induced High Calciuria. J. Transl. Med. 2014, 12, 133. [Google Scholar] [CrossRef] [Green Version]
- Torimoto, K.; Uchimura, N.; Roitmann, E.; Marumoto, M.; Hirakata, T.; Burtea, T. A large survey of nocturia related to sleep quality and daytime quality of life in a young Japanese population: NOCTURNE study. Neurourol. Urodyn. 2021, 40, 340–347. [Google Scholar] [CrossRef]
- Akil, I.; Kavukçu, S.; Inan, S.; Yilmaz, O.; Atilla, P.; Işlekel, H.; Neşe, N.; Müftüoğlu, S. Evaluation of Histologic Changes in the Urinary Tract of Hypercalciuric Rats. Pediatr. Nephrol. 2006, 21, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Laera, A.; Gouraud, S.; Pace, G.; Aceto, G.; Penza, R.; Selvaggi, F.P.; Svelto, M. Low-Calcium Diet in Hypercalciuric Enuretic Children Restores AQP2 Excretion and Improves Clinical Symptoms. Am. J. Physiol. Renal. Physiol. 2002, 283, F895–F903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staplen, F.B. The Southwest Pediatric Nephrology Study Group Idiopathic Hypercalciuria: Association with Isolated Hematuria and Risk for Urolithiasis in Children. Kidney Int. 1990, 37, 807–811. [Google Scholar]
- Matsushita, K.; Tanikawa, K. Significance of the Calcium to Creatinine Concentration Ratio of a Single-Voided Urine Specimen in Patients with Hypercalciuric Urolithiasis. Tokai J. Exp. Clin. Med. 1987, 12, 167–171. [Google Scholar] [PubMed]
- Sebert, J.L.; Cornaille, G.; San Juan, M.P.; Kha, T.D.; Blotman, F.; Simon, L. Fasting Urinary Excretion of Calcium, Phosphorus and Hydroxyproline in Relation to Creatinine. Normal Values and Correlations with 24-Hour Urine Values (Author’s Transl). Nouv. Presse Med. 1981, 10, 2799–2801. [Google Scholar]
- Gökçe, Ç.; Gökçe, Ö.; Baydinç, C.; Īlhan, N.; Alaşehirli, E.; Özküçük, F.; Taşçi, M.; Atikeler, M.K.; Çelebi, H.; Arslan, N.; et al. Use of Random Urine Samples to Estimate Total Urinary Calcium and Phosphate Excretion. Arch. Intern. Med. 1991, 151, 1587–1588. [Google Scholar] [CrossRef]
- Kaneko, K.; Tsuchiya, K.; Kawamura, R.; Ohtomo, Y.; Shimizu, T.; Yamashiro, Y.; Yamada, T.; Yamauchi, K.; Kitagawa, T. Low Prevalence of Hypercalciuria in Japanese Children. Nephron 2002, 91, 439–443. [Google Scholar] [CrossRef]
- Gül, A.; Özer, S.; Yılmaz, R.; Sönmezgöz, E.; Kasap, T.; Takçı, Ş.; Karaaslan, E.; Önder, Y.; Çıtıl, R.; Bütün, İ.; et al. Prevalence of Hypercalciuria and Urinary Calcium Excretion in School-aged Children in the Province of Tokat. Turk. Arch. Pediatrics 2016, 51, 193–197. [Google Scholar] [CrossRef]
- Esteghamati, M.; Ghasemi, K.; Nami, M.N.M. Prevalence of Idiopathic Hypercalciuria in Children with Urinary System Related Symptoms Attending a Pediatric Hospital in Bandar Abbas in 2014. Electron. Physician 2017, 9, 5261–5264. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, L.; Zhang, W.; Putnis, C.V. Role of Hyperoxaluria/Hypercalciuria in Controlling the Hydrate Phase Selection of Pathological Calcium Oxalate Mineralization. Cryst. Growth Des. 2021, 21, 683–691. [Google Scholar] [CrossRef]
- Rossi, M.; Barone, B.; Di Domenico, D.; Esposito, R.; Fabozzi, A.; D’errico, G.; Prezioso, D. Correlation between ion composition of oligomineral water and calcium oxalate crystal formation. Crystals 2021, 11, 1507. [Google Scholar] [CrossRef]
- Vachvanichsanong, P.; Malagon, M.; Moore, E.S. Urinary Incontinence Due to Idiopathic Hypercalciuria in Children. J. Urol. 1994, 152, 1226–1228. [Google Scholar] [CrossRef]
- Ishikawa, S.E.; Schrier, R.W. Pathophysiological Roles of Arginine Vasopressin and aquaporin-2 in Impaired Water Excretion. Clin. Endocrinol. 2003, 58, 1–17. [Google Scholar] [CrossRef] [PubMed]
- McCarron, D.A.; Pingree, P.A.; Rubin, R.J.; Gaucher, S.M.; Molitch, M.; Krutzik, S. Enhanced Parathyroid Function in Essential Hypertension: A Homeostatic Response to a Urinary Calcium Leak. Hypertension 1980, 2, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, T.; Miyata, Y.; Sakai, H. Daily Salt Intake Is an Independent Risk Factor for Pollakiuria and Nocturia. Int. J. Urol. 2017, 24, 384–389. [Google Scholar] [CrossRef] [Green Version]
- Pace, G.; Aceto, G.; Cormio, L.; Traficante, A.; Tempesta, A.; Lospalluti, M.L.; Selvaggi, F.P.; Penza, R. Nocturnal Enuresis Can Be Caused by Absorptive Hypercalciuria. Scand. J. Urol. Nephrol. 1999, 33, 111–114. [Google Scholar]
- Mayan, H.; Hourvitz, A.; Schiff, E.; Farfel, Z. Symptomatic Hypocalcaemia in Hypermagnesaemia-Induced Hypoparathyroidism, During Magnesium Tocolytic Therapy—Possible Involvement of the Calcium-Sensing Receptor. Nephrol. Dial. Transplant. 1999, 14, 1764–1766. [Google Scholar] [CrossRef] [Green Version]
- Bonny, O.; Rubin, A.; Huang, C.L.; Frawley, W.H.; Pak, C.Y.C.; Moe, O.W. Mechanism of Urinary Calcium Regulation by Urinary Magnesium and pH. J. Am. Soc. Nephrol. 2008, 19, 1530–1537. [Google Scholar] [CrossRef] [Green Version]
- Kirby, M.G.; Wagg, A.; Cardozo, L.; Chapple, C.; Castro-Diaz, D.; De Ridder, D.; Espuna-Pons, M.; Haab, F.; Kelleher, C.; Kölbl, H.; et al. Overactive Bladder: Is There a Link to the Metabolic Syndrome in Men? Neurourol. Urodyn. 2010, 29, 1360–1364. [Google Scholar] [CrossRef]
- Hall, T.J.; Schaueblin, M. Hydrochlorothiazide Inhibits Osteoclastic Bone Resorption In Vitro. Calcif. Tissue Int. 1994, 55, 266–268. [Google Scholar] [CrossRef]
- DeLellis, R.A.; Xia, L. Paraneoplastic Endocrine Syndromes: A Review. Endocr. Pathol. 2003, 14, 303–317. [Google Scholar] [CrossRef]
- Bailey, R.L.; Dodd, K.W.; Goldman, J.A.; Gahche, J.J.; Dwyer, J.T.; Moshfegh, A.J.; Sempos, C.T.; Picciano, M.F. Estimation of total usual calcium and vitamin D intakes in the United States. J. Nutr. 2010, 140, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French Agency for Food, Environmental and Occupational Health & Safety. Levels and Sources of Intake. Available online: https://www.anses.fr/en/content/calcium (accessed on 2 September 2019).
- Ministry of Health, Labour and Welfare. National health and Nutrition Survey. 2014. Available online: http://www.mhlw.go.jp/bunya/kenkou=/eiyou/dl/h26-houkoku.pdf (accessed on 2 September 2019).
- Ministry of Health, Labour and Welfare. Overview of Dietary Reference Intakes for Japanese. 2015. Available online: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf (accessed on 2 September 2019).
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary calcium intake and risk of stroke: A dose-response meta-analysis. Am. J. Clin. Nutr. 2013, 97, 951–957. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y.; et al. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Kogure, M.; Tsuchiya, N.; Narita, A.; Hirata, T.; Nakaya, N.; Nakamura, T.; Hozawa, A.; Hayakawa, T.; Okuda, N.; Miyagawa, N.; et al. of Daily Living in a Japanese Population: NIPPON DATA90. J. Epidemiol. 2021, 31, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; van Kerrebroeck, P.; Victor, A.; Wein, A.; Standardisation Sub-committee of the International Continence Society. The Standardisation of Terminology of Lower Urinary Tract Function: Report from the Standardisation Sub-Committee of the International Continence Society. Neurourol. Urodyn. 2002, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Yamaguchi, O.; Kageyama, S.; Nishizawa, O.; Yoshida, M.; Kawabe, K. Nocturia in the adult: Classification on the basis of largest voided volume and nocturnal urine production. J. Urol. 2000, 163, 777–781. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A Simple Method to Estimate Populational 24-h Urinary Sodium and Potassium Excretion Using a Casual Urine Specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Japanese Society of Hypertension Guidelines Subcommittee for the Management of Hypertension. Guidelines for the Management of Hypertension for General Practitioners. Hypertens Res. 2001, 24, 613–634. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, A.; Niimi, M.; Akaza, H.; Takeshima, H.; Otani, M.; Noguchi, R.; Ishikawa, S.; Ohashi, Y. Assessment of Reproducibility and Unidimensionality of International Prostate Symptom Score Japanese Version. Nihon Hinyokika Gakkai Zasshi. Jpn. J. Urol. 1997, 88, 1013–1020. [Google Scholar]
- Homma, Y.; Yoshida, M.; Seki, N.; Yokoyama, O.; Kakizaki, H.; Gotoh, M.; Yamanishi, T.; Yamaguchi, O.; Takeda, M.; Nishizawa, O. Symptom Assessment Tool for Overactive Bladder Syndrome--Overactive Bladder Symptom Score. Urology 2006, 68, 318–323. [Google Scholar] [CrossRef]
- Matsuo, T.; Miyata, Y.; Sakai, H. Effect of salt intake reduction on nocturia in patients with excessive salt intake. Neurourol. Urodyn. 2019, 38, 927–933. [Google Scholar] [CrossRef] [PubMed]


| Entire n = 512 | Non-Hypercalciuria n = 379 | Hypercalciuria n = 133 | p-Value | |
|---|---|---|---|---|
| Gender (male/female) | 241/271 | 192/187 | 49/84 | 0.006 |
| Age (years) | 63.9 ± 14.8 | 62.0 ± 15.3 | 69.5 ± 11.6 | <0.001 |
| Body mass index (Kg/m2) | 22.4 ± 10.1 | 22.9 ± 11.1 | 21.0 ± 6.4 | 0.016 |
| Urinary calcium excretion volume (urinary Ca/Cr) | 0.16 ± 0.12 | 0.10 ± 0.05 | 0.32 ± 0.11 | <0.001 |
| Estimated daily salt intake volume (g/day) | 8.9 ± 2.5 | 8.4 ± 2.2 | 10.2 ± 2.7 | <0.001 |
| Overactive bladder (%) | 139 (27.2) | 80 (21.1) | 59 (44.4) | <0.001 |
| Hypertension (%) | 180 (35.2) | 121 (31.9) | 59 (44.4) | 0.011 |
| Diabetes mellitus (%) | 47 (9.2) | 40 (10.6) | 7 (5.3) | 0.081 |
| Renal dysfunction (%) | 82 (16.0) | 60 (15.8) | 22 (16.5) | 0.891 |
| Hyperlipidemia (%) | 58 (11.3) | 42 (11.1) | 16 (12.0) | 0.752 |
| Osteoporosis (%) | 47 (9.2) | 34 (9.0) | 13 (9.2) | 0.862 |
| Entire n = 512 | Non-Hypercalciuria n = 379 | Hypercalciuria n = 133 | p-Value | |
|---|---|---|---|---|
| OABSS | ||||
| Q1 (daytime frequency) | 0.5 ± 0.6 | 0.4 ± 0.6 | 0.7 ± 0.7 | <0.001 |
| Q2 (nighttime frequency) | 1.5 ± 1.0 | 1.3 ± 1.0 | 2.1 ± 0.8 | <0.001 |
| Q3 (urgency) | 0.9 ± 1.2 | 0.7 ± 1.2 | 1.5 ± 1.2 | <0.001 |
| Q4 (urgency incontinence) | 0.3 ± 0.9 | 0.3 ± 0.8 | 0.5 ± 0.9 | <0.001 |
| Total score | 3.2 ± 2.9 | 2.7 ± 2.8 | 4.7 ± 2.8 | <0.001 |
| IPSS | ||||
| Q1 (incomplete emptying) | 1.4 ± 1.5 | 1.4 ± 1.6 | 1.3 ± 1.1 | 0.901 |
| Q2 (frequency) | 1.0 ± 1.2 | 0.8 ± 1.0 | 1.4 ± 1.5 | <0.001 |
| Q3 (intermittency) | 0.9 ± 1.2 | 0.9 ± 1.2 | 0.9 ± 1.3 | 0.834 |
| Q4 (urgency) | 0.9 ± 1.0 | 0.8 ± 1.0 | 1.2 ± 1.1 | <0.001 |
| Q5 (weak stream) | 1.7 ± 1.3 | 1.7 ± 1.3 | 1.6 ± 1.1 | 0.684 |
| Q6 (straining) | 1.3 ± 1.5 | 1.3 ± 1.5 | 1.4 ± 1.4 | 0.195 |
| Q7 (nighttime frequency) | 1.7 ± 1.1 | 1.5 ± 1.1 | 2.2 ± 1.2 | <0.001 |
| Storage symptoms (Q2 + Q4 + Q7) | 3.6 ± 2.4 | 3.1 ± 2.1 | 4.8 ± 2.6 | <0.001 |
| Voiding symptoms (Q1 + Q3 + Q5 + Q6) | 5.3 ± 3.7 | 5.3 ± 3.8 | 5.2 ± 3.2 | 0.722 |
| Total score | 8.8 ± 4.7 | 8.4 ± 4.7 | 10.0 ± 4.5 | <0.001 |
| QOL score | 3.7 ± 1.4 | 3.7 ± 1.4 | 3.7 ± 1.2 | 0.638 |
| Frequency volume chart | ||||
| Number of daytime frequency | 7.5 ± 2.3 | 7.3 ± 2.3 | 8.0 ± 2.2 | <0.001 |
| Number of nighttime frequency | 1.7 ± 1.3 | 1.4 ± 1.2 | 2.5 ± 1.3 | <0.001 |
| Nocturia ≥2 (%) | 274 (53.5) | 173 (45.6) | 101 (75.9) | <0.001 |
| 24-h urine volume (mL) | 2136.0 ± 561.5 | 2086.2 ± 568.2 | 2278.0 ± 518.1 | <0.001 |
| Diurnal urine volume (mL) | 1662.8 ± 437.6 | 1658.2 ± 450.4 | 1675.9 ± 400.1 | 0.460 |
| Nocturnal urine volume (mL) | 473.2 ± 230.5 | 428.0 ± 209.2 | 602.0 ± 240.4 | <0.001 |
| Nocturnal polyuria index (%) | 21.7 ± 8.0 | 20.1 ± 7.3 | 26.2 ± 8.1 | <0.001 |
| Presence of noctornal polyuria (%) | 162 (31.6) | 87 (23.0) | 75 (56.4) | <0.001 |
| Voided volume (mL) | 240.5 ± 38.3 | 246.7 ± 37.5 | 222.7 ± 34.8 | <0.001 |
| r | p-Value | |
|---|---|---|
| OABSS | ||
| Q1 (daytime frequency) | 0.232 | <0.001 |
| Q2 (nighttime frequency) | 0.317 | <0.001 |
| Q3 (urgency) | 0.302 | <0.001 |
| Q4 (urgency incontinence) | 0.193 | <0.001 |
| Total score | 0.347 | <0.001 |
| Number of daytime frequency | 0.144 | 0.001 |
| Number of nighttime frequency | 0.324 | <0.001 |
| 24-h urine volume (mL) | 0.185 | <0.001 |
| Diurnal urine volume (mL) | 0.028 | 0.533 |
| Nocturnal urine volume (mL) | 0.399 | <0.001 |
| Nocturnal polyuria index (%) | 0.407 | <0.001 |
| Voided volume (mL) | −0.180 | <0.001 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| For overactive bladder | |||||||
| Gender: male | 0.51 | 0.34–0.76 | <0.001 | 0.55 | 0.35–0.86 | 0.009 | |
| Age | 1.04 | 1.02–1.05 | <0.001 | 1.02 | 1.01–1.03 | 0.004 | |
| Body mass index | 1.00 | 0.98–1.02 | 0.656 | - | - | - | |
| Estimated daily salt intake volume | 1.03 | 0.96–1.12 | 0.387 | - | - | - | |
| Hypercalciuria: presence | 2.98 | 1.95–4.55 | <0.001 | 2.61 | 1.66–4.11 | <0.001 | |
| Hypertension: presence | 1.89 | 1.27–2.82 | 0.002 | 1.57 | 1.44–1.67 | 0.032 | |
| Diabetes mellitus: presence | 1.95 | 1.04–3.60 | 0.038 | 1.82 | 0.28–1.08 | 0.082 | |
| Renal dysfunction: presence | 2.48 | 1.52–4.05 | <0.001 | 2.04 | 1.17–3.54 | 0.014 | |
| Hyperlipidemia: presence | 1.24 | 0.67–2.21 | 0.480 | - | - | - | |
| Osteoporosis: presence | 2.15 | 1.15–3.97 | 0.017 | 1.42 | 0.71–2.83 | 0.324 | |
| For nocturia (≥2) | |||||||
| Gender: male | 0.75 | 0.50–1.13 | 0.171 | - | - | - | |
| Age | 1.00 | 0.99–1.03 | 0.965 | - | - | - | |
| Body mass index | 0.99 | 0.98–1.02 | 0.630 | - | - | - | |
| Estimated daily salt intake volume | 1.48 | 1.34–1.65 | <0.001 | 1.48 | 1.33–1.66 | <0.001 | |
| Hypercalciuria: presence | 3.87 | 2.52–5.97 | <0.001 | 2.16 | 1.33–3.5 | 0.002 | |
| Hypertension: presence | 1.99 | 1.32–2.99 | <0.001 | 2.91 | 1.81–4.73 | <0.001 | |
| Diabetes mellitus: presence | 5.45 | 1.94–22.8 | <0.001 | 7.00 | 2.23–31.5 | <0.001 | |
| Renal dysfunction: presence | 1.05 | 0.62–1.86 | 0.854 | - | - | - | |
| Hyperlipidemia: presence | 1.50 | 0.78–3.13 | 0.248 | - | - | - | |
| Osteoporosis: presence | 2.03 | 0.94–5.07 | 0.094 | - | - | - | |
| For nocturnal polyuria (>10 mL/kg body) | |||||||
| Gender: male | 0.83 | 0.57–1.28 | 0.336 | - | - | - | |
| Age | 1.03 | 1.01–1.05 | <0.001 | 1.02 | 1.01–1.06 | <0.001 | |
| Body mass index | 1.01 | 0.99–1.05 | 0.292 | - | - | - | |
| Estimated daily salt intake volume | 1.47 | 1.34–1.63 | <0.001 | 1.41 | 1.28–1.58 | <0.001 | |
| Hypercalciuria: presence | 4.34 | 2.87–6.62 | <0.001 | 2.73 | 1.69–441 | <0.001 | |
| Hypertension: presence | 1.37 | 0.93–2.01 | 0.111 | - | - | - | |
| Diabetes mellitus: presence | 2.41 | 1.16–5.68 | 0.017 | 1.87 | 0.82–4.78 | 0.141 | |
| Renal dysfunction: presence | 1.07 | 0.65–1.80 | 0.806 | - | - | - | |
| Hyperlipidemia: presence | 2.51 | 1.11–4.43 | 0.022 | 1.89 | 0.92–4.19 | 0.085 | |
| Osteoporosis: presence | 1.38 | 0.73–2.55 | 0.310 | - | - | - | |
| Non-Hypercalciuria n = 100 | Hypercalciuria n = 100 | p Value | Standardized Mean Differences | |
|---|---|---|---|---|
| Gender (male/female) | 36/64 | 43/57 | 0.386 | 0.085 |
| Age (years) | 68.2 ± 12.6 | 68.6 ± 12.6 | 0.801 | 0.036 |
| Body mass index (Kg/m2) | 21.6 ± 5.2 | 21.6 ± 5.2 | 0.958 | 0.015 |
| Estimated daily salt intake volume (g/day) | 9.5 ± 1.9 | 9.5 ± 2.0 | 0.951 | 0.009 |
| Hypertension (%) | 43 (43.0) | 42 (42.0) | 1.000 | 0.020 |
| Diabetes mellitus (%) | 9 (9.0) | 7 (7.0) | 0.795 | 0.074 |
| Renal dysfunction (%) | 20 (20.0) | 16 (16.0) | 0.581 | 0.094 |
| Hyperlipidemia (%) | 15 (15.0) | 13 (13.0) | 0.839 | 0.058 |
| Osteoporosis (%) | 14 (14.0) | 9 (9.0) | 0.376 | 0.008 |
| 24-h urine volume (mL) | 2255.4 ± 639.0 | 2246 ± 520.1 | 0.913 | 0.015 |
| Non-Hypercalciuria n = 100 | Hypercalciuria n = 100 | p Value | |
|---|---|---|---|
| OAB (%) | 19 (19.0) | 42 (42.0) | 0.001 |
| Nocturia ≥2 (%) | 55 (55.0) | 45 (45.0) | 0.028 |
| Nocturnal polyuria >10 mL/kg body (%) | 37 (37.0) | 52 (52.0) | 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, T.; Ito, H.; Mitsunari, K.; Ohba, K.; Miyata, Y. Relationship between Urinary Calcium Excretion and Lower Urinary Tract Symptoms. Metabolites 2022, 12, 229. https://doi.org/10.3390/metabo12030229
Matsuo T, Ito H, Mitsunari K, Ohba K, Miyata Y. Relationship between Urinary Calcium Excretion and Lower Urinary Tract Symptoms. Metabolites. 2022; 12(3):229. https://doi.org/10.3390/metabo12030229
Chicago/Turabian StyleMatsuo, Tomohiro, Hidenori Ito, Kensuke Mitsunari, Kojiro Ohba, and Yasuyoshi Miyata. 2022. "Relationship between Urinary Calcium Excretion and Lower Urinary Tract Symptoms" Metabolites 12, no. 3: 229. https://doi.org/10.3390/metabo12030229
APA StyleMatsuo, T., Ito, H., Mitsunari, K., Ohba, K., & Miyata, Y. (2022). Relationship between Urinary Calcium Excretion and Lower Urinary Tract Symptoms. Metabolites, 12(3), 229. https://doi.org/10.3390/metabo12030229