Metabolites in Cherry Buds to Detect Winter Dormancy
Abstract
:1. Introduction
2. Results
2.1. Metabolites in Cherry Buds, Identified by Global Profiling
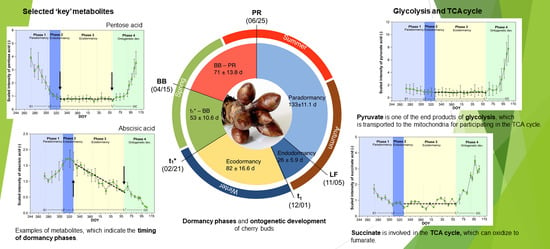
2.2. Metabolites of Glycolysis and TCA Cycle
2.3. Selection of Potential Metabolites That Indicate Dormancy Phases
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Sampling of Sweet Cherry Buds
4.3. Sampling of Twigs
4.4. Global Metabolite Profiling
4.5. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajula, S.N.R.; Nanjappan, S. Metabolomics: A recent advanced omics technology in herbal medicine research. In Medicinal and Aromatic Plants: Expanding Their Horizons through Omics; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 97–117. [Google Scholar]
- Vimont, N.; Fouché, M.; Campoy, J.A.; Tong, M.; Arkoun, M.; Yvin, J.C.; Wigge, P.A.; Dirlewanger, E.; Cortijo, S.; Wenden, B. From bud formation to flowering: Transcriptomic state defines the cherry developmental phases of sweet cherry bud Dormancy. BMC Genom. 2019, 20, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adetunji, C.O.; Palai, S.; Ekwuabu, C.P.; Egbuna, C.; Adetunji, J.B.; Ehis-Eriakha, C.B.; Kesh, S.S.; Mtewa, A.G. General principle of primary and secondary plant metabolites: Biogenesis, metabolism, and extraction. In Preparation of Phytopharmaceuticals for the Management of Disorders; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 3–23. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Adamakis, I.D.; Karamanoli, K.; Martens, S.; Molassiotis, A. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Bound, S.A.; Foo, E.; Gélinas-Marion, A.; Nichols, D.S.; Nissen, R. The impact of hormone breakers on hormone profiles, fruit growth and quality in sweet cherry. Agriculture 2022, 12, 270. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Dormant flower buds actively accumulate starch over winter in sweet cherry. Front. Plant Sci. 2018, 9, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branchereau, C.; Quero-Garcia, J.; Zaracho Echagüte, N.H.; Lambelin, L.; Fouché, M.; Wenden, B.; Donkpegan, A.; Le Dantec, L.; Barreneche, T.; Alletru, D.; et al. New insight into flowering date in Prunus: Fine mapping of a major QTL in sweet cherry. Hortic. Res. 2022, uhac042. [Google Scholar] [CrossRef] [PubMed]
- Sabir, I.A.; Manzoor, M.A.; Shah, I.H.; Liu, X.; Zahid, M.S.; Jui, S.; Wang, J.; Abdullah, M.; Zhang, C. BYB transcription factor family in sweet cherry (Prunus avium L.): Genome-wide investigation, evolution, structure, characterization and expression patterns. BMC Plant Biol. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Vimont, N.; Schwarzenberg, A.; Domijan, M.; Donkpegan, A.S.L.; Beauvieux, R.; Le Dantec, L.; Arkoun, M.; Jamois, F.; Yvin, J.C.; Wigge, P.A.; et al. Fine tuning of hormonal signaling is linked to dormancy status in sweet cherry flower buds. Tree Physiol. 2020, 41, 544–561. [Google Scholar] [CrossRef]
- Baldermann, S.; Homann, T.; Neugart, S.; Chmielewski, F.M.; Götz, K.P.; Gödeke, C.; Huschek, G.; Morlock, G.E.; Ravel, H.M. Selected plant metabolites involved in oxidation-reduction processes during bud dormancy and ontogenetic development in sweet cherry buds (Prunus avium L.). Molecules 2018, 23, 1197. [Google Scholar] [CrossRef] [Green Version]
- Velappan, Y.; Chabiwka, T.G.; Considine, J.A.; Agudelo-Romero, P.; Foyer, C.H.; Signorelli, S.; Considine, M.J. The bud dormancy disconnect: Latent buds of grapevine are dormant during summer despite a high metabolic rate. J. Exp. Bot. 2022, erac001. [Google Scholar] [CrossRef]
- Woitania, A.; Markiewicz, M.; Walígorski, P. Regulation of the bud dormancy development and release in micropropagated rhubarb ‘Malinowy’. Int. J. Mol. Sci. 2022, 23, 1480. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Ríos, G.; Leida, C.; Conejero, A.; Badenes, M.L. Epigenetic regulation of bud dormancy events in perennial plants. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A Conceptual Framework for Winter Dormancy in Deciduous Trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Lang, G.; Early, J.; Martin, G.; Darnell, R. Endo-, Para-, and ecodormancy: Physiological terminology and classification for dormancy research. Hortic. Sci. 1987, 22, 371–377. [Google Scholar]
- Chmielewski, F.M.; Götz, K.P. Identification and Timing of Dormant and Ontogenetic Phase for Sweet Cherries in Northeast Germany for Modelling Purposes. J. Hortic. 2017, 4, 205. [Google Scholar] [CrossRef] [Green Version]
- Cline, M.G.; Deppong, D.O. The Role of Apical Dominance in Paradormancy of Temperate Woody Plants: A Reappraisal. J. Plant Physiol. 1999, 155, 350–356. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Singh, R.K.; Svystun, T.; Al Dahmash, B.; Jönsson, A.M.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of phenology in trees—A molecular perspective. New Phytol. 2017, 213, 511–524. [Google Scholar] [CrossRef] [Green Version]
- Heide, O.M. Interaction of photoperiod and temperature in the control of growth and dormancy of Prunus species. Sci. Hortic. 2008, 115, 309–314. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Timm, S.; Arrivault, S. Regulation of Central Carbon and Amino Acid Metabolism. Plants 2021, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fernie, A.R. On the role of the tricarboxylic acid cycle in plant productivity. J. Integr. Plant Biol. 2018, 60, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, F.M.; Götz, K.P.; Homann, T.; Huschek, G.; Rawel, H.M. Identification of endodormancy release for cherries (Prunus avium L.) by abscisic acid and sugars. J. Hortic. 2017, 4, 210. [Google Scholar] [CrossRef]
- Götz, K.P.; Chmielewski, F.M. Response of sweet cherry buds and twigs to temperature changes—Evaluated by the determination of the degradation and synthesis of sucrose. Hortic. Sci. Prague 2021, 48, 149–157. [Google Scholar] [CrossRef]
- Plaxton, W.C. The organisation and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricaboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Lipavská, H.; Svobodá, H.; Albrechtová, J. Annual dynamics of the content of non-structural saccharides in the context of structural development of vegetative buds of Norway spruce. J. Plant Physiol. 2000, 157, 365–373. [Google Scholar] [CrossRef]
- Richardson, A.C.; Walton, E.F.; Meekings, J.S.; Boldingh, H.L. Carbohydrate changes in kiwifruit buds during the onset and release from dormancy. Sci. Hortic. 2010, 124, 463–468. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, F.; Wang, G.; Liu, Y.; Liu, D. Partial deficiency of isoleucine impairs root development and alters transcript levels of the genes involved in branched-chain amino acid and glucosinolate metabolism in Arabidopsis. J. Exp. Bot. 2013, 64, 599–612. [Google Scholar] [CrossRef]
- Or, E.; Belausov, E.; Popilevsky, I.; Ben Tal, Y. Changes in endogenous ABA level in relation to the dormancy cycle in grapevines grown in a hot climate. J. Hortic. Sci. Biotechnol. 2000, 75, 190–194. [Google Scholar] [CrossRef]
- Zheng, C.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic acid (ABA) promotes the induction and maintenance of pear (Pyrus pyrifolia white pear group) flower bud endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewski, F.M.; Baldermann, S.; Götz, K.P.; Homann, T.; Gödeke, K.; Schumacher, F.; Huschek, G.; Rawel, H.M. (2018) Abscisic Acid Related Metabolites in Sweet Cherry Buds (Prunus avium L.). J. Hortic. 2018, 5, 221. [Google Scholar] [CrossRef]
- Chuine, I.; Bonhomme, M.; Legave, J.M.; Garcia De Cortazar Atauri, I.; Charrier, G.; Lacointe, A.; Améglio, T. Can phenological models predict tree phenology accurately in the future? The unrevealed hurdle of endodormancy break. Glob. Chang. Biol. 2016, 22, 3444–3460. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth stages of mono-and dicotyledonous plants. In BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; 204p. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef] [Green Version]





| Phenological Phase | Phase ½ S1-LF/LF-t1 | Phase 2/3 LF-t1/t1-t1* | Phase 1/3 S1-LF/t1-t1* | Phase 1/4 S1-LF/t1*-OC | Phase 2/4 LF-t1/t1*-OC | Phase 3/4 t1-t1*/t1*-OC |
|---|---|---|---|---|---|---|
| n | 217 | 208 | 288 | 362 | 369 | 390 |
| increase | 114 | 119 | 159 | 305 | 323 | 338 |
| decrease | 103 | 89 | 129 | 57 | 46 | 52 |
| Phenological Stage | LF/t1 | t1/t1* | t1*/SB | SB/SG | SG/GT | GT/TC | TC/OC |
|---|---|---|---|---|---|---|---|
| Days between stages (2015/16) | 21 | 98 | 28 | 3 | 5 | 5 | 3 |
| n | 85 | 94 | 158 | 39 | 171 | 186 | 49 |
| increase|decrease in total | 16|69 | 84|10 | 150|8 | 38|1 | 142|29 | 141|45 | 38|11 |
| increase|decrease in % | 19|81 | 89|11 | 95|5 | 97|3 | 83|17 | 76|24 | 78|22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmielewski, F.-M.; Götz, K.-P. Metabolites in Cherry Buds to Detect Winter Dormancy. Metabolites 2022, 12, 247. https://doi.org/10.3390/metabo12030247
Chmielewski F-M, Götz K-P. Metabolites in Cherry Buds to Detect Winter Dormancy. Metabolites. 2022; 12(3):247. https://doi.org/10.3390/metabo12030247
Chicago/Turabian StyleChmielewski, Frank-M., and Klaus-P. Götz. 2022. "Metabolites in Cherry Buds to Detect Winter Dormancy" Metabolites 12, no. 3: 247. https://doi.org/10.3390/metabo12030247
APA StyleChmielewski, F.-M., & Götz, K.-P. (2022). Metabolites in Cherry Buds to Detect Winter Dormancy. Metabolites, 12(3), 247. https://doi.org/10.3390/metabo12030247