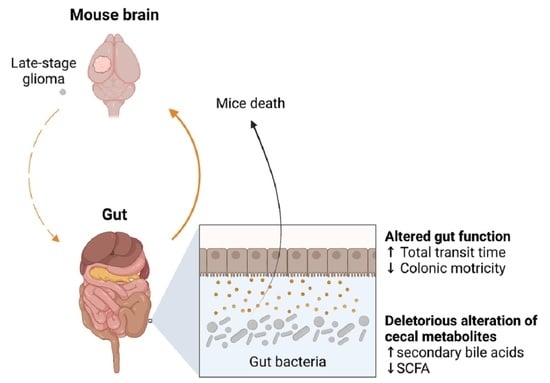
Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice
Abstract
1. Introduction
2. Results
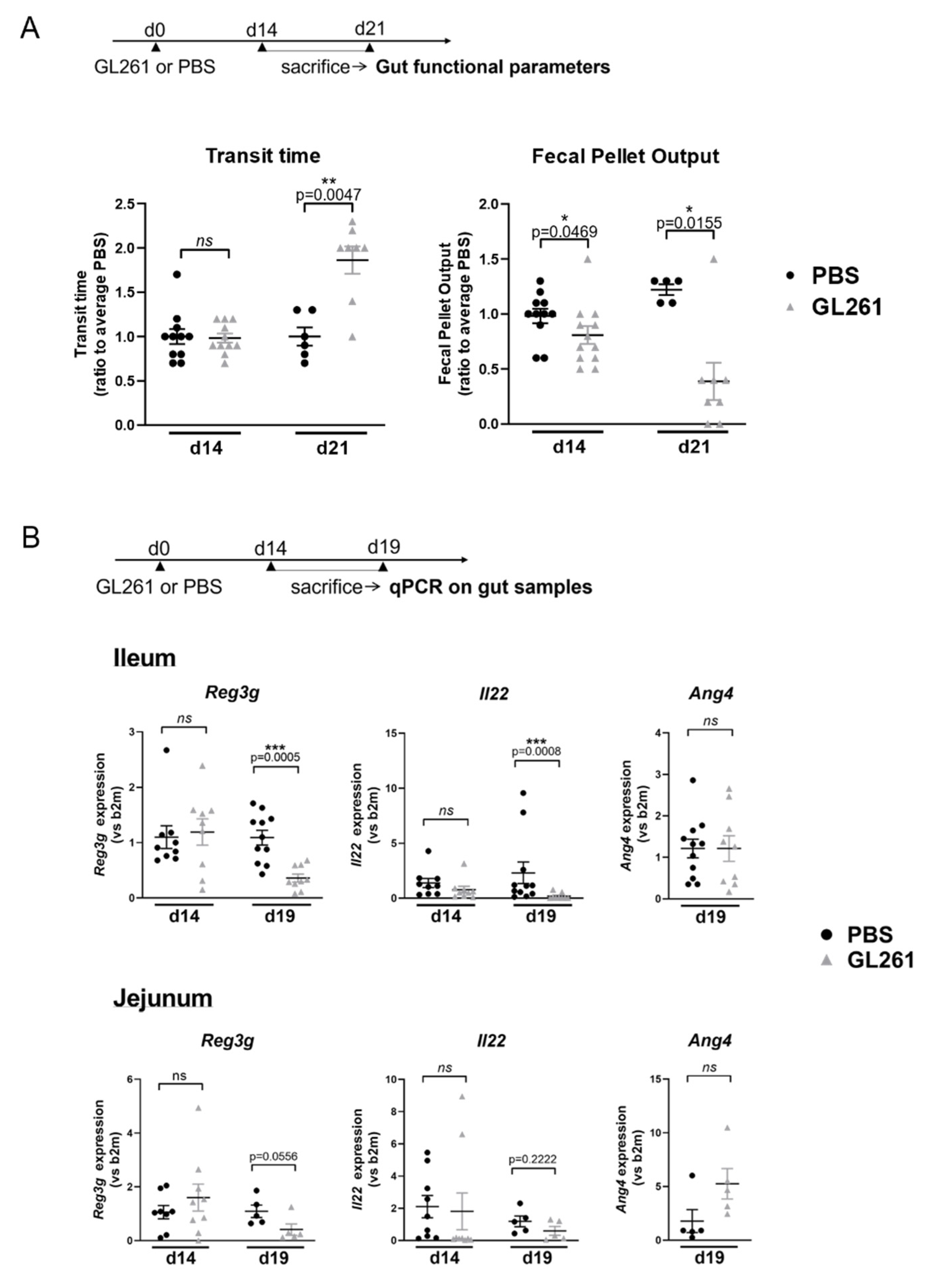
2.1. Late-Stage Glioma in Mice Is Associated with Changes in Gut Motricity and Expression Level of Genes Involved in Epithelial Antimicrobial Defense in the Jejunum and Ileum
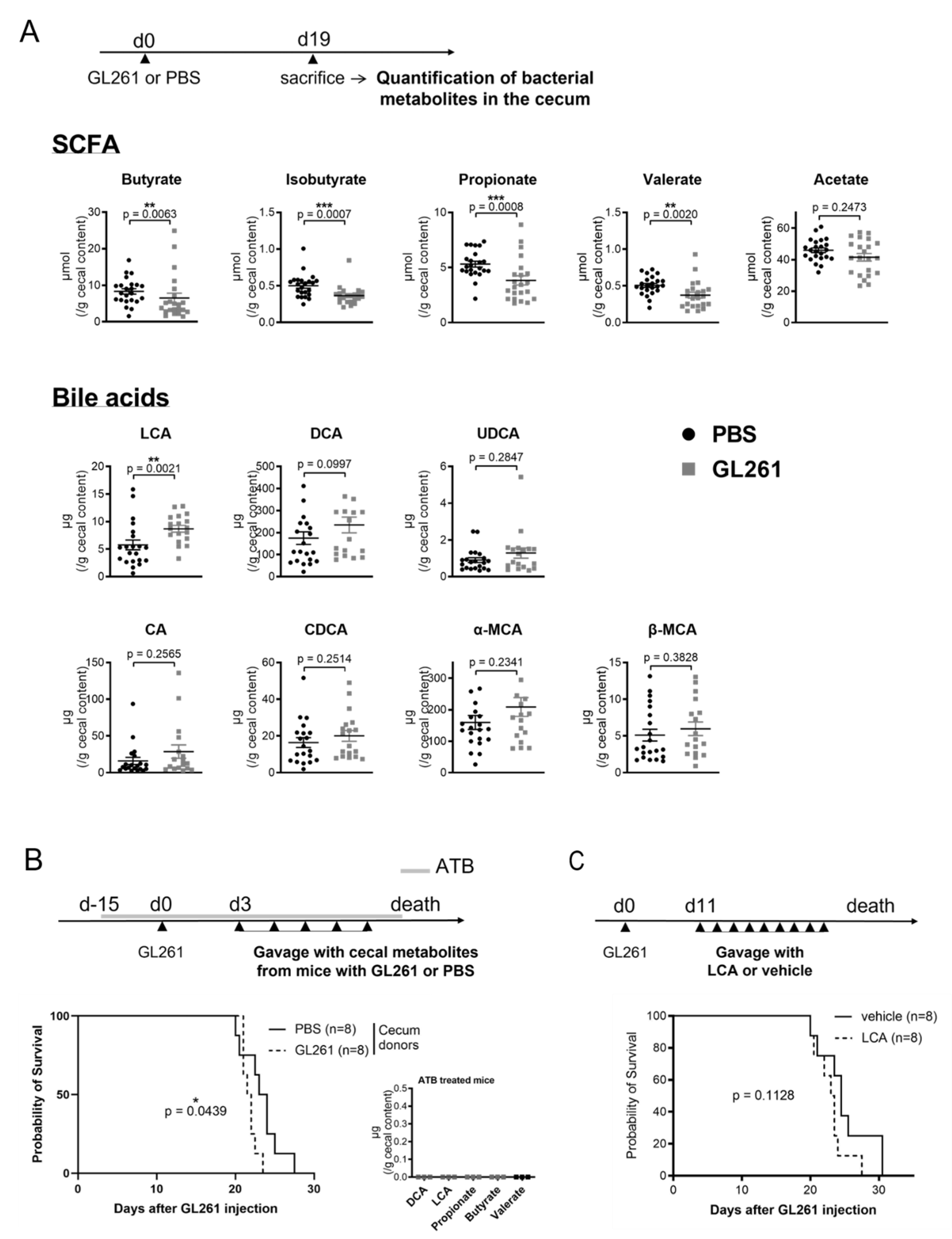
2.2. Late-Stage Glioma Is Associated with a Shift in Bacterial Metabolites in the Cecum That Contributes to Speed up Mice Death upon Transfer
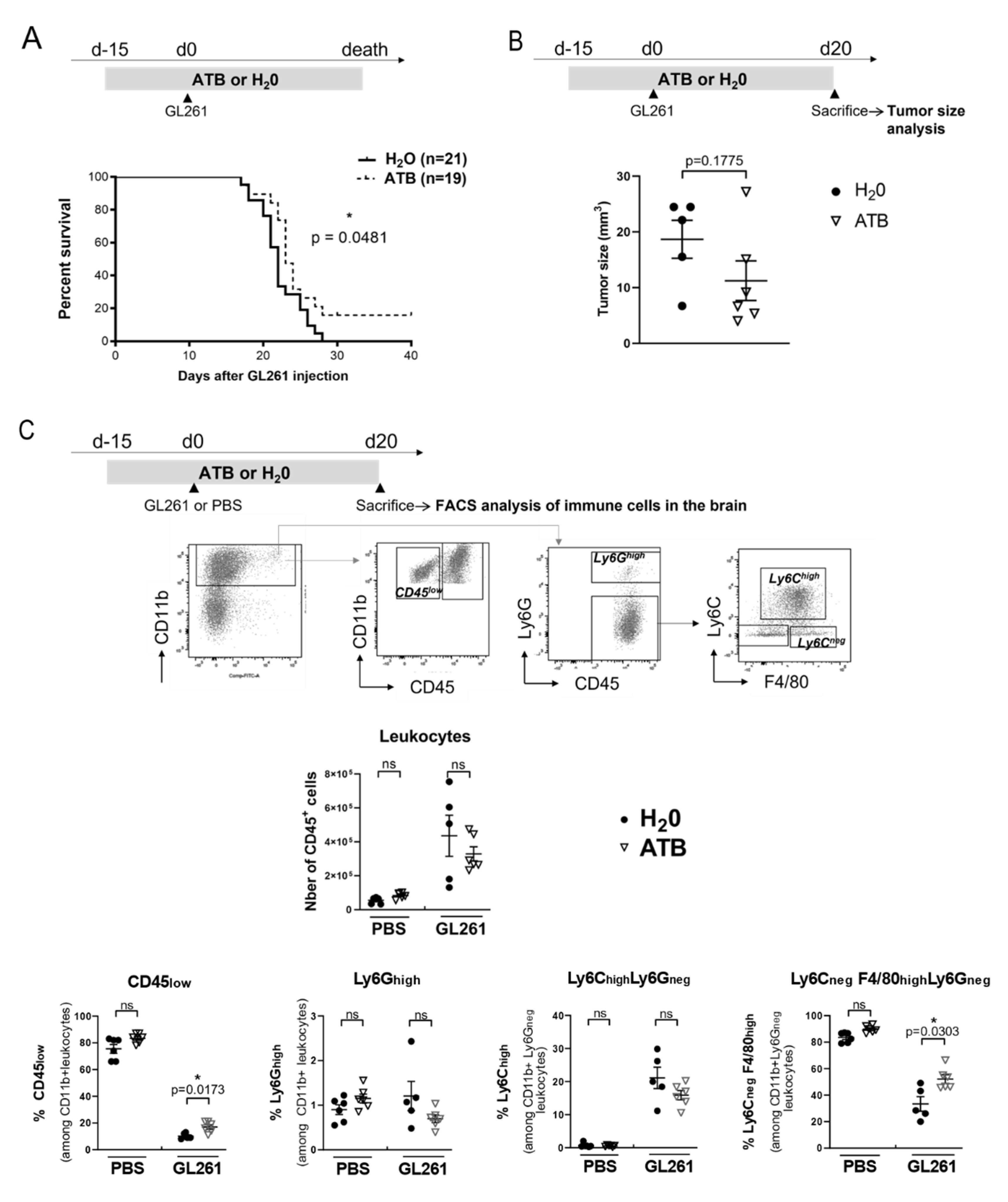
2.3. Oral Antibiotherapy Delay Mice Death and Is Associated with a Shift in the Proportion of Immune Myeloid Cells in the Brain Tumor Environment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. GL261 Tumor Cells Brain Injection
4.3. Antibiotic and Lithocholic Acid (LCA) Treatment
4.4. Cecal Metabolites Transfer Experiment
4.5. In Vivo and Ex Vivo Intestinal Parameters Monitoring
4.6. Gene Expression Analysis in Mouse Tissues
4.7. Quantification of Cecal Metabolites
4.8. Tumor Size Analysis
4.9. Flow Cytometry Analysis of Immune Cells in the Brain
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and differentiation of microglia. Front. Cell. Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Bennett, F.C.; Bennett, M.L.; Yaqoob, F.; Mulinyawe, S.B.; Grant, G.A.; Gephart, M.H.; Plowey, E.D.; Barres, B.A. A Combination of Ontogeny and CNS Environment Establishes Microglial Identity. Neuron 2018, 98, 1170–1183.e8. [Google Scholar] [CrossRef]
- Manjally, A.V.; Tay, T.L. Attack of the Clones: Microglia in Health and Disease. Front. Cell. Neurosci. 2022, 16, 831747. [Google Scholar] [CrossRef]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.; Simões, L.R.; Faller, C.J.; Ceretta, R.A.; Petronilho, F.; Lopes-Borges, J.; Valvassori, S.S.; Quevedo, J. Sodium Butyrate Prevents Memory Impairment by Re-establishing BDNF and GDNF Expression in Experimental Pneumococcal Meningitis. Mol. Neurobiol. 2015, 52, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-K.; Yao, S.-K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, X.; Guo, Y.; Yan, J.; Abuduwaili, A.; Aximujiang, K.; Yan, J.; Wu, M. Gut microbiota influence tumor development and Alter interactions with the human immune system. J. Exp. Clin. Cancer Res. 2021, 40, 42. [Google Scholar] [CrossRef] [PubMed]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Lam, K.C.; Araya, R.E.; Huang, A.; Chen, Q.; Di Modica, M.; Rodrigues, R.R.; Lopès, A.; Johnson, S.B.; Schwarz, B.; Bohrnsen, E.; et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell 2021, 184, 5338–5356.e21. [Google Scholar] [CrossRef] [PubMed]
- Coutzac, C.; Jouniaux, J.-M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2011, 59, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Brandenburg, S.; Turkowski, K.; Müller, S.; Vajkoczy, P. Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int. J. Cancer 2015, 137, 278–288. [Google Scholar] [CrossRef]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef]
- Patrizz, A.; Dono, A.; Zorofchian, S.; Hines, G.; Takayasu, T.; Husein, N.; Otani, Y.; Arevalo, O.; Choi, H.A.; Savarraj, J.; et al. Glioma and temozolomide induced alterations in gut microbiome. Sci. Rep. 2020, 10, 21002. [Google Scholar] [CrossRef]
- Dono, A.; Patrizz, A.; McCormack, R.M.; Putluri, N.; Ganesh, B.P.; Kaur, B.; McCullough, L.D.; Ballester, L.Y.; Esquenazi, Y. Glioma induced alterations in fecal short-chain fatty acids and neurotransmitters. CNS Oncol. 2020, 9, CNS57. [Google Scholar] [CrossRef]
- Yang, J.; Moon, H.E.; Park, H.W.; McDowell, A.; Shin, T.-S.; Jee, Y.-K.; Kym, S.; Paek, S.H.; Kim, Y.-K. Brain tumor diagnostic model and dietary effect based on extracellular vesicle microbiome data in serum. Exp. Mol. Med. 2020, 52, 1602–1613. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The Antibacterial Lectin RegIII Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. NeuroMol. Med. 2021, 23, 99–117. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Erofeev, A.; Sandvik, A.; Grcic, V.; Jahnsen, F.L.; Gaustad, P.; McCoy, K.D.; Macpherson, A.J.; Meza-Zepeda, L.A.; Johansen, F.-E. Depletion of murine intestinal microbiota: Effects on gut mucosa and epithelial gene expression. PLoS ONE 2011, 6, e17996. [Google Scholar] [CrossRef] [PubMed]
- Kovács, P.; Csonka, T.; Kovács, T.; Sári, Z.; Ujlaki, G.; Sipos, A.; Karányi, Z.; Szeőcs, D.; Hegedűs, C.; Uray, K.; et al. Lithocholic Acid, a Metabolite of the Microbiome, Increases Oxidative Stress in Breast Cancer. Cancers 2019, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.H.; Bard, J.-M.; Carbonnelle, D.; Chaillou, C.; Huvelin, J.-M.; Bobin-Dubigeon, C.; Nazih, H. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell. Oncol. 2018, 41, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.A.; Titorenko, V.I.; Beach, A.; Sanderson, J.T. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ 2013, 1, e122. [Google Scholar] [CrossRef]
- Gafar, A.A.; Draz, H.; Goldberg, A.A.; Bashandy, M.A.; Bakry, S.; Khalifa, M.; AbuShair, W.; Titorenko, V.I.; Sanderson, J.T. Lithocholic acid induces endoplasmic reticulum stress, autophagy and mitochondrial dysfunction in human prostate cancer cells. PeerJ 2016, 4, e2445. [Google Scholar] [CrossRef]
- Narisawa, T.; Magadia, N.E.; Weisburger, J.H.; Wynder, E.L. Promoting Effect of Bile Acids on Colon Carcinogenesis after Intrarectal Instillation of N-Methyl-N′ nitro-N-nitrosoguanidine in Rats. J. Natl. Cancer Inst. 1974, 53, 1093–1097. [Google Scholar] [CrossRef]
- Hill, M.J.; Drasar, B.S.; Williams, R.E.; Meade, T.W.; Cox, A.G.; Simpson, J.E.; Morson, B.C. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet 1975, 1, 535–539. [Google Scholar] [CrossRef]
- Owen, R.W.; Henly, P.J.; Thompson, M.H.; Hill, M.J. Steroids and cancer: Faecal bile acid screening for early detection of cancer risk. J. Steroid Biochem. 1986, 24, 391–394. [Google Scholar] [CrossRef]
- Ura, H.; Makino, T.; Ito, S.; Tsutsumi, M.; Kinugasa, T.; Kamano, T.; Ichimiya, H.; Konishi, Y. Combined effects of cholecystectomy and lithocholic acid on pancreatic carcinogenesis of N-nitrosobis(2-hydroxypropyl)amine in Syrian golden hamsters. Cancer Res. 1986, 46, 4782–4786. [Google Scholar]
- Kulkarni, M.S.; Cox, A.B.; Yielding, K.L. Requirements for induction of DNA strand breaks by lithocholic acid. Cancer Res. 1982, 42, 2792–2795. [Google Scholar]
- Pongracz, J.; Clark, P.; Neoptolemos, J.P.; Lord, J.M. Expression of protein kinase C isoenzymes in colorectal cancer tissue and their differential activation by different bile acids. Int. J. Cancer 1995, 61, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Farhana, L.; Nangia-Makker, P.; Arbit, E.; Shango, K.; Sarkar, S.; Mahmud, H.; Hadden, T.; Yu, Y.; Majumdar, A.P.N. Bile acid: A potential inducer of colon cancer stem cells. Stem Cell Res. Ther. 2016, 7, 181. [Google Scholar] [CrossRef] [PubMed]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef]
- Pan, X.; Elliott, C.T.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Hölscher, C.; McClean, P.L.; Graham, S.F.; Green, B.D. Metabolomic Profiling of Bile Acids in Clinical and Experimental Samples of Alzheimer’s Disease. Metabolites 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Su, Q.; Chen, J.; Wang, Y.; He, S. Gut Microbiome Alterations Affect Glioma Development and Foxp3 Expression in Tumor Microenvironment in Mice. Front. Oncol. 2022, 12, 836953. [Google Scholar] [CrossRef]
- Yonekura, S.; Terrisse, S.; Silva, C.A.C.; Lafarge, A.; Iebba, V.; Ferrere, G.; Goubet, A.G.; Fahrner, J.-E.; Lahmar, I.; Ueda, K.; et al. Cancer induces a stress ileopathy depending on B-adrenergic receptors and promoting dysbiosis that contribute to carcinogenesis. Cancer Discov. 2021, candisc.0999.2021. [Google Scholar] [CrossRef] [PubMed]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; Gomez de Agüero, M.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar] [CrossRef]
- Appleby, R.N.; Walters, J.R.F. The role of bile acids in functional GI disorders. Neurogastroenterol. Motil. 2014, 26, 1057–1069. [Google Scholar] [CrossRef]
- Li, N.; Koester, S.T.; Lachance, D.M.; Dutta, M.; Cui, J.Y.; Dey, N. Microbiome-encoded bile acid metabolism modulates colonic transit times. iScience 2021, 24, 102508. [Google Scholar] [CrossRef]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel Functions, Fecal Unconjugated Primary and Secondary Bile Acids, and Colonic Transit in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275.e1. [Google Scholar] [CrossRef]
- Tottey, W.; Feria-Gervasio, D.; Gaci, N.; Laillet, B.; Pujos, E.; Martin, J.-F.; Sebedio, J.-L.; Sion, B.; Jarrige, J.-F.; Alric, M.; et al. Colonic Transit Time Is a Driven Force of the Gut Microbiota Composition and Metabolism: In Vitro Evidence. J. Neurogastroenterol. Motil. 2017, 23, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, D.H.; Kettenmann, H. Microglia/Brain Macrophages as Central Drivers of Brain Tumor Pathobiology. Neuron 2019, 104, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014, 211, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, G.; Antonangeli, F.; Marrocco, F.; Porzia, A.; Lauro, C.; Santoni, A.; Limatola, C. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur. J. Immunol. 2020, 50, 705–711. [Google Scholar] [CrossRef]
- Ziemons, J.; Smidt, M.L.; Damink, S.O.; Rensen, S.S. Gut microbiota and metabolic aspects of cancer cachexia. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101508. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, I.C.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Salazar, N.; Taminiau, B.; Druart, C.; Muccioli, G.G.; François, E.; Blecker, C.; Richel, A.; Daube, G.; et al. Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PLoS ONE 2015, 10, e0131009. [Google Scholar] [CrossRef]
- Jarry, U.; Donnou, S.; Vincent, M.; Jeannin, P.; Pineau, L.; Fremaux, I.; Delneste, Y.; Couez, D. Treg depletion followed by intracerebral CpG-ODN injection induce brain tumor rejection. J. Neuroimmunol. 2014, 267, 35–42. [Google Scholar] [CrossRef][Green Version]
- Tasselli, M.; Chaumette, T.; Paillusson, S.; Monnet, Y.; Lafoux, A.; Huchet-Cadiou, C.; Aubert, P.; Hunot, S.; Derkinderen, P.; Neunlist, M.; et al. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol. Motil. 2013, 25, e183–e193. [Google Scholar] [CrossRef]
- Rincel, M.; Aubert, P.; Chevalier, J.; Grohard, P.-A.; Basso, L.; Monchaux de Oliveira, C.; Helbling, J.C.; Lévy, É.; Chevalier, G.; Leboyer, M.; et al. Multi-hit early life adversity affects gut microbiota, brain and behavior in a sex-dependent manner. Brain Behav. Immun. 2019, 80, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Aubert, P.; Oleynikova, E.; Rizvi, H.; Ndjim, M.; Le Berre-Scoul, C.; Grohard, P.A.; Chevalier, J.; Segain, J.-P.; Le Drean, G.; Neunlist, M.; et al. Maternal protein restriction induces gastrointestinal dysfunction and enteric nervous system remodeling in rat offspring. FASEB J. 2019, 33, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Coué, M.; Tesse, A.; Falewée, J.; Aguesse, A.; Croyal, M.; Fizanne, L.; Chaigneau, J.; Boursier, J.; Ouguerram, K. Spirulina Liquid Extract Protects against Fibrosis Related to Non-Alcoholic Steatohepatitis and Increases Ursodeoxycholic Acid. Nutrients 2019, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Cuffaro, B.; Assohoun, A.; Boutillier, D.; Peucelle, V.; Desramaut, J.; Boudebbouze, S.; Croyal, M.; Waligora-Dupriet, A.-J.; Rhimi, M.; Grangette, C.; et al. Identification of New Potential Biotherapeutics from Human Gut Microbiota-Derived Bacteria. Microorganisms 2021, 9, 565. [Google Scholar] [CrossRef]
- Donnou, S.; Fisson, S.; Mahe, D.; Montoni, A.; Couez, D. Identification of new CNS-resident macrophage subpopulation molecular markers for the discrimination with murine systemic macrophages. J. Neuroimmunol. 2005, 169, 39–49. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbreteau, A.; Aubert, P.; Croyal, M.; Naveilhan, P.; Billon-Crossouard, S.; Neunlist, M.; Delneste, Y.; Couez, D.; Aymeric, L. Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice. Metabolites 2022, 12, 290. https://doi.org/10.3390/metabo12040290
Herbreteau A, Aubert P, Croyal M, Naveilhan P, Billon-Crossouard S, Neunlist M, Delneste Y, Couez D, Aymeric L. Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice. Metabolites. 2022; 12(4):290. https://doi.org/10.3390/metabo12040290
Chicago/Turabian StyleHerbreteau, Aglae, Philippe Aubert, Mikaël Croyal, Philippe Naveilhan, Stéphanie Billon-Crossouard, Michel Neunlist, Yves Delneste, Dominique Couez, and Laetitia Aymeric. 2022. "Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice" Metabolites 12, no. 4: 290. https://doi.org/10.3390/metabo12040290
APA StyleHerbreteau, A., Aubert, P., Croyal, M., Naveilhan, P., Billon-Crossouard, S., Neunlist, M., Delneste, Y., Couez, D., & Aymeric, L. (2022). Late-Stage Glioma Is Associated with Deleterious Alteration of Gut Bacterial Metabolites in Mice. Metabolites, 12(4), 290. https://doi.org/10.3390/metabo12040290