Sulfonation Reactions behind the Fate of White Wine’s Shelf-Life
Abstract
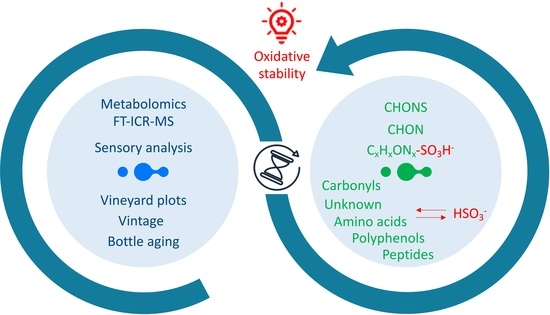
:1. Introduction
2. Results and Discussion
2.1. Multivariate FT-ICR-MS-Based Statistical Analysis of Wines
2.2. Compositional Networks for Metabolite Identification
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Design
3.3. Sensory Analysis
3.4. Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Analysis and Data Processing
3.5. Data Mining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, P.; Silva, M.A.; Pons, A.; Tominaga, T.; Lavigne, V.; Saucier, C.; Darriet, P.; Teissedre, P.L.; Dubourdieu, D. Impact of oxygen dissolved at bottling and transmitted through closures on the composition and sensory properties of a Sauvignon blanc wine during bottle storage. J. Agric. Food Chem. 2009, 57, 10261–10270. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of wine phenolics: A critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar]
- Ugliano, M.; Kwiatkowski, M.; Travis, B.; Francis, I.L.; Waters, E.J.; Herderich, M.; Pretorius, I.S. Post-bottling management of oxygen to reduce off-flavour formation and optimize wine style. Aust. N. Z. Wine Ind. J. 2009, 24, 24–28. [Google Scholar]
- Gougeon, R.D.; Lucio, M.; De Boel, A.; Frommberger, M.; Hertkorn, N.; Peyron, D.; Chassagne, D.; Feuillat, F.; Cayot, P.; Voilley, A.; et al. Expressing Forest Origins in the Chemical Composition of Cooperage Oak Woods and Corresponding Wines by Using FTICR-MS. Chem. Eur. J. 2009, 15, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Arbulu, M.; Sampedro, M.C.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Untargeted metabolomic analysis using liquid chromatography quadrupole time-of-flight mass spectrometry for non-volatile profiling of wines. Anal. Chim. Acta 2015, 858, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cuadros-Inostroza, A.; Giavalisco, P.; Hummel, J.; Eckardt, A.; Willmitzer, L.; Peña-Cortés, H. Discrimination of Wine Attributes by Metabolome Analysis. Anal. Chem. 2010, 82, 3573–3580. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef]
- Arapitsas, P.; Scholz, M.; Vrhovsek, U.; Di Blasi, S.; Bartolini, A.B.; Masuero, D.; Perenzoni, D.; Rigo, A.; Mattivi, F. A Metabolomic Approach to the Study of Wine Micro-Oxygenation. PLoS ONE 2012, 7, e37783. [Google Scholar] [CrossRef]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Witting, M.; Moritz, F.; Gil, R.B.; Goffette, D.; Valade, M.; Schmitt-Kopplin, P.; Gougeon, R.D. Natural oxygenation of Champagne wine during ageing on lees: A metabolomics picture of hormesis. Food Chem. 2016, 203, 207–215. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Karbowiak, T.; Crouvisier-Urion, K.; Lagorce, A.; Ballester, J.; Geoffroy, A.; Roullier-Gall, C.; Chanut, J.; Gougeon, R.D.; Schmitt-Kopplin, P.; Bellat, J.-P. Wine aging: A bottleneck story. NPJ Sci. Food 2019, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roullier-Gall, C.; Kanawati, B.; Hemmler, D.; Druschel, G.K.; Gougeon, R.D.; Schmitt-Kopplin, P. Electrochemical triggering of the Chardonnay wine metabolome. Food Chem. 2019, 286, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanet, R.; Coelho, C.; Liu, Y.; Bahut, F.; Ballester, J.; Nikolantonaki, M.; Gougeon, D.R. The Antioxidant Potential of White Wines Relies on the Chemistry of Sulfur-Containing Compounds: An Optimized DPPH Assay. Molecules 2019, 24, 1353. [Google Scholar] [CrossRef] [Green Version]
- Romanet, R.; Sarhane, Z.; Bahut, F.; Uhl, J.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Exploring the chemical space of white wine antioxidant capacity: A combined DPPH, EPR and FT-ICR-MS study. Food Chem. 2021, 355, 129566. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-Da-Silva, J.M.; Laureano, O.; Mullen, W.; Crozier, A. Effect of ellagitannins, ellagic acid and volatile compounds from oak wood on the (+)-catechin, procyanidin B1 and malvidin-3-glucoside content of model wines. Aust. J. Grape Wine Res. 2008, 14, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Nikolantonaki, M.; Waterhouse, A.L. A Method to Quantify Quinone Reaction Rates with Wine Relevant Nucleophiles: A Key to the Understanding of Oxidative Loss of Varietal Thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef]
- Bahut, F.; Liu, Y.; Romanet, R.; Coelho, C.; Sieczkowski, N.; Alexandre, H.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Metabolic diversity conveyed by the process leading to glutathione accumulation in inactivated dry yeast: A synthetic media study. Food Res. Int. 2019, 123, 762–770. [Google Scholar] [CrossRef]
- Bahut, F.; Romanet, R.; Sieczkowski, N.; Schmitt-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Antioxidant activity from inactivated yeast: Expanding knowledge beyond the glutathione-related oxidative stability of wine. Food Chem. 2020, 325, 126941. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Hemmler, D.; Gonsior, M.; Li, Y.; Nikolantonaki, M.; Aron, A.; Coelho, C.; Gougeon, R.D.; Schmitt-Kopplin, P. Sulfites and the wine metabolome. Food Chem. 2017, 237, 106–113. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Perenzoni, D.; Angeli, A.; Pangrazzi, P.; Mattivi, F. Wine metabolomics reveals new sulfonated products in bottled white wines, promoted by small amounts of oxygen. J. Chromatogr. A 2016, 1429, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gougeon, R.D.; Lucio, M.; Frommberger, M.; Peyron, D.; Chassagne, D.; Alexandre, H.; Feuillat, F.; Voilley, A.; Cayot, P.; Gebefugi, I.; et al. The chemodiversity of wines can reveal a metabologeography expression of cooperage oak wood. Proc. Natl. Acad. Sci. USA 2009, 106, 9174–9179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tziotis, D.; Hertkorn, N.; Schmitt-Kopplin, P. Kendrick-Analogous Network Visualisation of Ion Cyclotron Resonance Fourier Transform Mass Spectra: Improved Options for the Assignment of Elemental Compositions and the Classification of Organic Molecular Complexity. Eur. J. Mass Spectrom. 2011, 17, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Jackowetz, J.N.; de Orduña, R.M. Survey of SO2 binding carbonyls in 237 red and white table wines. Food Control 2013, 32, 687–692. [Google Scholar] [CrossRef]
- Tachtalidou, S.; Sok, N.; Denat, F.; Noret, L.; Schmit-Kopplin, P.; Nikolantonaki, M.; Gougeon, R.D. Direct NMR evidence for the dissociation of sulfur-dioxide-bound acetaldehyde under acidic conditions: Impact on wines oxidative stability. Food Chem. 2022, 373, 131679. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731. [Google Scholar] [CrossRef]
- Liu, Y.; Forcisi, S.; Lucio, M.; Harir, M.; Bahut, F.; Deleris-Bou, M.; Krieger-Weber, S.; Gougeon, R.D.; Alexandre, H.; Schmitt-Kopplin, P. Digging into the low molecular weight peptidome with the OligoNet web server. Sci. Rep. 2017, 7, 11692. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolantonaki, M.; Romanet, R.; Lucio, M.; Schmitt-Kopplin, P.; Gougeon, R. Sulfonation Reactions behind the Fate of White Wine’s Shelf-Life. Metabolites 2022, 12, 323. https://doi.org/10.3390/metabo12040323
Nikolantonaki M, Romanet R, Lucio M, Schmitt-Kopplin P, Gougeon R. Sulfonation Reactions behind the Fate of White Wine’s Shelf-Life. Metabolites. 2022; 12(4):323. https://doi.org/10.3390/metabo12040323
Chicago/Turabian StyleNikolantonaki, Maria, Rémy Romanet, Marianna Lucio, Philippe Schmitt-Kopplin, and Régis Gougeon. 2022. "Sulfonation Reactions behind the Fate of White Wine’s Shelf-Life" Metabolites 12, no. 4: 323. https://doi.org/10.3390/metabo12040323