Soy Phospholipids Exert a Renoprotective Effect by Inhibiting the Nuclear Factor Kappa B Pathway in Macrophages
Abstract
:1. Introduction
2. Results
2.1. Composition of Isoflavones and Phospholipids in the Insoluble Fraction
2.2. SPI and SPIEE Suppress Nephropathy in Zucker Rats
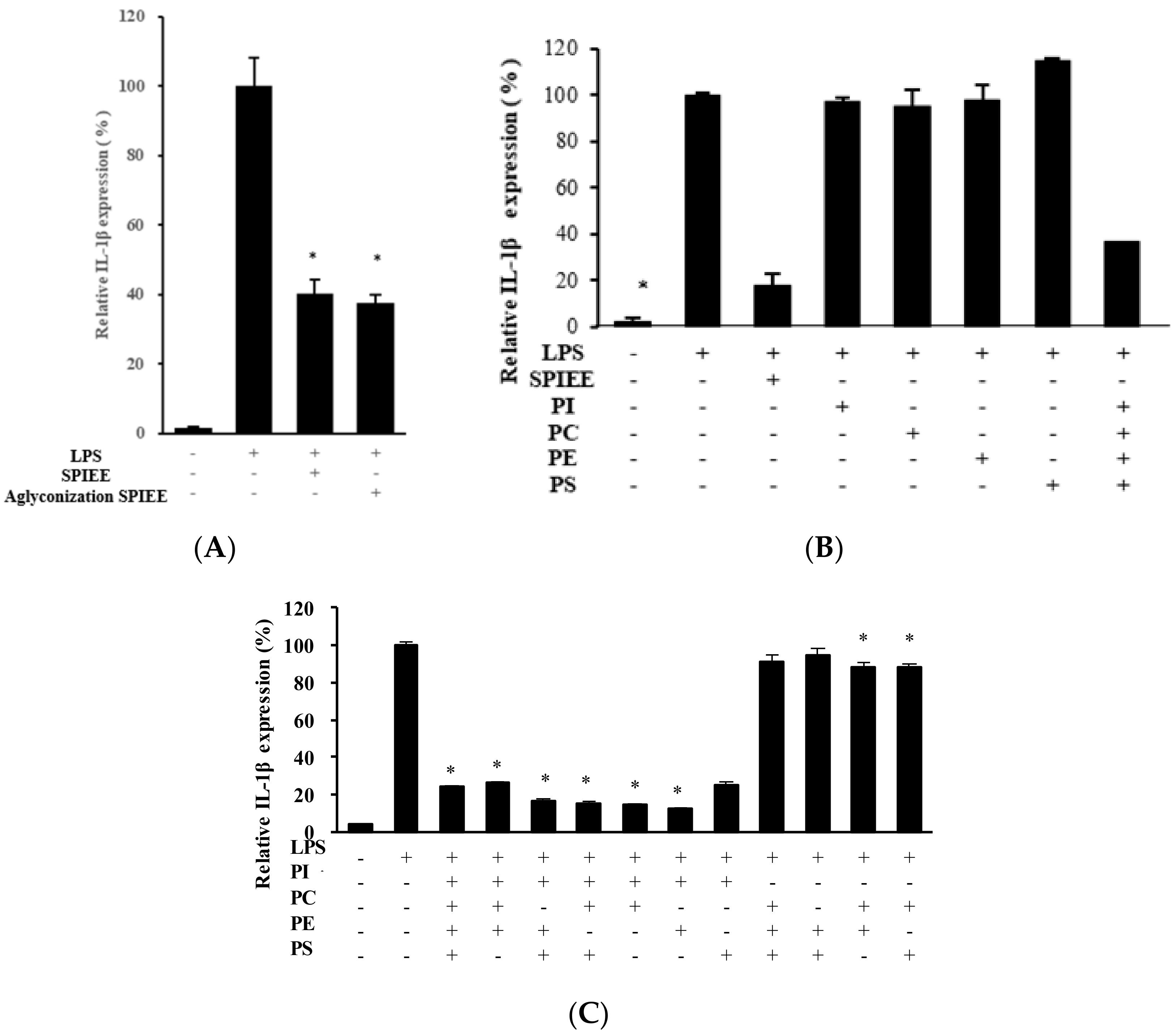
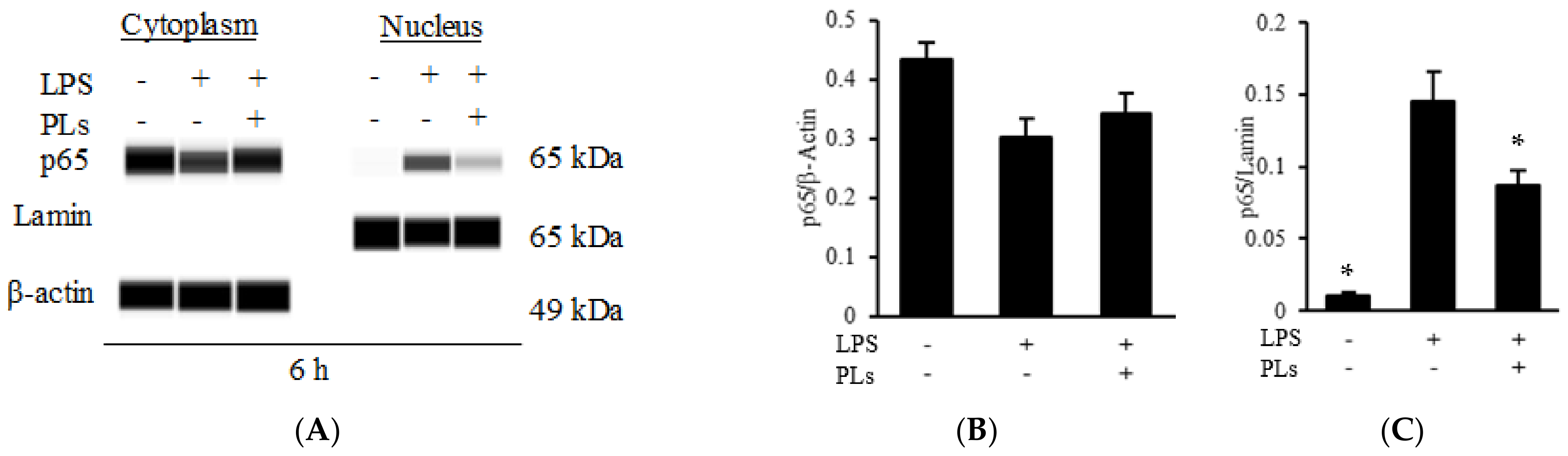
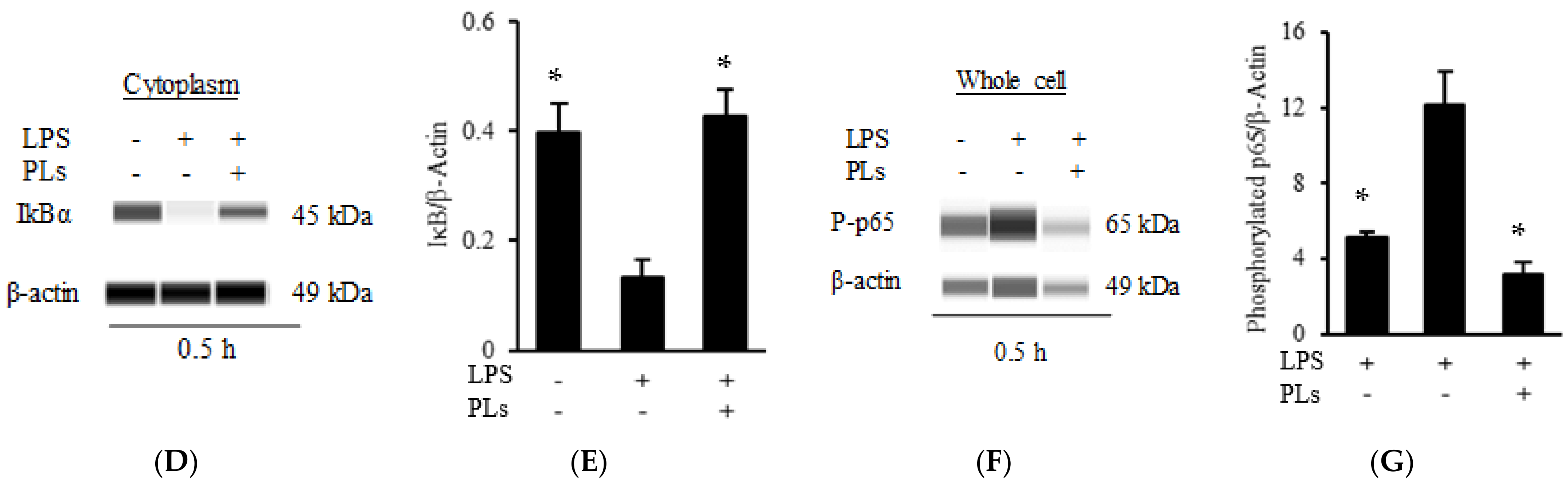
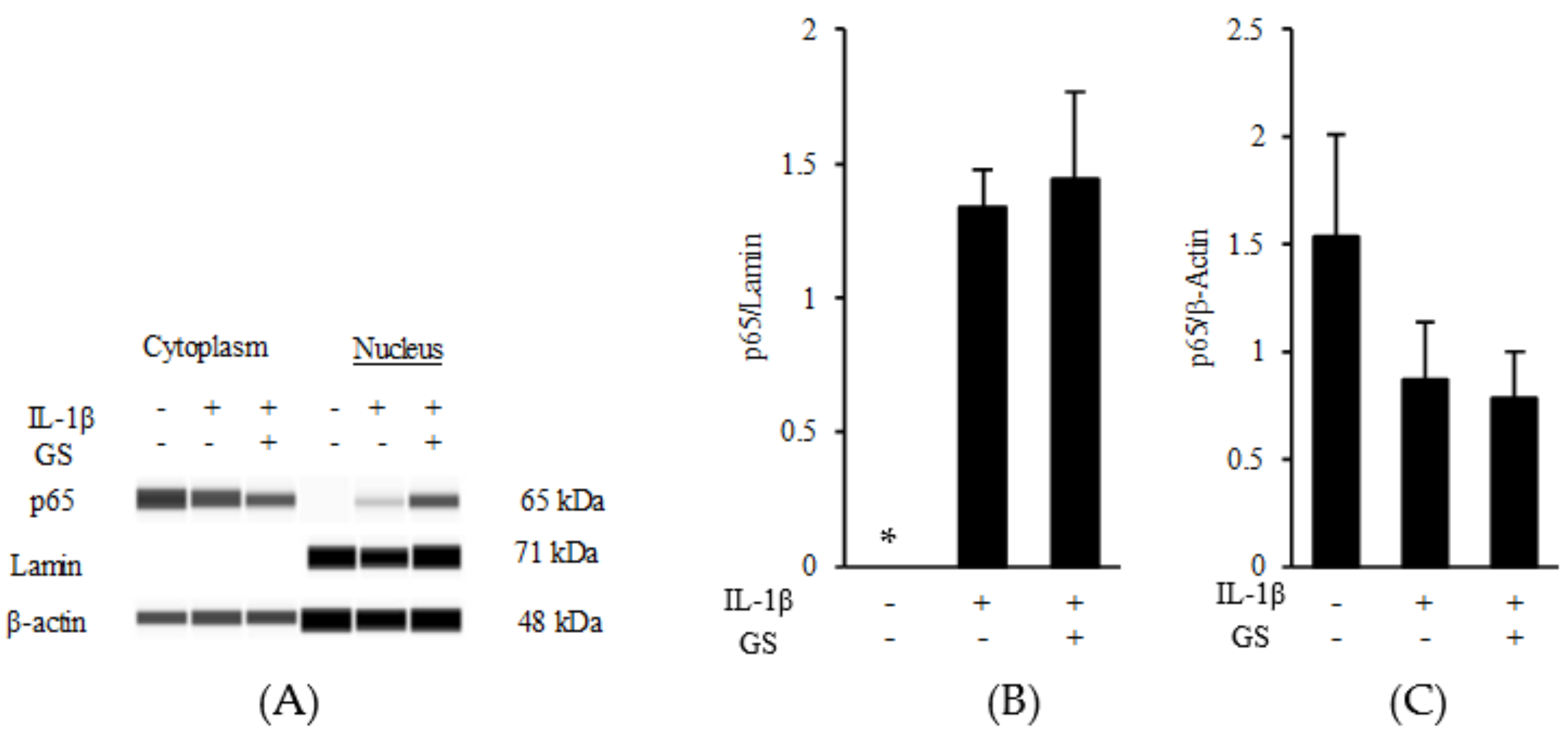
2.3. PLs Suppress IL-1β Expression in Macrophages via the NF-κB Pathway
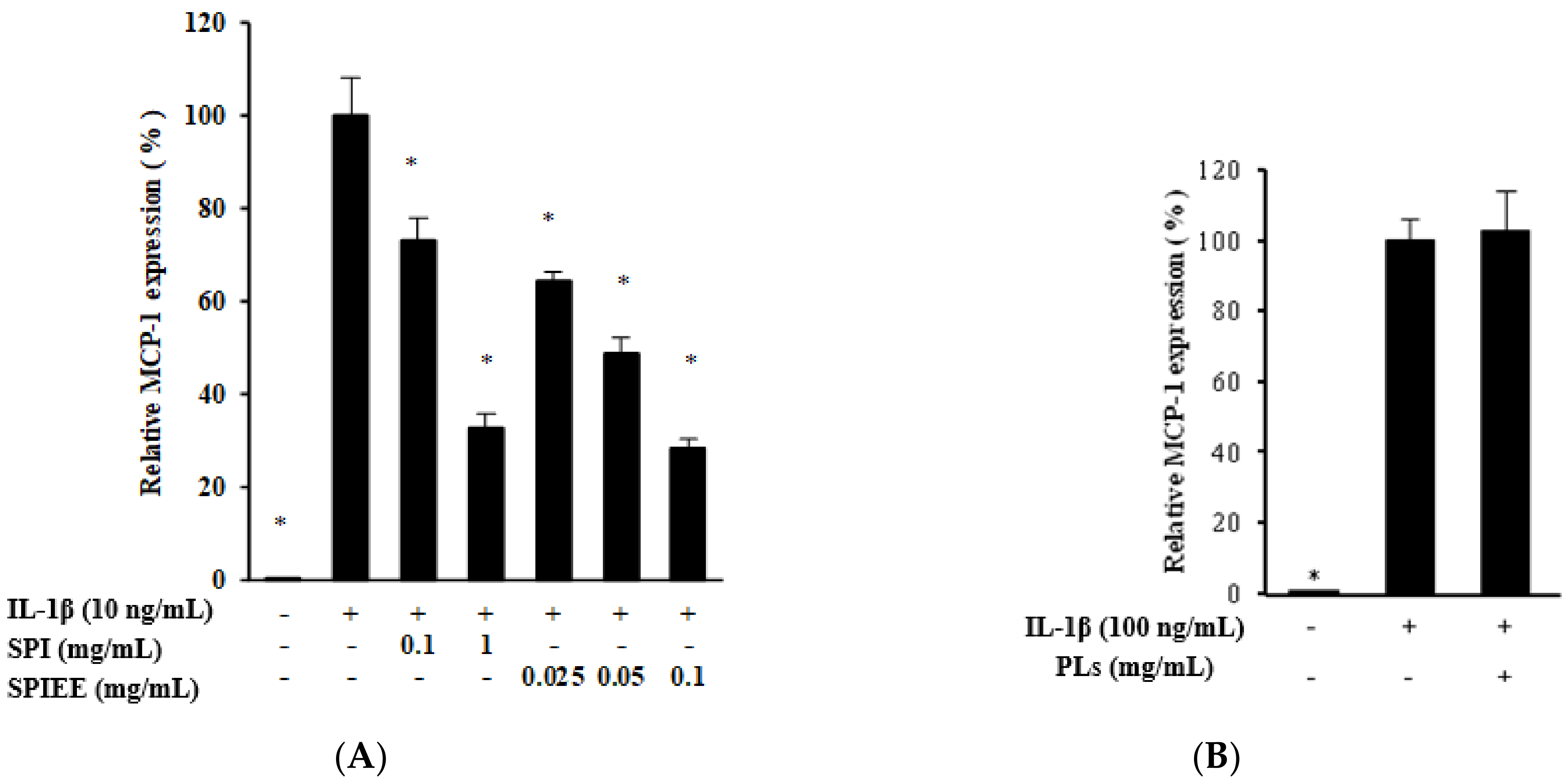
2.4. Genistein Suppresses MCP-1 Expression in Proximal Tubular Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of SPIEE and Fractionation
4.2. Detection of Isoflavonoid Species and PLs

4.3. Animals and Animal Experimental Design
4.4. Urinalysis
4.5. Histochemical Staining
4.6. Cell Culture and Treatments
4.7. Gene Expression Analysis
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lerman, L.O. The metabolic syndrome and chronic kidney disease. Transl. Res. 2017, 183, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahba, I.M.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Huang, Y.-F.; Wang, M.-Q.; Chen, D.-X.; Wan, H.; Wei, L.-B.; Xiao, W. Dietary fiber intake is associated with chronic kidney disease (CKD) progression and cardiovascular risk, but not protein nutritional status, in adults with CKD. Asia Pac. J. Clin. Nutr. 2017, 26, 598–605. [Google Scholar] [PubMed]
- Asghari, G.; Momenan, M.; Yuzbashian, E.; Mirmiran, P.; Azizi, F. Dietary pattern and incidence of chronic kidney disease among adults: A population-based study. Nutr. Metab. 2018, 15, 88. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.; Fu, X.; Wang, Z. Lipid accumulation and chronic kidney disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Palmer, S.C.; Wai, S.N.; Ruospo, M.; Carrero, J.-J.; Campbell, K.; Strippoli, G.F.M. Healthy dietary patterns and risk of mortality and ESRD in CKD: A meta-analysis of cohort studies. Clin. J. Am. Soc. Nephrol. 2016, 12, 272–279. [Google Scholar] [CrossRef]
- Caponio, G.R.; Wang, D.Q.-H.; Di Ciaula, A.; De Angelis, M.; Portincasa, P. Regulation of cholesterol metabolism by bioactive components of soy proteins: Novel translational evidence. Int. J. Mol. Sci. 2020, 22, 227. [Google Scholar] [CrossRef]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean.BETA.-conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, N.; Yamashita, Y.; Nagata, M.; Wanezaki, S.; Ashida, H.; Horio, F.; Kohno, M. Soy β-conglycinin improves glucose uptake in skeletal muscle and ameliorates hepatic insulin resistance in Goto-Kakizaki rats. Nutr. Res. 2014, 34, 160–167. [Google Scholar] [CrossRef]
- McGraw, N.J.; Krul, E.S.; Grunz-Borgmann, E.; Parrish, A.R. Soy-based renoprotection. World J. Nephrol. 2016, 5, 233–257. [Google Scholar] [CrossRef]
- Kafeshani, M.; Rafieian-Kopaei, M.; Beigrezaei, S.; Nasri, H. Soy protein and chronic kidney disease: An updated review. Int. J. Prev. Med. 2017, 8, 105. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and c-reactive protein in type 2 diabetes with nephropathy. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Jheng, H.-F.; Hirotsuka, M.; Goto, T.; Shibata, M.; Matsumura, Y.; Kawada, T. Dietary low-fat soy milk powder retards diabetic nephropathy progression via inhibition of renal fibrosis and renal inflammation. Mol. Nutr. Food Res. 2017, 61, 61. [Google Scholar] [CrossRef]
- Shikata, K.; Makino, H. Microinflammation in the pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2013, 4, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Black, L.M.; Lever, J.M.; Agarwal, A. Renal inflammation and fibrosis: A double-edged sword. J. Histochem. Cytochem. 2019, 67, 663–681. [Google Scholar] [CrossRef] [Green Version]
- Asanoma, M.; Tachibana, N.; Hirotsuka, M.; Kohno, M.; Watanabe, Y. Effects of soy protein isolate feeding on severe kidney damage in DOCA salt-treated obese zucker rats. J. Agric. Food Chem. 2012, 60, 5367–5372. [Google Scholar] [CrossRef]
- Palanisamy, N.; Kannappan, S.; Anuradha, C.V. Genistein modulates NF-κB-associated renal inflammation, fibrosis and podocyte abnormalities in fructose-fed rats. Eur. J. Pharmacol. 2011, 667, 355–364. [Google Scholar] [CrossRef]
- Palanisamy, N.; Viswanathan, P.; Anuradha, C.V. Effect of genistein, a soy isof lavone, on whole body insulin sensitivity and renal damage induced by a high-fructose diet. Ren. Fail. 2008, 30, 645–654. [Google Scholar] [CrossRef]
- Ogborn, M.R.; Nitschmann, E.; Bankovic-Calic, N.; Weiler, H.A.; Aukema, H.M. Dietary soy protein benefit in experimental kidney disease is preserved after isoflavone depletion of diet. Exp. Biol. Med. 2010, 235, 1315–1320. [Google Scholar] [CrossRef]
- Sato, N.; Kaneko, M.; Tamura, M.; Kurumatani, H. The prostacyclin analog beraprost sodium ameliorates characteristics of metabolic syndrome in obese zucker (fatty) rats. Diabetes 2010, 59, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, J.; Ramírez, V.; Pérez, J.; Torre-Villalvazo, I.; Torres, N.; Tovar, A.R.; Muñoz, R.M.; Uribe, N.; Gamba, G.; Bobadilla, N.A.; et al. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. Am. J. Physiol. Physiol. 2005, 288, F108–F116. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [Green Version]
- Balla, T.; Szentpetery, Z.; Kim, Y.J. Phosphoinositide signaling: New tools and insights. Physiology 2009, 24, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Tadaomi, T.; Ishitoya, J.; Homma, Y.; Kato, M.; Nagai, Y. Role of enhanced inositol phospholipid metabolism in neutrophil activation. Biochem. Pharmacol. 1985, 34, 1931–1935. [Google Scholar] [CrossRef]
- Takenawa, T.; Homma, Y.; Nagai, Y. Role of Ca2+ in phosphatidylinositol response and arachidonic acid release in formylated tripeptide- or Ca2+ ionophore A23187-stimulated guinea pig neutrophils. J. Immunol. 1983, 130, 2849–2855. [Google Scholar]
- Huang, Y.-H.; Wang, H.-M.; Cai, Z.-Y.; Xu, F.-Y.; Zhou, X.-Y. Lipoxin A4 Inhibits NF-κB activation and Cell Cycle Progression in RAW264.7 Cells. Inflammation 2014, 37, 1084–1090. [Google Scholar] [CrossRef]
- Kure, I.; Nishiumi, S.; Nishitani, Y.; Tanoue, T.; Ishida, T.; Mizuno, M.; Fujita, T.; Kutsumi, H.; Arita, M.; Azuma, T.; et al. Lipoxin A4Reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. J. Pharmacol. Exp. Ther. 2009, 332, 541–548. [Google Scholar] [CrossRef]
- Serio, K.J.; Johns, S.C.; Luo, L.; Hodulik, C.R.; Bigby, T.D. Lipopolysaccharide down-regulates the leukotriene C4Synthase gene in the monocyte-like cell line, THP-1. J. Immunol. 2003, 170, 2121–2128. [Google Scholar] [CrossRef] [Green Version]
- Riddick, C.A.; Ring, W.L.; Baker, J.R.; Hodulik, C.R.; Bigby, T.D. Dexamethasone increases expression of 5-lipoxygenase and its activating protein in human monocytes and THP-1 Cells. Eur. J. Biochem. 1997, 246, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weibel, G.L.; Joshi, M.R.; Wei, C.; Bates, S.R.; Blair, I.A.; Rothblat, G.H. 15(S)-Lipoxygenase-1 associates with neutral lipid droplets in macrophage foam cells: Evidence of lipid droplet metabolism. J. Lipid Res. 2009, 50, 2371–2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Hosojima, M.; Kabasawa, H.; Kuwahara, S.; Goto, S.; Toba, K.; Kaseda, R.; Tanaka, T.; Kitamura, N.; Takihara, H.; et al. Rice endosperm protein administration to juvenile mice regulates gut microbiota and suppresses the development of high-fat diet-induced obesity and related disorders in adulthood. Nutrients 2019, 11, 2919. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Harris, D.C.H.; Wang, Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology 2015, 30, 183–194. [Google Scholar] [CrossRef]
- Kitagawa, K.; Wada, T.; Furuichi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Takeya, M.; Kuziel, W.A.; Matsushima, K.; Mukaida, N.; et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 2004, 165, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Furuichi, K.; Sakai, N.; Iwata, Y.; Kitagawa, K.; Ishida, Y.; Kondo, T.; Hashimoto, H.; Ishiwata, Y.; Mukaida, N.; et al. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J. Am. Soc. Nephrol. 2004, 15, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, O.; Stepanovska, B.; Starck, M.; Erhardt, M.; Römer, I.; Zu Heringdorf, D.M.; Pfeilschifter, J.; Zangemeister-Wittke, U.; Huwiler, A. Downregulation of the S1P transporter spinster homology protein 2 (Spns2) exerts an anti-fibrotic and anti-inflammatory effect in human renal proximal tubular epithelial cells. Int. J. Mol. Sci. 2018, 19, 1498. [Google Scholar] [CrossRef] [Green Version]
- Sarközi, R.; Corazza, U.; Osterkamp, J.-P.; Pirklbauer, M.; Mayer, G.; Schramek, H. Synergistic induction of CCL2/MCP-1 expression driven by oncostatin M and IL-1β in human proximal tubular cells depends on STAT3 and p65 NFκB/RelA. Physiol. Rep. 2015, 3, e12298. [Google Scholar] [CrossRef]
- Sung, M.J.; Kim, D.H.; Jung, Y.J.; Kang, K.P.; Lee, A.S.; Lee, S.; Kim, W.; Davaatseren, M.; Hwang, J.-T.; Kim, H.-J.; et al. Genistein protects the kidney from cisplatin-induced injury. Kidney Int. 2008, 74, 1538–1547. [Google Scholar] [CrossRef] [Green Version]
- Rovin, B.H.; Lu, L.; Cosio, A. Cyclopentenone prostaglandins inhibit cytokine-induced nf-kappab activation and chemokine production by human mesangial cells. J. Am. Soc. Nephrol. 2001, 12, 1659–1667. [Google Scholar] [CrossRef]
- Lee, M.-J.; Yang, C.W.; Jin, D.C.; Chang, Y.S.; Bang, B.K.; Kim, Y.-S. Bone morphogenetic protein-7 Inhibits constitutive and interleukin-1β-induced monocyte chemoattractant protein-1 expression in human mesangial cells: Role for JNK/AP-1 pathway. J. Immunol. 2003, 170, 2557–2563. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-C.; Hsieh, C.-C.; Kuo, H.-F.; Tsai, M.-K.; Yang, S.-N.; Kuo, C.-H.; Lee, M.-S.; Hung, C.-H. Effect of vitamin D3 on monocyte chemoattractant protein 1 production in monocytes and macrophages. Acta Cardiol. Sin. 2014, 30, 144–150. [Google Scholar]
- Nomura, J.; Busso, N.; Ives, A.; Tsujimoto, S.; Tamura, M.; So, A.; Yamanaka, Y. Febuxostat, an inhibitor of xanthine oxidase, suppresses lipopolysaccharide-induced MCP-1 production via MAPK phosphatase-1-mediated inactivation of JNK. PLoS ONE 2013, 8, e75527. [Google Scholar] [CrossRef]
- Guha, M.; O’Connell, M.A.; Pawlinski, R.; Hollis, A.; McGovern, P.; Yan, S.F.; Stern, D.; Mackman, N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 2001, 98, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Setchell, K.D.R.; Faughnan, M.S.; Avades, T.; Zimmer-Nechemias, L.; Brown, N.M.; Wolfe, B.E.; Brashear, W.T.; Desai, P.; Oldfield, M.F.; Botting, N.P.; et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am. J. Clin. Nutr. 2003, 77, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Zierenberg, O.; Grundy, S.M. Intestinal absorption of polyene phosphatidylcholine in man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Fukui, K.; Tachibana, N.; Wanezaki, S.; Tsuzaki, S.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Shimoda, T. Isoflavone-free soy protein prepared by column chromatography reduces plasma cholesterol in rats. J. Agric. Food Chem. 2002, 50, 5717–5721. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, Y.L.; Hyde, W.; Hendrich, S.; Murphy, P.A. Human fecal metabolism of soyasaponin I. J. Agric. Food Chem. 2004, 52, 2689–2696. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Reddy, M.B.; Hendrich, S.; Murphy, P.A. Soyasaponin I and Sapongenol B have limited absorption by Caco-2 intestinal cells and limited bioavailability in women. J. Nutr. 2004, 134, 1867–1873. [Google Scholar] [CrossRef] [Green Version]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-Derived Di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J. Nutr. 2011, 142, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Arito, M.; Mitsui, H.; Kurokawa, M.S.; Yudoh, K.; Kamada, T.; Niki, H.; Kato, T. Effects of soy peptides on IL-1β-induced matrix-degrading enzymes in human articular chondrocytes. Integr. Mol. Med. 2016, 3, 661–665. [Google Scholar] [CrossRef] [Green Version]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef]
- Kudou, S.; Fleury, Y.; Welti, D.; Magnolato, D.; Uchida, T.; Kitamura, K.; Okubo, K. Malonyl isoflavone glycosides in soybean seeds ( Glycine max Merrill). Agric. Biol. Chem. 1991, 55, 2227–2233. [Google Scholar] [CrossRef] [Green Version]
- Lott, J.A.; Stephan, V.A.; Pritchard, K.A. Evaluation of the coomassie brilliant blue G-250 method for urinary protein. Clin. Chem. 1983, 29, 1946–1950. [Google Scholar] [CrossRef]
- Noto, A.; Ogawa, Y.; Mori, S.; Yoshioka, M.; Kitakaze, T.; Hori, T.; Nakamura, M.; Miyake, T. Simple, rapid spectrophotometry of urinary N-acetyl-beta-D-glucosaminidase, with use of a new chromogenic substrate. Clin. Chem. 1983, 29, 1713–1716. [Google Scholar] [CrossRef] [PubMed]






| PC | 10.9 |
| PE | 3.94 |
| PI | 7.04 |
| PS | 0.64 |
| Daidzein | 1.31 |
| Glycitein | 0.23 |
| Genistein | 1.98 |
| (g/100g) |
| Cas | SPI | SPIEE | p-Values (vs. Cas) | ||
|---|---|---|---|---|---|
| (n) | 5 | 5 | 5 | (SPI, SPIEE) | |
| Initial weight (g) | 313.2 ± 6.0 | 314.3 ± 4.4 | 314.3 ± 5.1 | 0.983, 0.983 | |
| Final weight (g) | 715.1 ± 22.2 | 698.6 ± 9.0 | 733.4 ± 16.4 | 0.715, 0.663 | |
| Food intake (g/day) | 25.4 ± 0.9 | 23.4 ± 0.5 | 24.6 ± 0.7 | 0.109, 0.637 | |
| Food efficiency ratio | 0.139 ± 0.005 | 0.145 ± 0.005 | 0.150 ±0.003 | 0.548, 0.16 |
| Casein | SPI | SPIEE | |
|---|---|---|---|
| Protein source | |||
| Casein § | 227 | 227 | |
| SPI † | 234 | ||
| SPIEE | 18.1 | ||
| Soybean oil ‡ | 70 | 70 | 70 |
| Cornstarch | 373 | 367 | 355 |
| Sucrose | 100 | 100 | 100 |
| Dextrinized cornstarch | 132 | 132 | 132 |
| Cellulose powder | 50 | 50 | 50 |
| Mineral mixture ║ | 35 | 35 | 35 |
| Vitamin mixture ║ | 10 | 10 | 10 |
| Choline bitartrate | 3 | 3 | 3 |
| Total (g) | 1000 | 1000 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohta, S.; Asanoma, M.; Irie, N.; Tachibana, N.; Kohno, M. Soy Phospholipids Exert a Renoprotective Effect by Inhibiting the Nuclear Factor Kappa B Pathway in Macrophages. Metabolites 2022, 12, 330. https://doi.org/10.3390/metabo12040330
Ohta S, Asanoma M, Irie N, Tachibana N, Kohno M. Soy Phospholipids Exert a Renoprotective Effect by Inhibiting the Nuclear Factor Kappa B Pathway in Macrophages. Metabolites. 2022; 12(4):330. https://doi.org/10.3390/metabo12040330
Chicago/Turabian StyleOhta, Satoshi, Masashi Asanoma, Nao Irie, Nobuhiko Tachibana, and Mitsutaka Kohno. 2022. "Soy Phospholipids Exert a Renoprotective Effect by Inhibiting the Nuclear Factor Kappa B Pathway in Macrophages" Metabolites 12, no. 4: 330. https://doi.org/10.3390/metabo12040330
APA StyleOhta, S., Asanoma, M., Irie, N., Tachibana, N., & Kohno, M. (2022). Soy Phospholipids Exert a Renoprotective Effect by Inhibiting the Nuclear Factor Kappa B Pathway in Macrophages. Metabolites, 12(4), 330. https://doi.org/10.3390/metabo12040330





