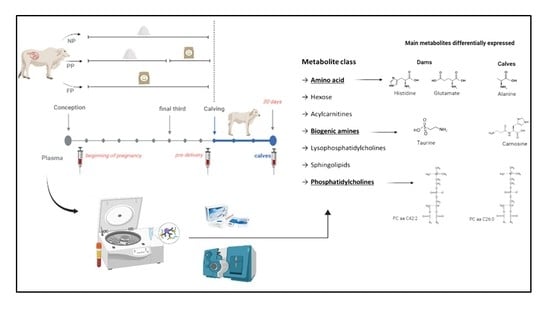
Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves
Abstract
:1. Introduction
2. Results
2.1. Univariate Analysis of Metabolome
2.2. Principal Component Analysis (PCA)
2.3. Partial Least Squares Discriminant Analysis (PLS-DA)
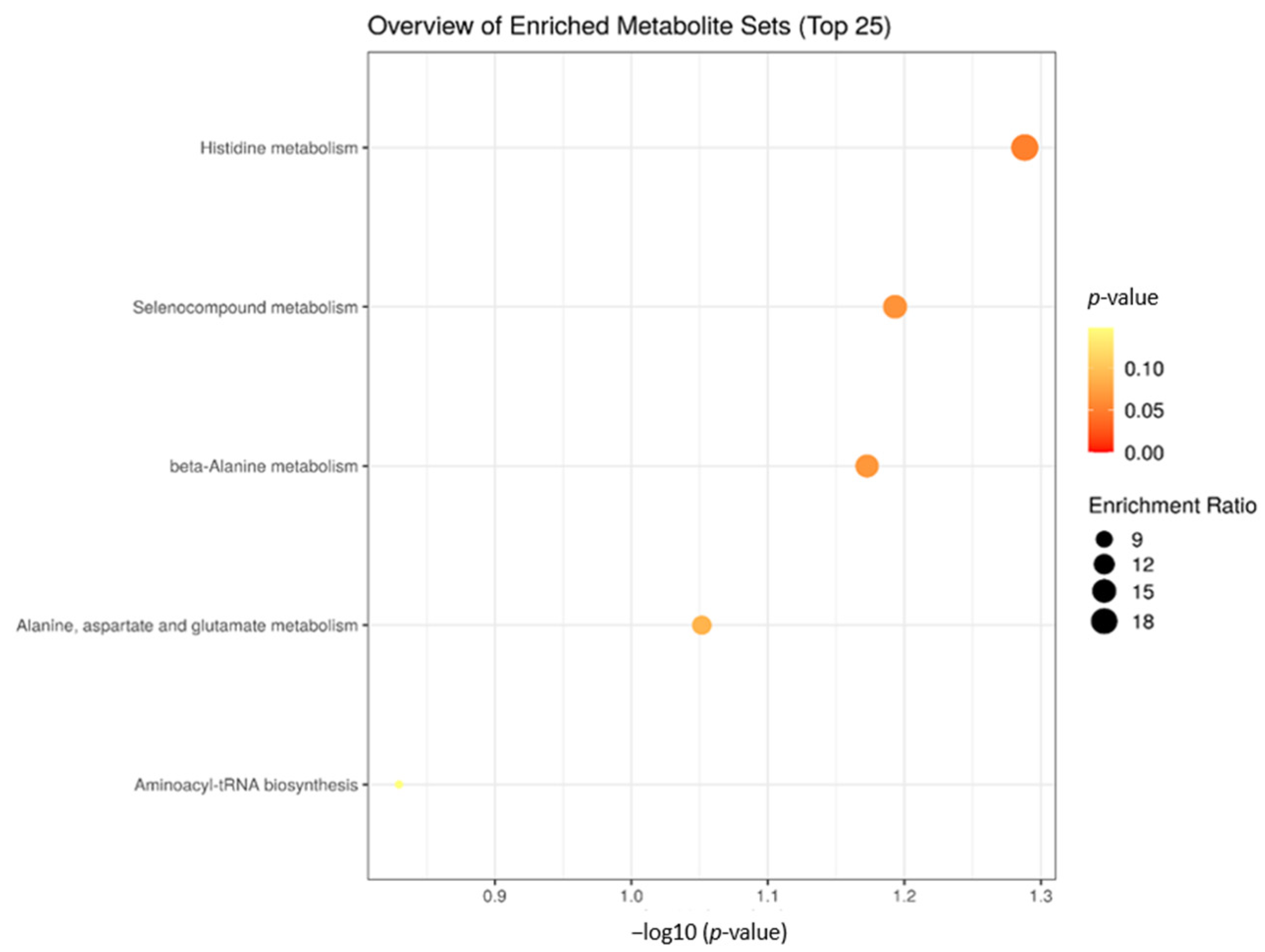
2.4. Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Plasma Sample Collection and Preparation
4.3. Targeted Metabolomics
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paradis, F.; Wood, K.M.; Swanson, K.C.; Miller, S.P.; McBride, B.W.; Fitzsimmons, C. Maternal Nutrient Restriction in Mid-to-Late Gestation Influences Fetal MRNA Expression in Muscle Tissues in Beef Cattle. BMC Genom. 2017, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Ward, A.K.; Caton, J.S. Epigenetics and Developmental Programming in Ruminants Long-Term Impacts on Growth and Development. In Biology of Domestic Animals; CRC Press: Boca Raton, FL, USA, 2017; pp. 85–121. ISBN 9781498747875. [Google Scholar]
- Vonnahme, K.A.; Tanner, A.R.; Hildago, M.A.V. Effect of Maternal Diet on Placental Development, Uteroplacental Blood Flow, and Offspring Development in Beef Cattle. Anim. Reprod. 2018, 15, 912–922. [Google Scholar] [CrossRef]
- Van Emon, M.; Sanford, C.; McCoski, S. Impacts of Bovine Trace Mineral Supplementation on Maternal and Offspring Production and Health. Animals 2020, 10, 2404. [Google Scholar] [CrossRef] [PubMed]
- Long, N.M.; Vonnahme, K.A.; Hess, B.W.; Nathanielsz, P.W.; Ford, S.P. Effects of Early Gestational Undernutrition on Fetal Growth, Organ Development, and Placentomal Composition in the Bovine. J. Anim. Sci. 2009, 87, 1950–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavatte-Palmer, P.; Tarrade, A.; Kiefer, H.; Duranthon, V.; Jammes, H.; Chavatte-Palmer, P.; Tarrade, A.; Kiefer, H.; Duranthon, V.; Jammes, H. Breeding Animals for Quality Products: Not Only Genetics. Reprod. Fertil. Dev. 2016, 28, 94–111. [Google Scholar] [CrossRef]
- Moriel, P.; Vedovatto, M.; Palmer, E.A.; Oliveira, R.A.; Silva, H.M.; Ranches, J.; Vendramini, J.M.B. Maternal Supplementation of Energy and Protein, but Not Methionine Hydroxy Analog, Enhanced Postnatal Growth and Response to Vaccination in Bos Indicus-Influenced Beef Offspring. J. Anim. Sci. 2020, 98, skaa123. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; de Francisco Strefezzi, R.; Cracco, R.C.; Fernandes, A.C.; Zuca, C.B.; Castellar, H.H.; Baldin, G.C.; de Almeida Santana, M.H. Effects of Different Maternal Nutrition Approaches on Weight Gain and on Adipose and Muscle Tissue Development of Young Bulls in the Rearing Phase. Trop. Anim. Health Prod. 2021, 53, 536. [Google Scholar] [CrossRef]
- Ramírez, M.; Testa, L.M.; López Valiente, S.; Latorre, M.E.; Long, N.M.; Rodriguez, A.M.; Pavan, E.; Maresca, S. Maternal Energy Status during Late Gestation: Effects on Growth Performance, Carcass Characteristics and Meat Quality of Steers Progeny. Meat Sci. 2020, 164, 108095. [Google Scholar] [CrossRef]
- Silva, G.M.; Chalk, C.D.; Ranches, J.; Schulmeister, T.M.; Henry, D.D.; DiLorenzo, N.; Arthington, J.D.; Moriel, P.; Lancaster, P.A. Effect of Rumen-Protected Methionine Supplementation to Beef Cows during the Periconception Period on Performance of Cows, Calves, and Subsequent Offspring. Animal 2021, 15, 100055. [Google Scholar] [CrossRef]
- Polizel, G.H.G.; Fantinato-Neto, P.; Rangel, R.B.; Grigoletto, L.; de Oliveira Bussiman, F.; Cracco, R.C.; Garcia, N.P.; Ruy, I.M.; Ferraz, J.B.S.; de Almeida Santana, M.H. Evaluation of Reproductive Traits and the Effect of Nutrigenetics on Bulls Submitted to Fetal Programming. Livest. Sci. 2021, 247, 104487. [Google Scholar] [CrossRef]
- Cracco, R.C.; de Oliveira Bussiman, F.; Polizel, G.H.G.; Furlan, É.; Garcia, N.P.; Poit, D.A.S.; Pugliesi, G.; de Almeida Santana, M.H. Effects of Maternal Nutrition on Female Offspring Weight Gain and Sexual Development. Front. Genet. 2021, 12, 2059. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock Metabolomics and the Livestock Metabolome: A Systematic Review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [Green Version]
- Hellmuth, C.; Lindsay, K.L.; Uhl, O.; Buss, C.; Wadhwa, P.D.; Koletzko, B.; Entringer, S. Maternal Metabolomic Profile and Fetal Programming of Offspring Adiposity: Identification of Potentially Protective Lipid Metabolites. Mol. Nutr. Food Res. 2019, 63, 1700889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abarghuei, M.J.; Rouzbehan, Y.; Salem, A.Z.M.; Zamiri, M.J. Nitrogen Balance, Blood Metabolites and Milk Fatty Acid Composition of Dairy Cows Fed Pomegranate-Peel Extract. Livest. Sci. 2014, 164, 72–80. [Google Scholar] [CrossRef]
- Saleem, F.; Ametaj, B.N.; Bouatra, S.; Mandal, R.; Zebeli, Q.; Dunn, S.M.; Wishart, D.S. A Metabolomics Approach to Uncover the Effects of Grain Diets on Rumen Health in Dairy Cows. J. Dairy Sci. 2012, 95, 6606–6623. [Google Scholar] [CrossRef] [Green Version]
- Karisa, B.K.; Thomson, J.; Wang, Z.; Li, C.; Montanholi, Y.R.; Miller, S.P.; Moore, S.S.; Plastow, G.S. Plasma Metabolites Associated with Residual Feed Intake and Other Productivity Performance Traits in Beef Cattle. Livest. Sci. 2014, 165, 200–211. [Google Scholar] [CrossRef]
- Novais, F.J.; Pires, P.R.L.; Alexandre, P.A.; Dromms, R.A.; Iglesias, A.H.; Ferraz, J.B.S.; Styczynski, M.P.W.; Fukumasu, H. Identification of a Metabolomic Signature Associated with Feed Efficiency in Beef Cattle. BMC Genom. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muroya, S.; Zhang, Y.; Kinoshita, A.; Otomaru, K.; Oshima, K.; Gotoh, Y.; Oshima, I.; Sano, M.; Roh, S.; Oe, M.; et al. Maternal Undernutrition during Pregnancy Alters Amino Acid Metabolism and Gene Expression Associated with Energy Metabolism and Angiogenesis in Fetal Calf Muscle. Metabolites 2021, 11, 582. [Google Scholar] [CrossRef]
- Muroya, S.; Zhang, Y.; Otomaru, K.; Oshima, K.; Oshima, I.; Sano, M.; Roh, S.; Ojima, K.; Gotoh, T. Maternal Nutrient Restriction Disrupts Gene Expression and Metabolites Associated with Urea Cycle, Steroid Synthesis, Glucose Homeostasis, and Glucuronidation in Fetal Calf Liver. Metabolites 2022, 12, 203. [Google Scholar] [CrossRef]
- Arany, E. Maternal Taurine Supplementation Prevents Misprogramming. In Diet, Nutrition, and Fetal Programming; Humana Press: Totowa, NJ, USA, 2017; pp. 309–324. [Google Scholar]
- Ghaffari, M.H.; Sadri, H.; Schuh, K.; Dusel, G.; Frieten, D.; Koch, C.; Prehn, C.; Adamski, J.; Sauerwein, H. Biogenic Amines: Concentrations in Serum and Skeletal Muscle from Late Pregnancy until Early Lactation in Dairy Cows with High versus Normal Body Condition Score. J. Dairy Sci. 2019, 102, 6571–6586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, L.; Van Assche, F.A. Taurine and Taurine-Deficiency in the Perinatal Period. J. Perinat. Med. 2002, 30, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.M.; Choi, M.J. Relation of Taurine Intake During Pregnancy and Newborns’ Growth. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1155, pp. 283–292. [Google Scholar]
- Wu, M.; Xiao, H.; Ren, W.; Yin, J.; Tan, B.; Liu, G.; Li, L.; Nyachoti, C.M.; Xiong, X.; Wu, G. Therapeutic Effects of Glutamic Acid in Piglets Challenged with Deoxynivalenol. PLoS ONE 2014, 9, e100591. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wu, M.; Luo, W.; Huang, R.; Yin, Y.; Li, Y.; Li, T.; Yu, X. Dietary Supplementation with Proline Confers a Positive Effect in Both Porcine Circovirus-Infected Pregnant and Non-Pregnant Mice. Br. J. Nutr. 2013, 110, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Ruth, M.R.; Field, C.J. The Immune Modifying Effects of Amino Acids on Gut-Associated Lymphoid Tissue. J. Anim. Sci. Biotechnol. 2013, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Caroprese, M.; Albenzio, M.; Marino, R.; Santillo, A.; Sevi, A. Immune Response and Milk Production of Dairy Cows Fed Graded Levels of Rumen-Protected Glutamine. Res. Vet. Sci. 2012, 93, 202–209. [Google Scholar] [CrossRef]
- Watford, M. Glutamine Metabolism and Function in Relation to Proline Synthesis and the Safety of Glutamine and Proline Supplementation. J. Nutr. 2008, 138, 2003S–2007S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yin, Y.; Shu, X.G.; Li, T.; Li, F.; Tan, B.; Wu, Z.; Wu, G. Oral Administration of MSG Increases Expression of Glutamate Receptors and Transporters in the Gastrointestinal Tract of Young Piglets. Amino Acids 2013, 45, 1169–1177. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Zhou, X.; Wu, F.; Yao, K.; Kong, X.; Li, T.; Blachier, F.; Yin, Y. Both Dietary Supplementation with Monosodium L-Glutamate and Fat Modify Circulating and Tissue Amino Acid Pools in Growing Pigs, but with Little Interactive Effect. PLoS ONE 2014, 9, e84533. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kadarmideen, H.N. Metabolomics Analyses in High-Low Feed Efficient Dairy Cows Reveal Novel Biochemical Mechanisms and Predictive Biomarkers. Metabolites 2019, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.C.; Azzout-Marniche, D. Histidine: A Systematic Review on Metabolism and Physiological Effects in Human and Different Animal Species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef] [PubMed]
- Schoof, A.; Gabel, M.; Voigt, J.; Schönhusen, U.; Kluth, H. Investigations on the Influence of Duodenal Histidine Infusion on Nitrogen and Amino Acid Turnover of Growing German Holstein Bulls. Arch. Anim. Nutr. 2000, 53, 303–321. [Google Scholar] [CrossRef]
- Doelman, J.; Purdie, N.G.; Osborne, V.R.; Cant, J.P. Short Communication: The Effects of Histidine-Supplemented Drinking Water on the Performance of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3998–4001. [Google Scholar] [CrossRef] [Green Version]
- Giallongo, F.; Harper, M.T.; Oh, J.; Parys, C.; Shinzato, I.; Hristov, A.N. Histidine Deficiency Has a Negative Effect on Lactational Performance of Dairy Cows. J. Dairy Sci. 2017, 100, 2784–2800. [Google Scholar] [CrossRef]
- Yang, T.; Cheng, Z.; Jiang, M.; Ma, X.; Datsomor, O.; Zhao, G.; Zhan, K. Histidine Promotes the Glucose Synthesis through Activation of the Gluconeogenic Pathway in Bovine Hepatocytes. Animals 2021, 11, 3295. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, N.V.; Ha, C.-E. Lipids II. In Essentials of Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 299–320. ISBN 978-0-12-416687-5. [Google Scholar]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Edidin, M. Lipids on the Frontier: A Century of Cell-Membrane Bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Marchlewicz, E.H.; Dolinoy, D.C.; Tang, L.; Milewski, S.; Jones, T.R.; Goodrich, J.M.; Soni, T.; Domino, S.E.; Song, P.X.K.; Burant, C.F.; et al. Lipid Metabolism Is Associated with Developmental Epigenetic Programming. Sci. Rep. 2016, 6, 34857. [Google Scholar] [CrossRef]
- Klopp, R.N.; Ferreira, C.R.; Casey, T.M.; Boerman, J.P. Relationship of Cow and Calf Circulating Lipidomes with Colostrum Lipid Composition and Metabolic Status of the Cow. J. Dairy Sci. 2021, 105, 1768–1787. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.; Staples, C.R.; Thatcher, W.W. Effects of Dietary Fatty Acids on Reproduction in Ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of Beta-Alanine Supplementation on Muscle Carnosine Concentrations and Exercise Performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and Pathophysiology of Carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Barca, A.; Ippati, S.; Urso, E.; Vetrugno, C.; Storelli, C.; Maffia, M.; Romano, A.; Verri, T. Carnosine Modulates the Sp1-Slc31a1/Ctr1 Copper-Sensing System and Influences Copper Homeostasis in Murine CNS-Derived Cells. Am. J. Physiol.—Cell Physiol. 2019, 316, C235–C245. [Google Scholar] [CrossRef]
- Liu, Q. Concentrations of Creatine, Creatinine, Carnosine, and Anserine in Bovine Longissimus Muscle and Their Correlations with Carcass and Palatability Traits; Iowa State University: Ames, IA, USA, 2011. [Google Scholar]
- Cônsolo, N.R.B.; Buarque, V.L.M.; Silva, J.; Poleti, M.D.; Barbosa, L.C.G.S.; Higuera-Padilla, A.; Gómez, J.F.M.; Colnago, L.A.; Gerrard, D.E.; Saran Netto, A.; et al. Muscle and Liver Metabolomic Signatures Associated with Residual Feed Intake in Nellore Cattle. Anim. Feed Sci. Technol. 2021, 271, 114757. [Google Scholar] [CrossRef]
- Felig, P.; Pozefsk, T.; Marlis, E.; Cahill, G.F. Alanine: Key Role in Gluconeogenesis. Science 1970, 167, 1003–1004. [Google Scholar] [CrossRef]
- Foroutan, A.; Fitzsimmons, C.; Mandal, R.; Piri-moghadam, H.; Zheng, J.; Guo, A.; Li, C.; Guan, L.L.; Wishart, D.S. The Bovine Metabolome. Metabolites 2020, 10, 233. [Google Scholar] [CrossRef]
- Miles, E.D.; McBride, B.W.; Jia, Y.; Liao, S.F.; Boling, J.A.; Bridges, P.J.; Matthews, J.C. Glutamine Synthetase and Alanine Transaminase Expression Are Decreased in Livers of Aged vs. Young Beef Cows and GS Can Be Upregulated by 17β-Estradiol Implants. J. Anim. Sci. 2015, 93, 4500–4509. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Wesolowski, S.R.; Gilje, E.A.; Baker, P.R.; Reisz, J.A.; D’Alessandro, A.; Hay, W.W.; Rozance, P.J.; Brown, L.D. Hormones, Reproduction and Development: Skeletal Muscle Amino Acid Uptake Is Lower and Alanine Production Is Greater in Late Gestation Intrauterine Growth-Restricted Fetal Sheep Hindlimb. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2019, 317, R615. [Google Scholar] [CrossRef] [PubMed]
- Diniz, W.J.S.; Reynolds, L.P.; Borowicz, P.P.; Ward, A.K.; Sedivec, K.K.; McCarthy, K.L.; Kassetas, C.J.; Baumgaertner, F.; Kirsch, J.D.; Dorsam, S.T.; et al. Maternal Vitamin and Mineral Supplementation and Rate of Maternal Weight Gain Affects Placental Expression of Energy Metabolism and Transport-Related Genes. Genes 2021, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Queiroz, A. Análise de Alimentos: Métodos Químicos e Biológicos, 3rd ed.; UFV: Viçosa, Brazil, 2009. [Google Scholar]
- Van Soest, P.J. Development of a Comprehensive System of Feed Analyses and Its Application to Forages. J. Anim. Sci. 1967, 26, 119–128. [Google Scholar] [CrossRef]
- Van Soest, P. Nutritional Ecology of the Ruminant, 4th ed.; Cornell University Press: Ithaca, NY, USA, 1995. [Google Scholar]
- Sindirações Métodos Analíticos—Minerais Por Espectrometria de Emissão Atômica Por Plasma Indutivamente Acoplado (ICP-OES). In Compêndio Brasileiro de Alimentação Animal; SINDI LAÇÕES: São Paulo, Brazil, 2013; pp. 175–180.
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]





| Metabolites (µM) | NP | PP | FP | p Value |
|---|---|---|---|---|
| PC aa C30:0 | 1.44 ± 0.05 a,b | 1.26 ± 0.04 a | 1.58 ± 0.06 b | <0.001 |
| Histidine | 61.3 ± 4.03 a | 43.1 ± 3.31 b | 57.5 ± 2.83 a | 0.003 |
| PC ae C30:2 | 254.1 ± 16.0 a,b | 235.2 ± 8.11 a | 289.3 ± 6.17 b | 0.004 |
| PC ae C30:0 | 110.3 ± 5.80 a,b | 101.0 ± 3.76 a | 127.0 ± 7.51 b | 0.009 |
| PC ae C30:1 | 413.2 ± 37.3 a | 419.1 ± 21.4 a,b | 505.1 ± 19.9 b | 0.044 |
| Metabolites (µM) | NP | PP | FP | p Value |
|---|---|---|---|---|
| SDMA | 0.86 ± 0.03 a | 0.65 ± 0.03 b | 0.67 ± 0.04 b | 0.001 |
| PC aa C34:4 | 10.5 ± 1.21 a | 17.9 ± 2.09 b | 17.0 ± 1.82 b | 0.002 |
| PC aa C38:3 | 61.9 ± 4.35 a | 89.6 ± 6.73 b | 97.8 ± 10.6 b | 0.003 |
| PC aa C40:3 | 4.14 ± 0.38 a | 6.71 ± 0.59 b | 7.35 ± 0.84 b | 0.005 |
| Taurine | 16.2 ± 2.03 a | 30.7 ± 4.54 b | 23.0 ± 1.86 a,b | 0.005 |
| Glutamic acid | 48.0 ± 3.34 a | 55.3 ± 2.66 a,b | 70.2 ± 6.06 b | 0.005 |
| PC ae C38:1 | 12.8 ± 1.00 a | 14.4 ± 2.02 b | 14.4 ± 2.31 b | 0.009 |
| PC ae C34:3 | 30.6 ± 3.60 a | 51.7 ± 5.92 b | 44.1 ± 4.29 a,b | 0.014 |
| PC aa C40:4 | 11.9 ± 1.01 a | 14.8 ± 1.09 a,b | 17.0 ± 1.32 b | 0.015 |
| PC aa C42:4 | 60.7 ± 5.83 a | 79.3 ± 8.37 a,b | 92.0 ± 8.31 b | 0.030 |
| PC aa C42:2 | 51.0 ± 5.67 a | 69.0 ± 5.75 b | 55.1 ± 4.22 a,b | 0.035 |
| PC ae C42:4 | 0.55 ± 0.05 a | 0.75 ± 0.06 b | 0.73 ± 0.06 a,b | 0.037 |
| PC aa C42:6 | 0.92 ± 0.06 a | 0.81 ± 0.06 a,b | 0.70 ± 0.05 b | 0.040 |
| Histidine | 74.2 ± 4.17 a,b | 70.3 ± 4.91 a | 88.2 ± 5.36 b | 0.042 |
| Carnosine | 25.4 ± 2.03 a | 31.1 ± 2.39 a,b | 33.7 ± 2.80 b | 0.044 |
| PC aa C36:2 | 289.1 ± 41.1 a | 457.2 ± 54.3 b | 414.0 ± 48.31 a,b | 0.048 |
| Metabolites (µM) | NP | PP | FP | p Value |
|---|---|---|---|---|
| PC aa C42:6 | 1.45 ± 0.15 a | 1.24 ± 0.07 a,b | 0.96 ± 0.06 b | 0.010 |
| PC ae C38:4 | 6.14 ± 0.42 a | 8.43 ± 0.59 b | 7.50 ± 0.51 a,b | 0.011 |
| Carnosine | 31.8 ± 3.20 a | 30.3 ± 3.46 a,b | 20.6 ± 1.88 b | 0.030 |
| Alanine | 261.0 ± 17.8 a,b | 279.2 ± 14.8 a | 226.0 ± 8.40 b | 0.048 |
| PC aa C26:0 | 7.64 ± 0.24 a,b | 9.07 ± 0.85 a | 6.57 ± 0.72 b | 0.049 |
| PC ae C40:4 | 1.56 ± 0.12 a | 2.09 ± 0.16 b | 1.94 ± 0.21 a,b | 0.049 |
| Ingredients/Nutrients | Mineral Supplement | Protein-Energy Supplement |
|---|---|---|
| Corn (%) | 35.00 | 60.00 |
| Soybean meal (%) | - | 30.00 |
| Dicalcium phosphate (%) | 10.00 | - |
| Urea 45% (%) | - | 2.50 |
| Salt (%) | 30.00 | 5.00 |
| Minerthal 160 MD (%) * | 25.00 | 2.50 |
| Total digestible nutrients (%) | 26.76 | 67.55 |
| Crude protein (%) | 2.79 | 24.78 |
| Non-protein nitrogen (%) | - | 7.03 |
| Acid detergent fiber (%) | 1.25 | 4.76 |
| Neutral detergent fiber (%) | 4.29 | 11.24 |
| Fat (%) | 1.26 | 2.61 |
| Calcium (g/kg) | 74.11 | 6.20 |
| Phosphorus (g/kg) | 59.38 | 7.24 |
| Forage Nutrients | NP | PP | FP |
|---|---|---|---|
| CP % (crude protein) | 7.38 ± 0.70 | 7.82 ± 0.93 | 7.40 ± 0.93 |
| TDN % (total digestible nutrients) | 63.1 ± 0.59 | 64.1 ± 0.95 | 61.4 ± 0.86 |
| NDF % (neutral detergent fiber) | 59.0 ± 1.49 | 61.4 ± 2.06 | 58.4 ± 1.67 |
| Ca % (calcium) | 0.38 ± 0.04 | 0.35 ± 0.02 | 0.39 ± 0.03 |
| P % (phosphorus) | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.17 ± 0.01 |
| Traits | NP | PP | FP | p Value |
|---|---|---|---|---|
| Initial BW (kg) | 461.1 ± 6.90 | 451.2 ± 9.38 | 454.1 ± 8.76 | 0.85 |
| Pre-delivery BW (kg) | 508.0 ± 7.23 a | 524.2 ± 9.07 a | 541.2 ± 10.1 b | <0.01 |
| Initial SFT (mm) | 4.28 ± 0.61 | 4.31 ± 0.61 | 4.33 ± 0.61 | 0.92 |
| Pre-delivery SFT (mm) | 7.23 ± 0.66 a | 9.24 ± 0.67 a | 12.5 ± 0.98 b | <0.01 |
| Initial BCS | 4.50 ± 0.09 | 4.60 ± 0.12 | 4.50 ± 0.09 | 0.34 |
| Pre-delivery BCS | 5.40 ± 0.13 a | 5.60 ± 0.13 a,b | 5.90 ± 0.13 b | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schalch Junior, F.J.; Polizel, G.H.G.; Cançado, F.A.C.Q.; Fernandes, A.C.; Mortari, I.; Pires, P.R.L.; Fukumasu, H.; Santana, M.H.d.A.; Saran Netto, A. Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites 2022, 12, 347. https://doi.org/10.3390/metabo12040347
Schalch Junior FJ, Polizel GHG, Cançado FACQ, Fernandes AC, Mortari I, Pires PRL, Fukumasu H, Santana MHdA, Saran Netto A. Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites. 2022; 12(4):347. https://doi.org/10.3390/metabo12040347
Chicago/Turabian StyleSchalch Junior, Fernando José, Guilherme Henrique Gebim Polizel, Fernando Augusto Correia Queiroz Cançado, Arícia Christofaro Fernandes, Isabela Mortari, Pedro Ratto Lisboa Pires, Heidge Fukumasu, Miguel Henrique de Almeida Santana, and Arlindo Saran Netto. 2022. "Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves" Metabolites 12, no. 4: 347. https://doi.org/10.3390/metabo12040347
APA StyleSchalch Junior, F. J., Polizel, G. H. G., Cançado, F. A. C. Q., Fernandes, A. C., Mortari, I., Pires, P. R. L., Fukumasu, H., Santana, M. H. d. A., & Saran Netto, A. (2022). Prenatal Supplementation in Beef Cattle and Its Effects on Plasma Metabolome of Dams and Calves. Metabolites, 12(4), 347. https://doi.org/10.3390/metabo12040347