Accumulation of Secondary Metabolites of Rhodiola semenovii Boriss. In Situ in the Dynamics of Growth and Development
Abstract
:1. Introduction
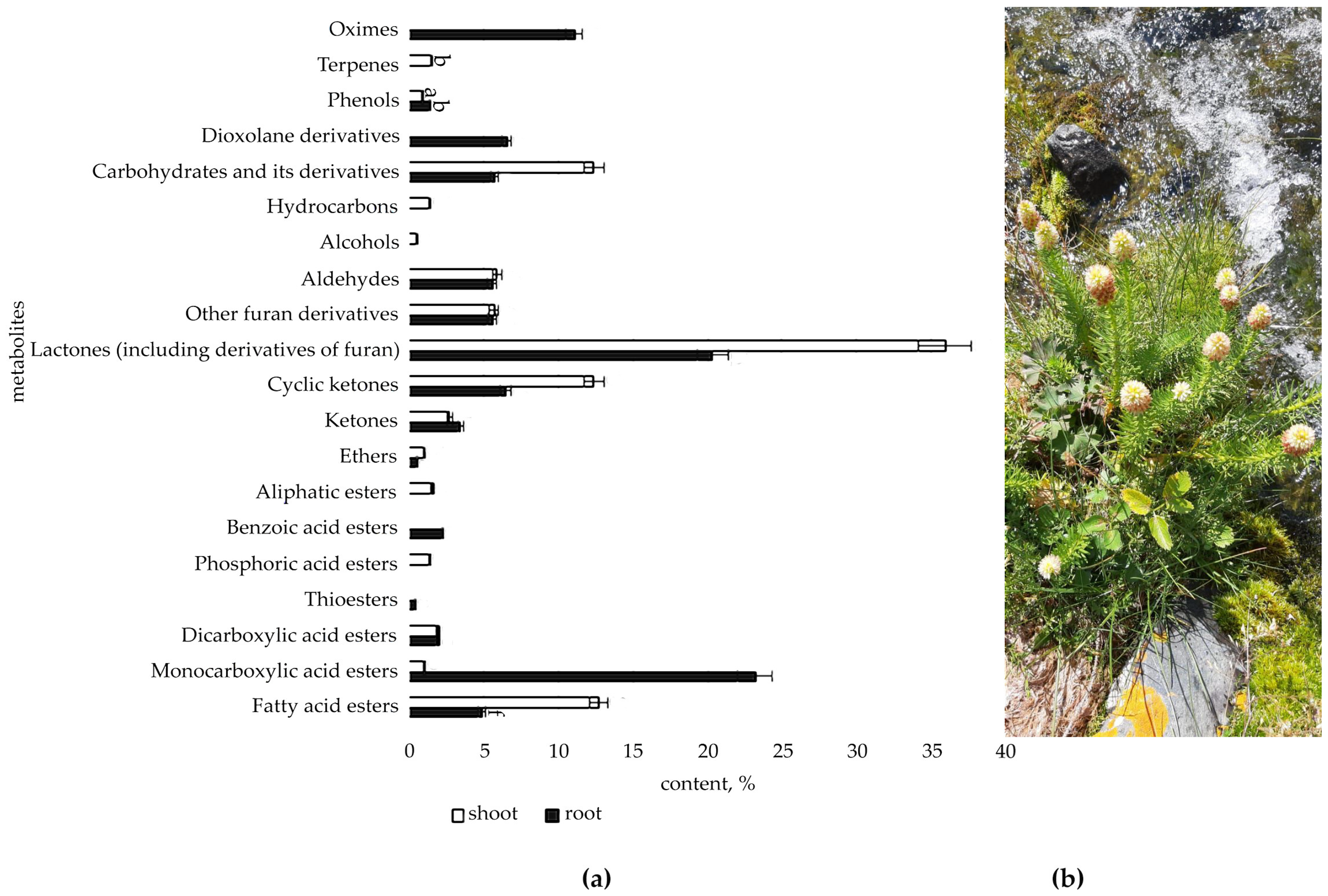
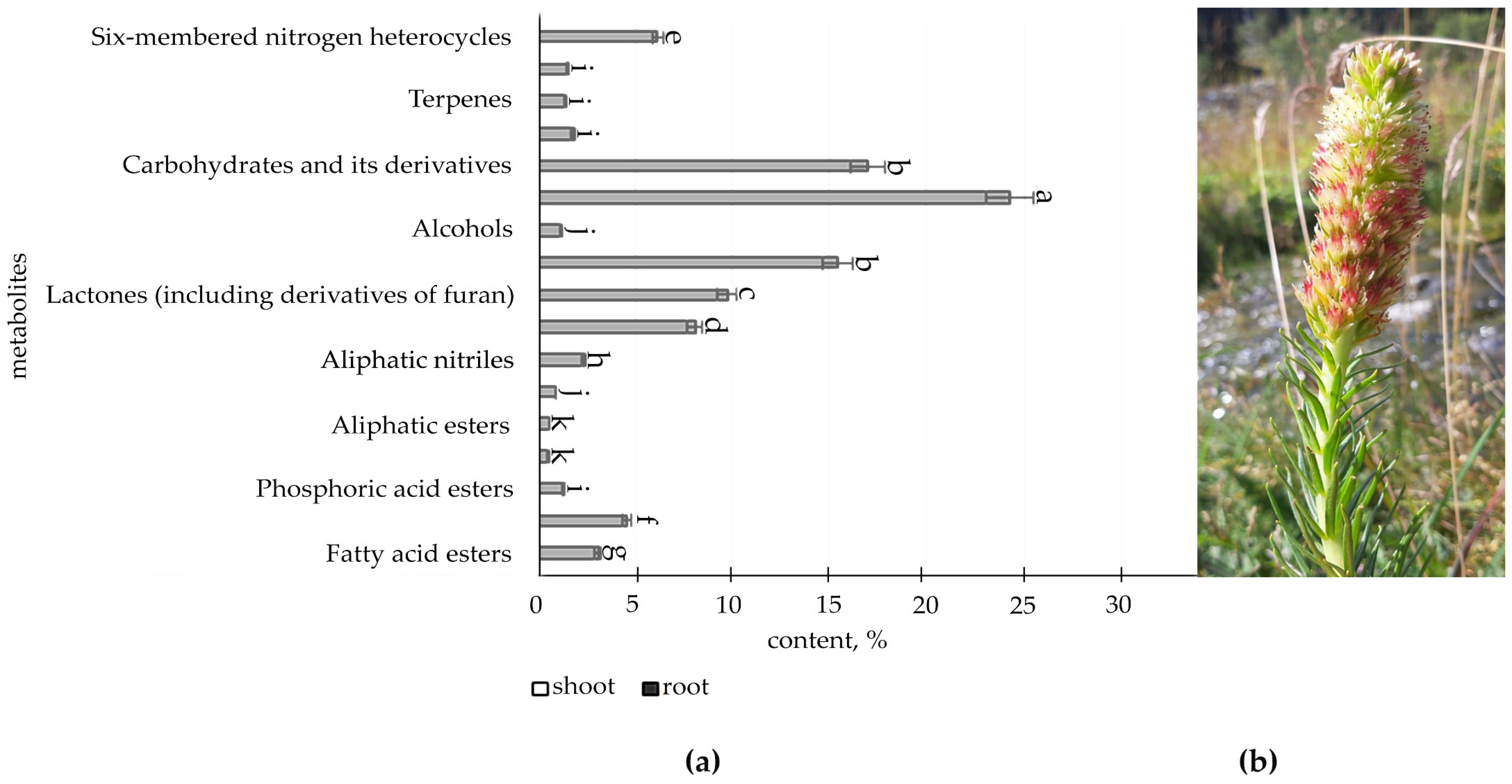
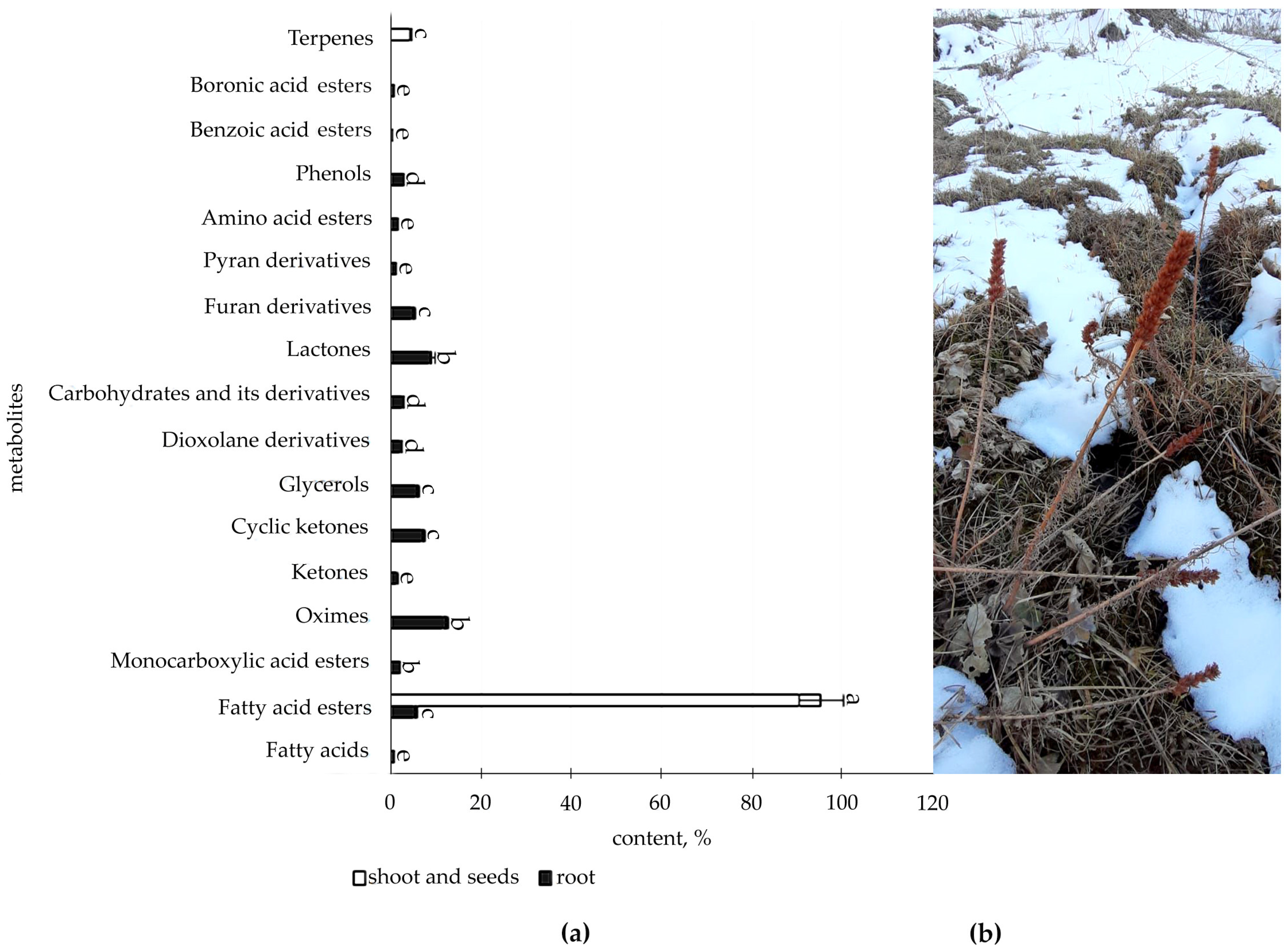
2. Results
2.1. Determination of Organic Compounds in Root and Shoot of R. semenovii during Vegetation
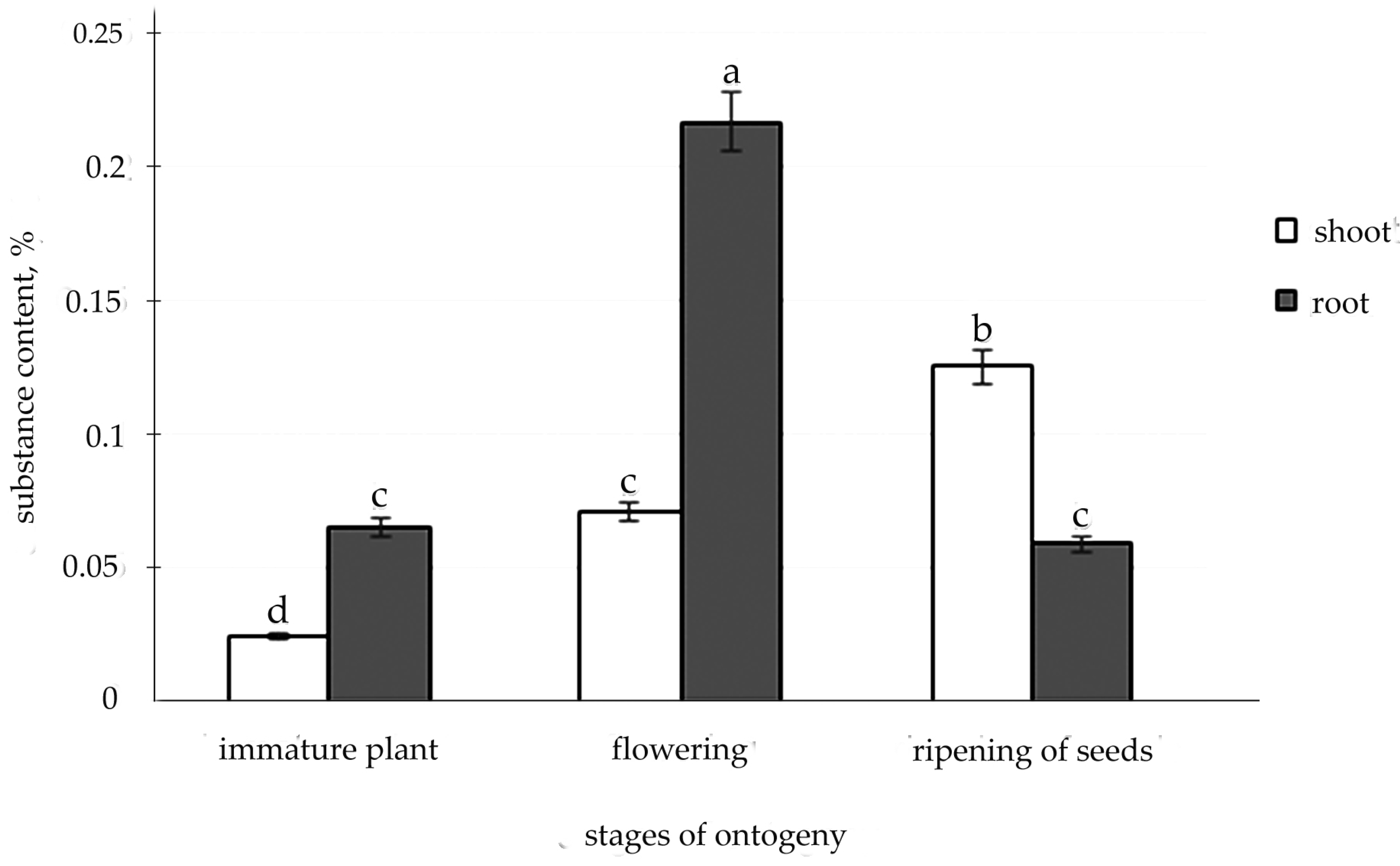
2.2. Determination of Salidrosid in Root and Shoot of R. semenovii during Vegetation
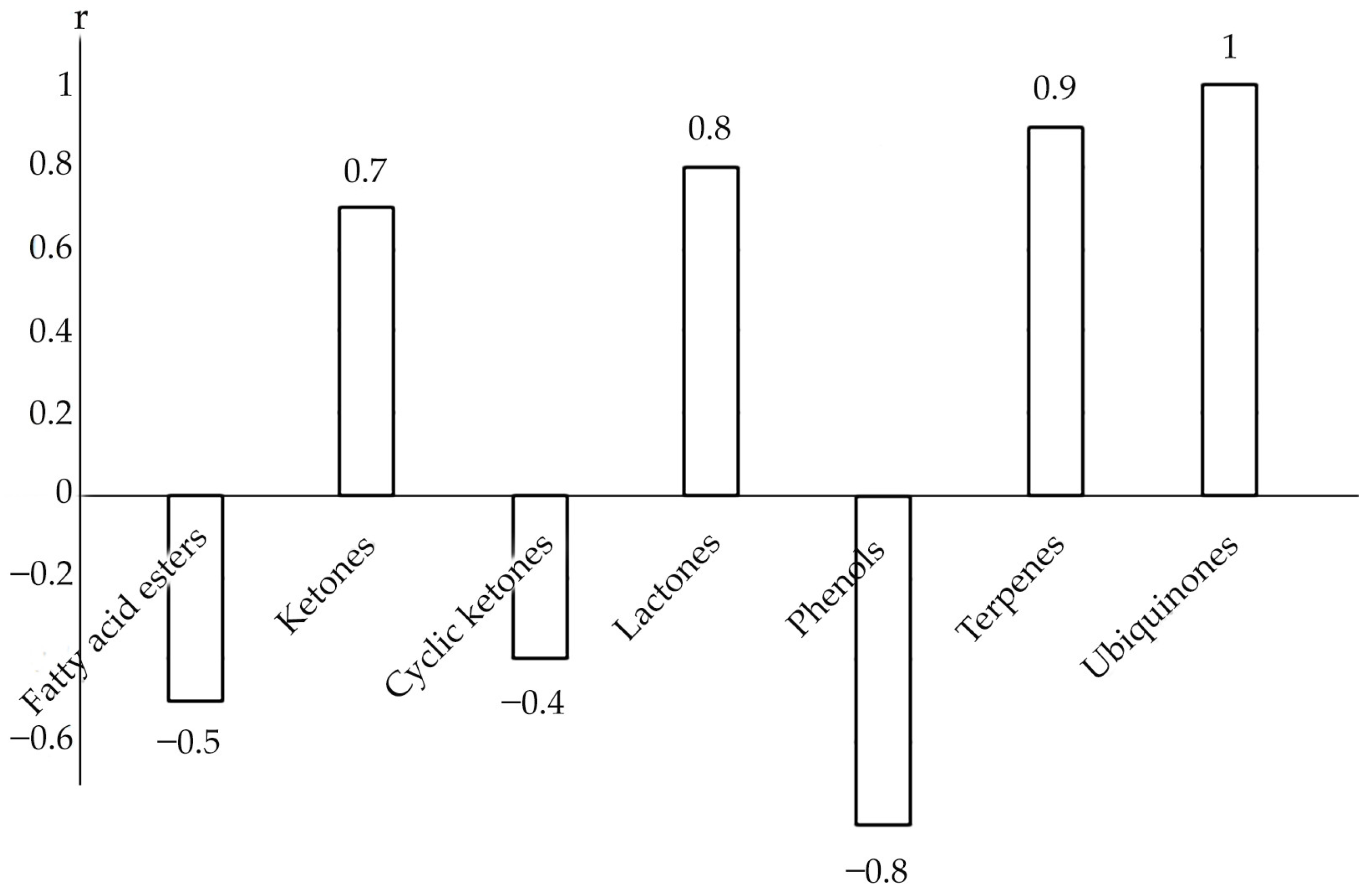
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Organic Compounds in Extracts
4.3. Determination of Salidrosid
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurmakov, A.G.; Belolipov, I.V. Wild Medicinal Plants of Uzbekistan (Botany, Chemistry, Pharmacology, Medicine); Extremum Press: Tashkent, Uzbekistan, 2012; 221p. (In Russian) [Google Scholar]
- Kuliev, R.Z.; Akhmedov, U.; Khalmatov, K.K.; Kuliev, Z.A. Dimeric Proanthocyanidins from Rhodiola semenovii. Chem. Nat. Compd. 2004, 40, 94–95. [Google Scholar] [CrossRef]
- Kuliev, Z.A.; Kim, K.K.; Vdovin, A.D.; Abdullaev, N.; Khushbaktova, Z.A.; Syrov, V.N. Oligomeric proanthocyanidin glycosides ofClementsia semenovii and their biological activity. III. Chem. Nat. Compd. 2000, 36, 60–67. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Korbozova, N.K.; Kudrina, N.O.; Kobylina, T.N.; Kurmanbayeva, M.S.; Meduntseva, N.T.; Tolstikova, T.G. The Influence of Abiotic Stress Factors on the Morphophysiological and Phytochemical Aspects of the Acclimation of the Plant Rhodiola semenowii Boriss. Plants 2021, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Koricheva, J.; Barton, K.E. Temporal changes in plant secondary metabolite production: Patterns, causes and consequences. In The Ecology of Plant Secondary Metabolites: From Genes to Global Processes; Iason, G., Dicke, M., Hartley, S., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 34–55. [Google Scholar] [CrossRef]
- Płonka, J.; Górny, A.; Kokoszka, K.; Barchanska, H. Metabolic profiles in the course of the shikimic acid pathway of Raphanus sativus var. longipinnatus exposed to mesotrione and its degradation products. Chemosphere 2020, 245, 125616. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Holeski, L.M.; Hillstrom, M.L.; Whitham, T.G.; Lindroth, R.L. Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia 2012, 170, 695–707. [Google Scholar] [CrossRef]
- Kursar, T.A.; Dexter, K.G.; Lokvam, J.; Pennington, R.T.; Richardson, J.E.; Weber, M.G.; Murakami, E.T.; Drake, C.; McGregor, R.; Coley, P.D. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc. Natl. Acad. Sci. USA 2009, 106, 18073–18078. [Google Scholar] [CrossRef] [Green Version]
- Endara, M.-J.; Soule, A.J.; Forrister, D.L.; Dexter, K.G.; Pennington, R.T.; Nicholls, J.A.; Loiseau, O.; Kursar, T.A.; Coley, P.D. The role of plant secondary metabolites in shaping regional and local plant community assembly. J. Ecol. 2021, 110, 34–45. [Google Scholar] [CrossRef]
- Tollenaar, M.; Daynard, T.B. Effect of source-sink ratio on dry matter accumulation and leaf senesence of Maize. Can. J. Plant Sci. 2022, 62, 855–860. [Google Scholar] [CrossRef]
- Gaia, A.M.; Yamaguchi, L.F.; Guerrero-Perilla, C.; Kato, M.J. Ontogenetic Changes in the Chemical Profiles of Piper Species. Plants 2021, 10, 1085. [Google Scholar] [CrossRef]
- Marak, H.B.; Biere, A.; Van Damme, J.M.M. Fitness costs of chemical defense in Plantago lanceolata L.: Effects of nutrient and competition stress. Evolution 2003, 57, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Stamp, N. Out Of The Quagmire Of Plant Defense Hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Barton, K.E.; Koricheva, J. The Ontogeny of Plant Defense and Herbivory: Characterizing General Patterns Using Meta-Analysis. Am. Nat. 2010, 175, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Mirajkar, S.J.; Suprasanna, P.; Vaidya, E.R. Spatial distribution and dynamics of sucrose metabolising enzymes in radiation induced mutants of sugarcane. Plant Physiol. Biochem. 2016, 100, 85–93. [Google Scholar] [CrossRef]
- Walters, D.; Heil, M. Costs and trade-offs associated with induced resistance. Physiol. Mol. Plant Pathol. 2007, 71, 3–17. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.-C. Sugars as signaling molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Andersen, C.P. Source–sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Farrar, J.F.; Jones, D.L. The control of carbon acquisition by roots. New Phytol. 2000, 147, 43–53. [Google Scholar] [CrossRef]
- Cara, C.; Gauvin-Lepage, J.; Lefebvre, H.; Létourneau, D.; Alderson, M.; LaRue, C.; Beauchamp, J.; Gagnon, L.; Casimir, M.; Girard, F.; et al. Le Modèle humaniste des soins infirmiers -UdeM: Perspective novatrice et pragmatique. Rech. Soins Infirm. 2016, 125, 20–31. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Paul, M.; Foyer, C.H. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Zakhozhiy, I.G.; Dalke, I.V.; Nizovtsev, A.N.; Golovko, T.K. Bioaccumulation and physiological responses of plants to man-made contamination of the environment by mercury. Theor. Appl. Ecol. 2011, 2, 37–44. (In Russian) [Google Scholar]
- Li, B.; Förster, C.; Robert, C.A.M.; Züst, T.; Hu, L.; Machado, R.A.R.; Berset, J.-D.; Handrick, V.; Knauer, T.; Hensel, G.; et al. Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci. Adv. 2018, 4, eaat6797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslani, L.; Gholami, M.; Mobli, M.; Sabzalian, M.R. The influence of altered sink-source balance on the plant growth and yield of greenhouse tomato. Physiol. Mol. Biol. Plants 2020, 26, 2109–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, B.-L.; Hu, Y.; Liu, J. Source–Sink Adjustment: A Mechanistic Understanding of the Timing and Severity of Drought Stress on Photosynthesis and Grain Yields of Two Contrasting Oat (Avena sativa L.) Genotypes. J. Plant Growth Regul. 2021, 40, 263–276. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Camas, N.; Caliskan, O.; Odabas, M.S. Changes in the contents of main secondary metabolites in two Turkish Hypericum species during plant development. Pharm. Biol. 2012, 51, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Peña, R.; Schlereth, A.; Höhne, M.; Encke, B.; Morcuende, R.; Nieto-Taladriz, M.T.; Araus, J.L.; Aparicio, N.; Vicente, R. Source-Sink Dynamics in Field-Grown Durum Wheat Under Contrasting Nitrogen Supplies: Key Role of Non-Foliar Organs During Grain Filling. Front. Plant Sci. 2022, 13, 869680. [Google Scholar] [CrossRef]
- Chang, T.-G.; Zhu, X.-G. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef]
- Chang, Y.; Yao, H.; Hsieh, S.; Lu, T.; Yeh, T. Quantitative determination of salidroside in rat plasma by on-line solid-phase extraction integrated with high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B 2007, 857, 164–169. [Google Scholar] [CrossRef]
- Meldau, S.; Erb, M.; Baldwin, I.T. Defence on demand: Mechanisms behind optimal defence patterns. Ann. Bot. 2012, 110, 1503–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barton, K.E.; Boege, K.; Barton, K.E.; Boege, K. Future directions in the ontogeny of plant defence: Understanding the evolutionary causes and consequences. Ecol. Lett. 2017, 20, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Mateo, P.; Ye, M.; Zhang, X.; Berset, J.D.; Handrick, V.; Radisch, D.; Grabe, V.; Köllner, T.G.; Gershenzon, J.; et al. Plant iron acquisition strategy exploited by an insect herbivore. Science 2018, 361, 694–697. [Google Scholar] [CrossRef] [Green Version]
- van Miltenburg, J.C.; Oonk, H.A.J.; Ventola, L. Heat Capacities and Derived Thermodynamic Functions of 1-Octadecanol, 1-Nonadecanol, 1-Eicosanol, and 1-Docosanol between 10 K and 370 K. J. Chem. Eng. Data 2000, 46, 90–97. [Google Scholar] [CrossRef]
- Borisova, G.G.; Ermoshin, A.A.; Maleva, M.G.; Chukina, N.V. Fundamentals of biochemistry of the secondary metabolism of plants. In Learning Method. Allowance; Borisova, G.G., Ed.; Publishing House Ural.: Yekaterinburg, Russia, 2014; 128p. (In Russian) [Google Scholar]
- Rohmer, M.; Seemann, M.; Horbach, S.; Bringer-Meyer, S.; Sahm, H. Glyceraldehyde 3-Phosphate and Pyruvate as Precursors of Isoprenic Units in an Alternative Non-mevalonate Pathway for Terpenoid Biosynthesis. J. Am. Chem. Soc. 1996, 118, 2564–2566. [Google Scholar] [CrossRef]
- Temelli, F.; Saldaña, M.D.A.; Comin, L. Application of Supercritical Fluid Extraction in Food Processing. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 415–440. [Google Scholar]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: "Talking Trees" in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [Green Version]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [Green Version]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.-M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Rontani, J.-F.; Nassiry, M.; Mouzdahir, A. Free radical oxidation (autoxidation) of α-tocopherol (vitamin E): A potential source of 4,8,12,16-tetramethylheptadecan-4-olide in the environment. Org. Geochem. 2007, 38, 37–47. [Google Scholar] [CrossRef]
- Lyutikova, M.N.; Turov, Y.P.; Botirov, E.H. Application of chromatography-mass spectrometry to determine free and esterified fatty acids in their combined presence in plant raw materials. Fine Chem. Technol. 2013, 8, 52–57. (In Russian) [Google Scholar]
- Iba, K. Acclimative response to temperature stress in higher plants: Approaches of Gene Engineering for Temperature Tolerance. Annu. Rev. Plant Biol. 2002, 53, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose Utilization for Improved Crop Yields: A Review Article. Int. J. Mol. Sci. 2021, 22, 4704. [Google Scholar] [CrossRef] [PubMed]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.-P.; Meghji, M.; Primavesi, L.F.; Paul, M.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dong, X.; Choi, K.; Song, H.; Yi, H.; Hur, Y. Identification of source-sink tissues in the leaf of Chinese cabbage (Brassica rapa ssp. pekinensis) by carbohydrate content and transcriptomic analysis. Genes Genom. 2020, 42, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, Y.; Zhang, Y.; Fan, S.; Kong, L. Source-sink modifications affect leaf senescence and grain mass in wheat as revealed by proteomic analysis. BMC Plant Biol. 2020, 20, 257. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.C. Metabolism and Compartmentation of Imported Sugars in Sink Organs in Relation to Sink Strength. Annu. Rev. Plant Phys. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Erst, A.A.; Petruk, A.A.; Zibareva, L.N.; Erst, A.S. Morphological, Histochemical and Biochemical Features of Cultivated Rhodiola rosea (Altai Mountains Ecotype). Contemp. Probl. Ecol. 2021, 14, 701–710. [Google Scholar] [CrossRef]
- Borghi, M.; Fernie, A.R. Outstanding questions in flower metabolism. Plant J. 2020, 103, 1275–1288. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Borghi, M.; Fernie, A.R. Floral Metabolism of Sugars and Amino Acids: Implications for Pollinators’ Preferences and Seed and Fruit Set. Plant Physiol. 2017, 175, 1510–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegeder, M.; Hammes, U.Z. The way out and in: Phloem loading and unloading of amino acids. Curr. Opin. Plant Biol. 2018, 43, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Southwick, E.E. Photosynthate Allocation to Floral Nectar: A Neglected Energy Investment. Ecology 1984, 65, 1775–1779. [Google Scholar] [CrossRef]
- Borghi, M.; De Souza, L.P.; Yoshida, T.; Fernie, A.R. Flowers and climate change: A metabolic perspective. New Phytol. 2019, 224, 1425–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybakova, G.R.; Tikhomirov, A.A.; Chepeleva, G.G. Research and study of the spectral composition of light when grown in light conditions at the exit of salidroside in Rhodiola rosea. Chem. Plant Raw Mater. 2002, 3, 77–83. (In Russian) [Google Scholar]
- Aniszewski, T. Alkaloids—Secrets of Life; Elsevier: Amsterdam, The Netherlands, 2007; 335p. [Google Scholar] [CrossRef]
- Jovanović, S.; Zlatković, B.K.; Stojanović, G.S. The chemical composition of Sedum rupestre L. ssp. rupestre epicuticular waxes: Horticultural versus the natural plant habitat. Facta Univ.-Ser. Phys. Chem. Technol. 2015, 13, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Terletskaya, N.V.; Seitimova, G.A.; Kudrina, N.O.; Meduntseva, N.D.; Ashimuly, K. The Reactions of Photosynthetic Capacity and Plant Metabolites of Sedum hybridum L. in Response to Mild and Moderate Abiotic Stresses. Plants 2022, 11, 828. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Huang, Z.; Wang, Z.; Zhu, Q.; Liu, L. Correlation of Cytokinin Levels in the Endosperms and Roots with Cell Number and Cell Division Activity during Endosperm Development in Rice. Ann. Bot. 2002, 90, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, P.K.; Panigrahi, R.; Turner, N.C. Physiology of Spikelet Development on the Rice Panicle: Is manipulation of apical dominance crucial for grain yield improvement? Adv. Agron. 2011, 110, 333–359. [Google Scholar] [CrossRef]
- Chikov, V.; Abdrakhimov, F.; Bakirova, G.; Batasheva, S. The role of sink-source relationships between different organs in regulation of photosynthesis and productivity. In Proceedings of the International Symposium on Source-Sink Relationships in Plants, Kaliningrad, Russia, 21–26 May 2007; pp. 87–98. [Google Scholar] [CrossRef]
- Suzich, J.A.; Dean, J.F.D.; Herrmann, K.M. 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase from Carrot Root (Daucus carota) Is a Hysteretic Enzyme. Plant Physiol. 1985, 79, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Zakhozhiy, I.G. The chemical composition of the underground organs of the plant Rhodiola rosea L., cultivated in the middle-taiga subzone of the Komi Republic. Bull. Inf. Secur. 2009, 8, 9–12. (In Russian) [Google Scholar]
- Sleptsov, I.V.; Zhuravskaya, A.N. Dynamics of flavonoid accumulation in the leaves of Amaranthus retroflexus, Agastache rugosa and Thlaspi arvense collected in Central Yakutia. Chem. Plant Raw Mater. 2016, 3, 67–72. (In Russian) [Google Scholar] [CrossRef]
- Andysheva, E.V.; Khramova, E.P. A chemotaxonomic study of phenolic compounds in the species of the genus Dasiphora (Rosaceae) from the Russian Far East and Eastern Siberia. Bot. Pacifica 2020, 9, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Kiryanov, A.A.; Bondarenko, L.T.; Kurkin, V.A.; Zapesochnaya, G.G. Dynamics of accumulation of rosavidin and salidroside in the raw materials of Rhodiola rosea cultivated in the Moscow region. Chem.-Farm. J. 1989, 4, 449–452. (In Russian) [Google Scholar]
- Kurkin, V.A.; Kurkina, T.V.; Zapesochnaya, G.G. Study of the dynamics of accumulation of active substances in the rhizomes of Rhodiola rosea, introduced in the Samara region. In Proceedings of the Scintific Conference “Biodiversity. Plant Introduction”, Saint-Petersburg, Russia, 12–15 December 1995; pp. 154–155. (In Russian). [Google Scholar]
- Kim, E.F. The content and age dynamics of the accumulation of salidroside in the aerial organs of Rhodiola rosea in connection with its introduction in different altitudinal zones of Altai. In Flora and vegetation of Siberia and the Far East; Krasnoyarsk Pedagogical University: Krasnoyarsk, Russia, 1996; pp. 255–257. (In Russian) [Google Scholar]
- Borisova, A.G. Abstract of the Crassulaceae DC family system. flora of the USSR (additions and changes). News Taxon. High. Plants 1969, 6, 112–121. (In Russian) [Google Scholar]
- Alekseeva, V.A.; Golotova, T.P.; Mikhailova, G.S. State Pharmacopoeia, 11th ed.; Medicine: Moskow, Russia, 1990; Volume 2, pp. 143–159. (In Russian) [Google Scholar]
- Kuliev, R.Z. Anatomical diagnosis of rhizomes and roots of Rhodiola Semenovii. Pharm. J. 2004, 2, 32–34. (In Russian) [Google Scholar]
- Mazurenko, M.; Andreeva, A. Succulents in the Kolyma. In The World of Plants; Capstone: Mankato, MN, USA, 2002; Volume 12, p. 14. (In Russian) [Google Scholar]
- Salnikov, V.; Turulina, G.; Polyakova, S.; Petrova, Y.; Skakova, A. Climate change in Kazakhstan during the past 70 years. Quat. Int. 2015, 358, 77–82. [Google Scholar] [CrossRef]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J. Food Sci. Technol. 2015, 52, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Theodoridis, G.A.; Gika, H.G.; Plumb, R.; Wilson, I.D. Liquid chromatographic methods combined with mass spectrometry in metabolomics. In Proteomic and Metabolomic Approaches to Biomarker Discovery, 2nd ed.; Issaq, H.J., Veenstra, T.D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 149–169. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terletskaya, N.V.; Korbozova, N.K.; Grazhdannikov, A.E.; Seitimova, G.A.; Meduntseva, N.D.; Kudrina, N.O. Accumulation of Secondary Metabolites of Rhodiola semenovii Boriss. In Situ in the Dynamics of Growth and Development. Metabolites 2022, 12, 622. https://doi.org/10.3390/metabo12070622
Terletskaya NV, Korbozova NK, Grazhdannikov AE, Seitimova GA, Meduntseva ND, Kudrina NO. Accumulation of Secondary Metabolites of Rhodiola semenovii Boriss. In Situ in the Dynamics of Growth and Development. Metabolites. 2022; 12(7):622. https://doi.org/10.3390/metabo12070622
Chicago/Turabian StyleTerletskaya, Nina V., Nazym K. Korbozova, Alexander E. Grazhdannikov, Gulnaz A. Seitimova, Nataliya D. Meduntseva, and Nataliya O. Kudrina. 2022. "Accumulation of Secondary Metabolites of Rhodiola semenovii Boriss. In Situ in the Dynamics of Growth and Development" Metabolites 12, no. 7: 622. https://doi.org/10.3390/metabo12070622
APA StyleTerletskaya, N. V., Korbozova, N. K., Grazhdannikov, A. E., Seitimova, G. A., Meduntseva, N. D., & Kudrina, N. O. (2022). Accumulation of Secondary Metabolites of Rhodiola semenovii Boriss. In Situ in the Dynamics of Growth and Development. Metabolites, 12(7), 622. https://doi.org/10.3390/metabo12070622






