Persistence of Metabolomic Changes in Patients during Post-COVID Phase: A Prospective, Observational Study
Abstract
:1. Introduction
2. Results
3. Discussion
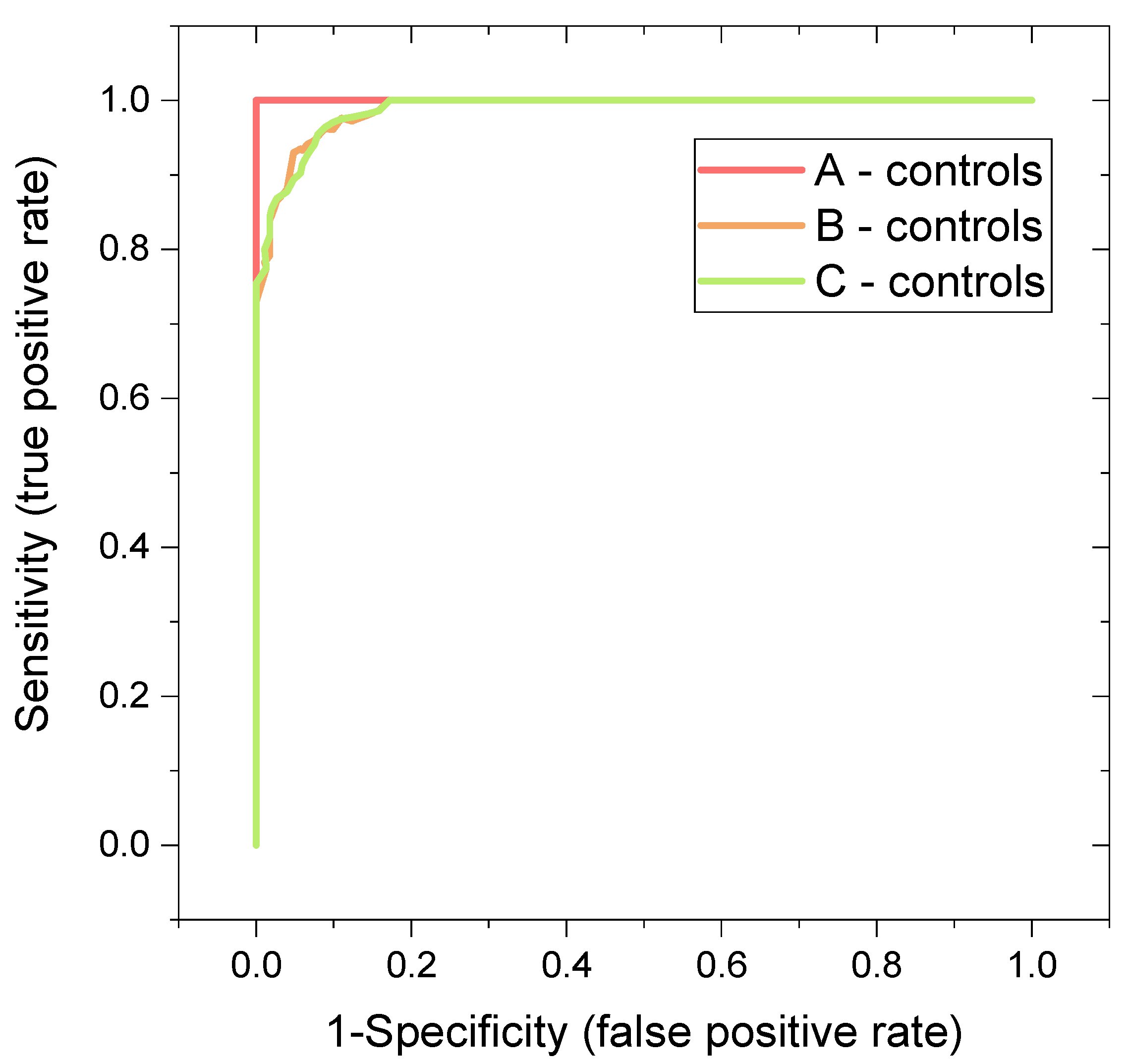
Multivariate and Discriminatory Analyses
4. Materials and Methods
4.1. Sample Preparation
4.2. NMR Data Acquisition
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasan, M.R.; Suleiman, M.; Pérez-López, A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front. Genet. 2021, 12, 1358. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhou, L.; Sun, X.; Yan, Z.; Hu, C.; Wu, J.; Xu, L.; Li, X.; Liu, H.; Yin, P.; et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci. Rep. 2017, 7, 9110. [Google Scholar] [CrossRef] [PubMed]
- Byers, N.; Fleshman, A.; Perera, R.; Molins, C. Metabolomic Insights into Human Arboviral Infections: Dengue, Chikungunya, and Zika Viruses. Viruses 2019, 11, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiogi, M.; Camargo, C.A., Jr.; Raita, Y.; Bochkov, Y.A.; Gern, J.E.; Mansbach, J.M.; Piedra, P.A.; Hasegawa, K. Respiratory viruses are associated with serum metabolome among infants hospitalized for bronchiolitis: A multicenter study. Pediatr. Allergy Immunol. 2020, 31, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Baranovicova, E.; Bobcakova, A.; Vysehradsky, R.; Dankova, Z.; Halasova, E.; Nosal, V.; Lehotsky, J. The Ability to Normalise Energy Metabolism in Advanced COVID-19 Disease Seems to Be One of the Key Factors Determining the Disease Progression—A Metabolomic NMR Study on Blood Plasma. Appl. Sci. 2021, 11, 4231. [Google Scholar] [CrossRef]
- Jia, H.; Liu, C.; Li, D.; Huang, Q.; Liu, D.; Zhang, Y.; Ye, C.; Zhou, D.; Wang, Y.; Tan, Y.; et al. Metabolomic analyses reveal new stage-specific features of COVID-19. Eur. Respir. J. 2021, 59, 2100284. [Google Scholar] [CrossRef]
- Wu, D.; Shu, T.; Yang, X.; Song, J.-X.; Zhang, M.; Yao, C.; Liu, W.; Huang, M.; Yu, Y.; Yang, Q.; et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020, 7, 1157–1168. [Google Scholar] [CrossRef]
- Danlos, F.-X.; Grajeda-Iglesias, C.; Durand, S.; Sauvat, A.; Roumier, M.; Cantin, D.; Colomba, E.; Rohmer, J.; Pommeret, F.; Baciarello, G.; et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 2021, 12, 258. [Google Scholar] [CrossRef]
- Shi, D.; Yan, R.; Lv, L.; Jiang, H.; Lu, Y.; Sheng, J.; Xie, J.; Wu, W.; Xia, J.; Xu, K.; et al. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism 2021, 118, 154739. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, P.; Xu, J.; Yang, L.; Ma, P.; Tan, X.; Chen, Q.; Zhou, M.; Song, S.; Xia, H.; et al. Plasma Metabolomic Profiles in Recovered COVID-19 Patients without Previous Underlying Diseases 3 Months after Discharge. J. Inflamm. Res. 2021, 14, 4485–4501. [Google Scholar] [CrossRef]
- Valdés, A.; Moreno, L.O.; Rello, S.R.; Orduña, A.; Bernardo, D.; Cifuentes, A. Metabolomics study of COVID-19 patients in four different clinical stages. Sci. Rep. 2022, 12, 1650. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, M.; Luo, P.; Yin, Z.; Wang, S.; Liao, T.; Yang, F.; Wang, Z.; Yang, D.; Peng, Y.; et al. Plasma Metabolomic Profiling of Patients Recovered From Coronavirus Disease 2019 (COVID-19) with Pulmonary Sequelae 3 Months after Discharge. Clin. Infect. Dis. 2021, 73, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, R.; Fernández, L.; Marco, S. Overoptimism in cross-validation when using partial least squares-discriminant analysis for omics data: A systematic study. Anal. Bioanal. Chem. 2018, 410, 5981–5992. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Broadhurst, D.; Wilson, M.; Wishart, D.S. Translational biomarker discovery in clinical metabolomics: An introductory tutorial. Metabolomics 2012, 9, 280–299. [Google Scholar] [CrossRef] [Green Version]
- Chicco, D.; Tötsch, N.; Jurman, G. The matthews correlation coefficient (Mcc) is more reliable than balanced accuracy, bookmaker informedness, and markedness in two-class confusion matrix evaluation. BioData Min. 2021, 14, 1–22. [Google Scholar] [CrossRef]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alblihed, M.; Guerreiro, S.G.; Cruz-Martins, N.; Batiha, G.E.-S. COVID-19 in Relation to Hyperglycemia and Diabetes Mellitus. Front. Cardiovasc. Med. 2021, 8, 644095. [Google Scholar] [CrossRef]
- He, X.; Liu, C.; Peng, J.; Li, Z.; Li, F.; Wang, J.; Hu, A.; Peng, M.; Huang, K.; Fan, D.; et al. COVID-19 induces new-onset insulin resistance and lipid metabolic dysregulation via regulation of secreted metabolic factors. Signal Transduct. Target. Ther. 2021, 6, 427. [Google Scholar] [CrossRef]
- Rayman, G.; Lumb, A.; Kennon, B.; Cottrell, C.; Nagi, D.; Page, E.; Voigt, D.; Courtney, H.C.; Atkins, H.; Higgins, K.; et al. Dexamethasone therapy in COVID-19 patients: Implications and guidance for the management of blood glucose in people with and without diabetes. Diabet. Med. 2020, 38, e14378. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. 2015, 872, 99–126. [Google Scholar]
- Pasternak, J.J.; McGregor, D.G.; Lanier, W.L. Effect of Single-Dose Dexamethasone on Blood Glucose Concentration in Patients Undergoing Craniotomy. J. Neurosurg. Anesthesiol. 2004, 16, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Tamez-Pérez, H.E.; Quintanilla-Flores, D.L.; Rodríguez-Gutiérrez, R.; González-González, J.G.; Tamez-Peña, A.L. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J. Diabetes 2015, 6, 1073–1081. [Google Scholar] [CrossRef]
- Mathioudakis, D.; Engel, J.; Welters, I.D.; Dehne, M.G.; Matejec, R.; Harbach, H.; Henrich, M.; Schwandner, T.; Fuchs, M.; Weismüller, K.; et al. Pyruvate: Immunonutritional effects on neutrophil intracellular amino or alpha-keto acid profiles and reactive oxygen species production. Amino Acids 2010, 40, 1077–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, A.; Brea-Diakite, D.; Cezard, A.; Wacquiez, A.; Baranek, T.; Bourgeais, J.; Picou, F.; Vasseur, V.; Meyer, L.; Chevalier, C.; et al. Host succinate inhibits influenza virus infection through succinylation and nuclear retention of the viral nucleoprotein. EMBO J. 2022, 41, e108306. [Google Scholar] [CrossRef]
- Yoon, M.-S. The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Zhenyukh, O.; González-Amor, M.; Díez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Branched-Chain Amino Acids and Brain Function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef]
- Branco, A.C.C.C.; Yoshikawa, F.S.Y.; Pietrobon, A.J.; Sato, M.N. Role of Histamine in Modulating the Immune Response and Inflammation. Mediat. Inflamm. 2018, 2018, 9524075. [Google Scholar] [CrossRef]
- Barberis, E.; Timo, S.; Amede, E.; Vanella, V.V.; Puricelli, C.; Cappellano, G.; Raineri, D.; Cittone, M.G.; Rizzi, E.; Pedrinelli, A.R.; et al. Large-Scale Plasma Analysis Revealed New Mechanisms and Molecules Associated with the Host Response to SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 8623. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines. 2021. Available online: https://www.covid19treatmentguidelines.nih.gov (accessed on 13 March 2022).
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-H.; Liu, T.-C.; Yin, M.-C. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem. Toxicol. 2008, 46, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-T.; Hsu, C.-C.; Lin, M.-H.; Liu, K.-S.; Yin, M.-C. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Gowda, G.A.N.; Gowda, Y.N.; Raftery, D. Expanding the Limits of Human Blood Metabolite Quantitation Using NMR Spectroscopy. Anal. Chem. 2014, 87, 706–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
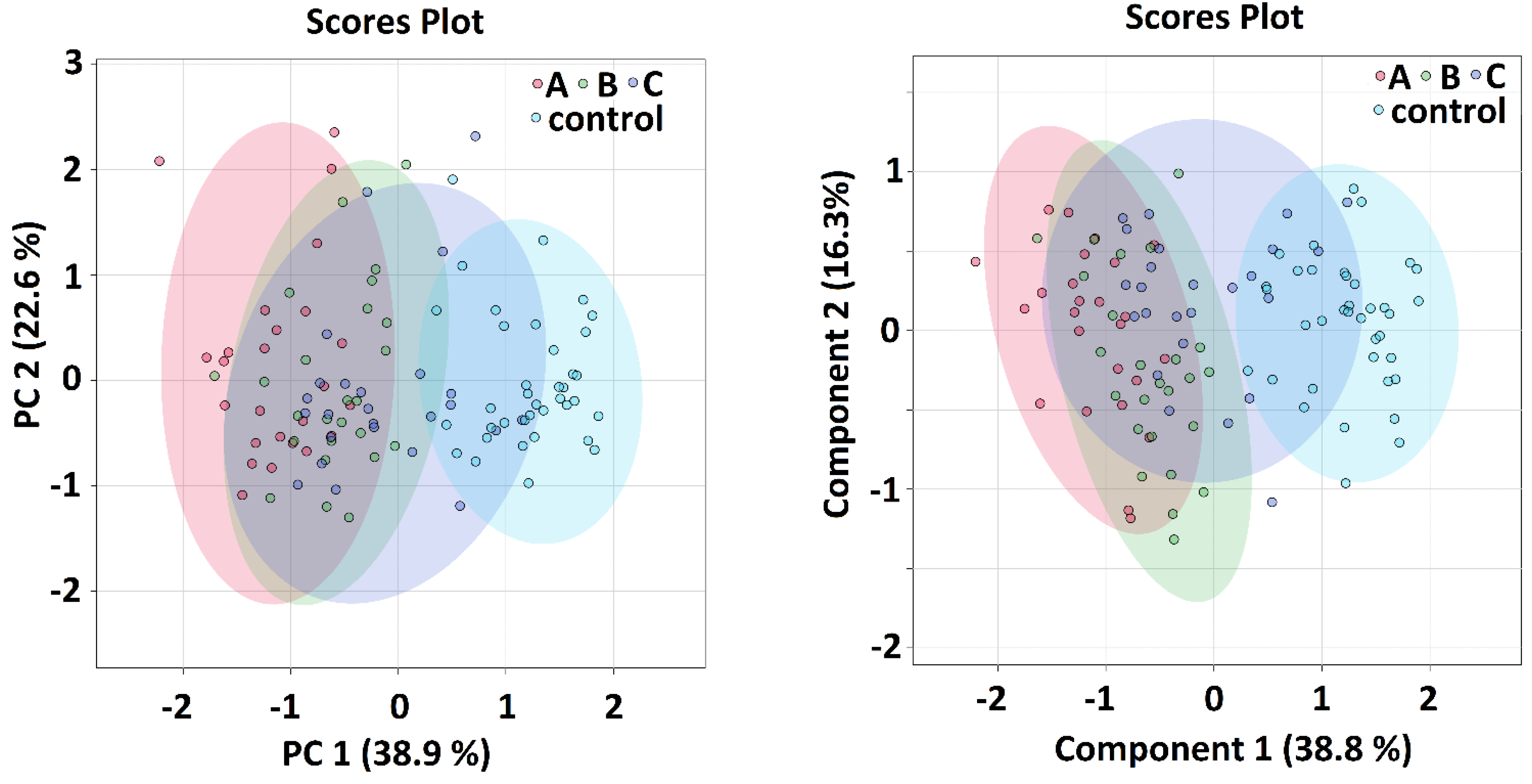
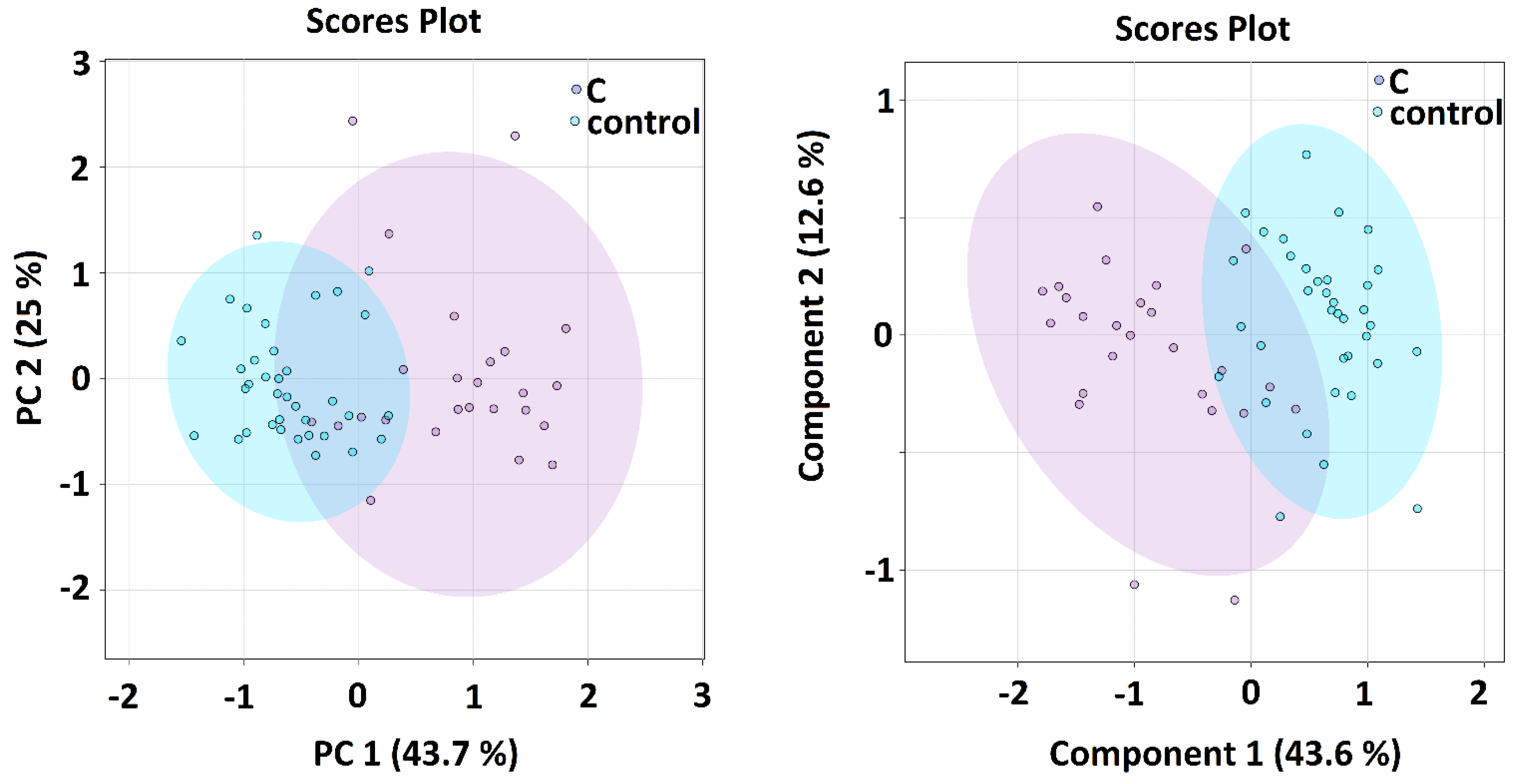
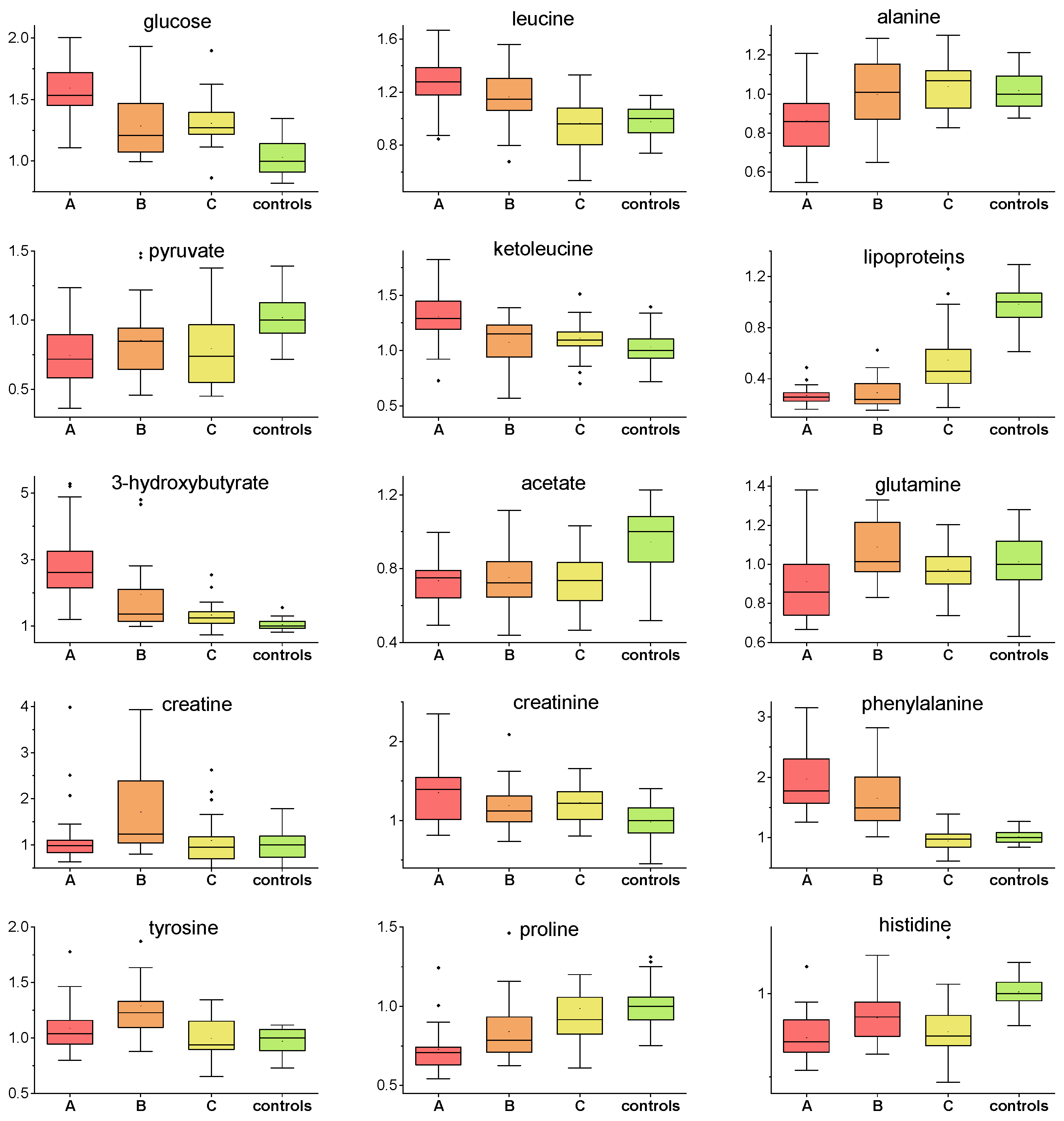
| Kruskal–Wallis | A-Controls | B-Controls | C-Controls | ||||
|---|---|---|---|---|---|---|---|
| p Value | p Value | % Change | p Value | % Change | p Value | % Change | |
| alanine | 0.0071 | 0.0031 | −15 | 0.85 | x | 0.71 | x |
| valine | 0.00082 | 0.00099 | 15 | 0.00051 | 16 | 0.012 | x |
| glucose | 0.00011 | 6.3 × 10−6 | 54 | 0.20 | 21 | 0.15 | 26 |
| leucine | 8.4 × 10−7 | 9.6 × 10−7 | 37 | 0.00077 | 15 | 0.63 | x |
| isoleucine | 2.6 × 10−7 | 2.6 × 10−8 | 32 | 0.00029 | 21 | 0.0016 | 17 |
| acetate | 0.0000092 | 0.00037 | −25 | 2.5 × 10−5 | −28 | 5.2 × 10−5 | −28 |
| pyruvate | 0.000020 | 4.4 × 10−6 | −28 | 0.0066 | −16 | 0.00049 | −26 |
| citrate | 6.4 × 10−10 | 0.041 | x | 0.00030 | −26 | 0.00078 | 21 |
| phenylalanine | 1.1 × 10−14 | 2.4 × 10−9 | 77 | 7.6 × 10−8 | 49 | 0.40 | x |
| tyrosine | 0.000051 | 0.042 | x | 6.1 × 10−6 | 22 | 0.52 | x |
| glutamine | 0.0089 | 0.024 | −15 | 0.24 | x | 0.17 | x |
| lipoproteins | 1.1 × 10−16 | 1.4 × 10−13 | −75 | 1.9 × 10−13 | −77 | 0.00014 | −54 |
| ketoleucine | 0.00036 | 3.0 × 10−5 | 29 | 0.37 | x | 0.060 | x |
| ketoisoleucine | 0.00025 | 6.5 × 10−5 | 37 | 0.059 | x | 0.0011 | 19 |
| ketovaline | 0.043 | 0.0057 | 20 | 0.11 | x | 0.090 | x |
| 3-hydroxy-butyrate | 1.6 × 10−12 | 9.3 × 10−14 | 261 | 8.4 × 10−6 | 34 | 0.0010 | 24 |
| creatine | 0.0028 | 0.82 | x | 0.00067 | 25 | 0.81 | x |
| creatinine | 0.0025 | 0.0040 | 39 | 0.052 | x | 0.00066 | 22 |
| histidine | 4.0 × 10−10 | 5.8 × 10−10 | −29 | 0.00026 | −15 | 2.8 × 10−7 | −26 |
| succinate | 0.0013 | 0.0083 | 28 | 0.023 | x | 0.00020 | 48 |
| proline | 3.3 × 10−8 | 8.6 × 10−9 | −30 | 0.00018 | −22 | 0.14 | x |
| System | Features | Oob Error (Based on Predicted Class Probabilities) | Average Accuracy Based on 100 Cross-Validations | AUC | MCC |
|---|---|---|---|---|---|
| A-control | 3 most important metabolites: histidine, proline, 3-hydroxybutyrate | 0 | 0.999 | 1 | 1 |
| 5 most important metabolites: histidine, proline, 3-hydroxyburtyrate, acetate, citrate | 0 | 0.999 | 1 | 1 | |
| all evaluated metabolites | 0 | 1 | 1 | 1 | |
| B-control | 3 most important metabolites: histidine, proline, 3-hydroxybutyrate | 5/62 | 0.884 | 0.966 | 0.839 |
| 5 most important metabolites: histidine, proline, 3-hydroxyburtyrate, pyruvate, citrate | 5/62 | 0.923 | 0.981 | 0.839 | |
| all evaluated metabolites | 2/62 | 0.948 | 0.992 | 0.936 | |
| C-control | 3 most important metabolites: histidine, glucose, pyruvate | 5/62 | 0.929 | 0.969 | 0.829 |
| 5 most important metabolites: histidine, glucose, pyruvate, phenylalanine, glutamine | 5/62 | 0.929 | 0.987 | 0.829 | |
| all evaluated metabolites | 3/62 | 0.932 | 0.991 | 0.895 |
| Median (IQR) | |
|---|---|
| Patients n = 25 | |
| Age [years] | 58 (21) |
| Sex: Female/Male | 7/18 |
| Weight [kg] | 82.6 (26) |
| Height [cm] | 171 (8) |
| BMI | 29 (9) |
| Chronic liver disease | 3 |
| Chronic kidney disease | 3 |
| Ischemic cardiac disease | 3 |
| Diabetes Mellitus | 3 |
| Thyroidal disease | 4 |
| Rheumatic disease | 0 |
| Other relevant | NA |
| Samples A | Samples B | Samples C | p Value (Multiple Comparison) | |
|---|---|---|---|---|
| Na | 133.32 (5.5) | 140 (6) | 139.4 (3.0) | <0.001 |
| K | 3.972 (0.6) | 4.2 (0.65) | 4.2 (0.45) | 0.017 |
| Cl | 99.48 (6.0) | 104 (6) | 104 (3.0) | <0.001 |
| Glucose | 8.068 (1.35) | 5.8 (3.05) | 5.6 (1.5) | 0.0021 |
| Cretinine | 83 (38.5) | 60 (22.5) one missing | 68 (32.5) | 0.32 |
| CRP | 116.5 (123.4) | 16.6 (31.45) one missing | 2.2 (4.55) | <0.001 |
| AST | 1.2604 (0.68) | 0.92 (1.13) eight missing | 0.508 (0.285) one missing | <0.001 |
| ALT | 1.0365 (0.715) one missing | 1.465 (1.325) nine missing | 0.575 (0.49) one missing | <0.01 |
| GMT | 1.6815 (1.715) five missing | 1.47 (2.48) three missing | 0.815 (1.08) one missing | 0.037 |
| Bilirubin | 10.7 (5.85) | 11.4 (6.65) eight missing | 9.6 (6.3) one missing | 0.41 |
| Leukocytes | 6.7 (3.05) | 7.7 (3.75) one missing | 8.2 (2.5) | 0.029 |
| Hemoglobine [g/l] | 142 (13) | 135.5 (18.5) one missing | 138 (13.5) | 0.16 |
| Platelets count | 190 (162.5) | 360.5 (215.5) one missing | 259 (133) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liptak, P.; Baranovicova, E.; Rosolanka, R.; Simekova, K.; Bobcakova, A.; Vysehradsky, R.; Duricek, M.; Dankova, Z.; Kapinova, A.; Dvorska, D.; et al. Persistence of Metabolomic Changes in Patients during Post-COVID Phase: A Prospective, Observational Study. Metabolites 2022, 12, 641. https://doi.org/10.3390/metabo12070641
Liptak P, Baranovicova E, Rosolanka R, Simekova K, Bobcakova A, Vysehradsky R, Duricek M, Dankova Z, Kapinova A, Dvorska D, et al. Persistence of Metabolomic Changes in Patients during Post-COVID Phase: A Prospective, Observational Study. Metabolites. 2022; 12(7):641. https://doi.org/10.3390/metabo12070641
Chicago/Turabian StyleLiptak, Peter, Eva Baranovicova, Robert Rosolanka, Katarina Simekova, Anna Bobcakova, Robert Vysehradsky, Martin Duricek, Zuzana Dankova, Andrea Kapinova, Dana Dvorska, and et al. 2022. "Persistence of Metabolomic Changes in Patients during Post-COVID Phase: A Prospective, Observational Study" Metabolites 12, no. 7: 641. https://doi.org/10.3390/metabo12070641
APA StyleLiptak, P., Baranovicova, E., Rosolanka, R., Simekova, K., Bobcakova, A., Vysehradsky, R., Duricek, M., Dankova, Z., Kapinova, A., Dvorska, D., Halasova, E., & Banovcin, P. (2022). Persistence of Metabolomic Changes in Patients during Post-COVID Phase: A Prospective, Observational Study. Metabolites, 12(7), 641. https://doi.org/10.3390/metabo12070641






