Sustained Endurance Training Leads to Metabolomic Adaptation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
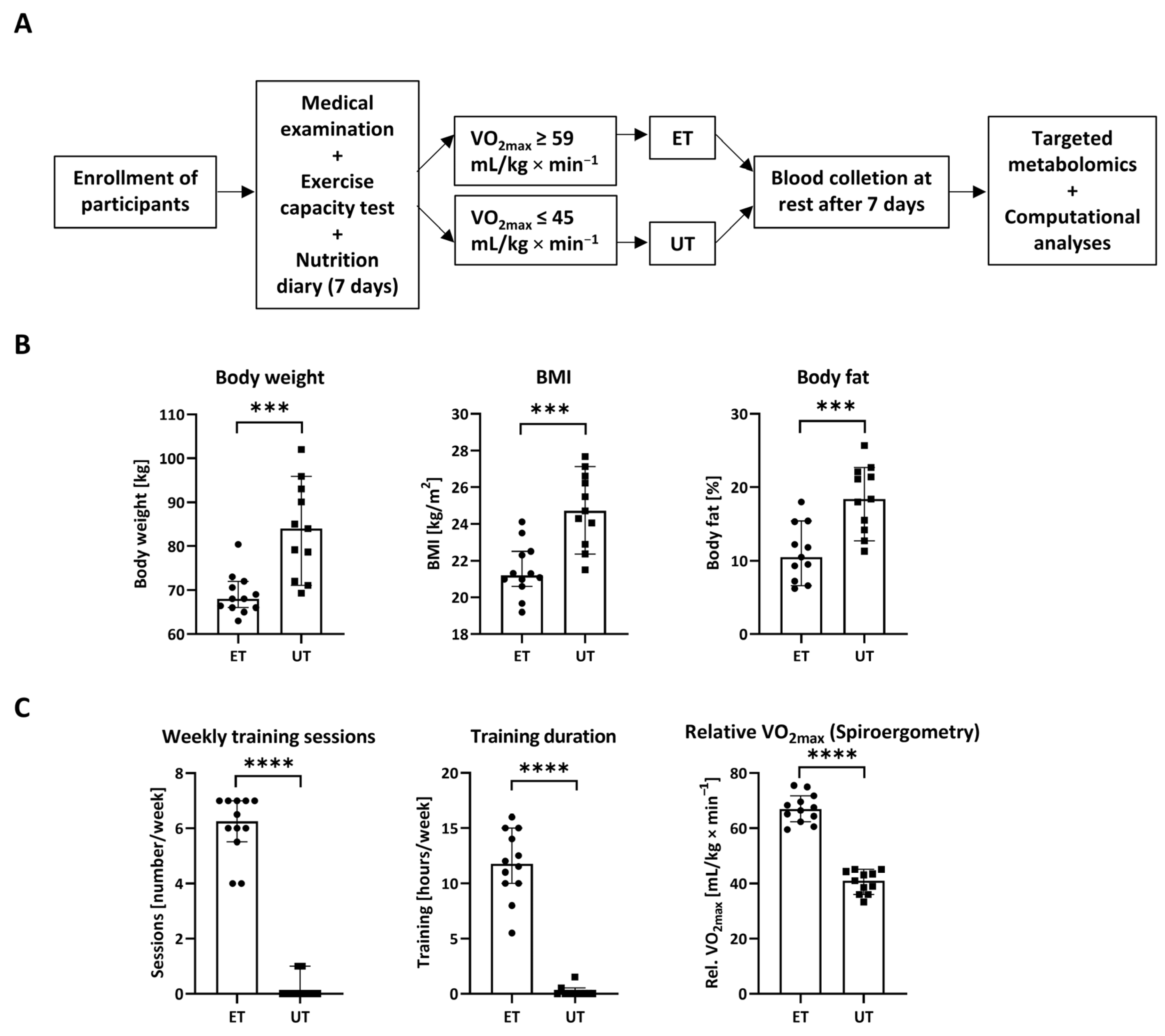
2.2. Study Design
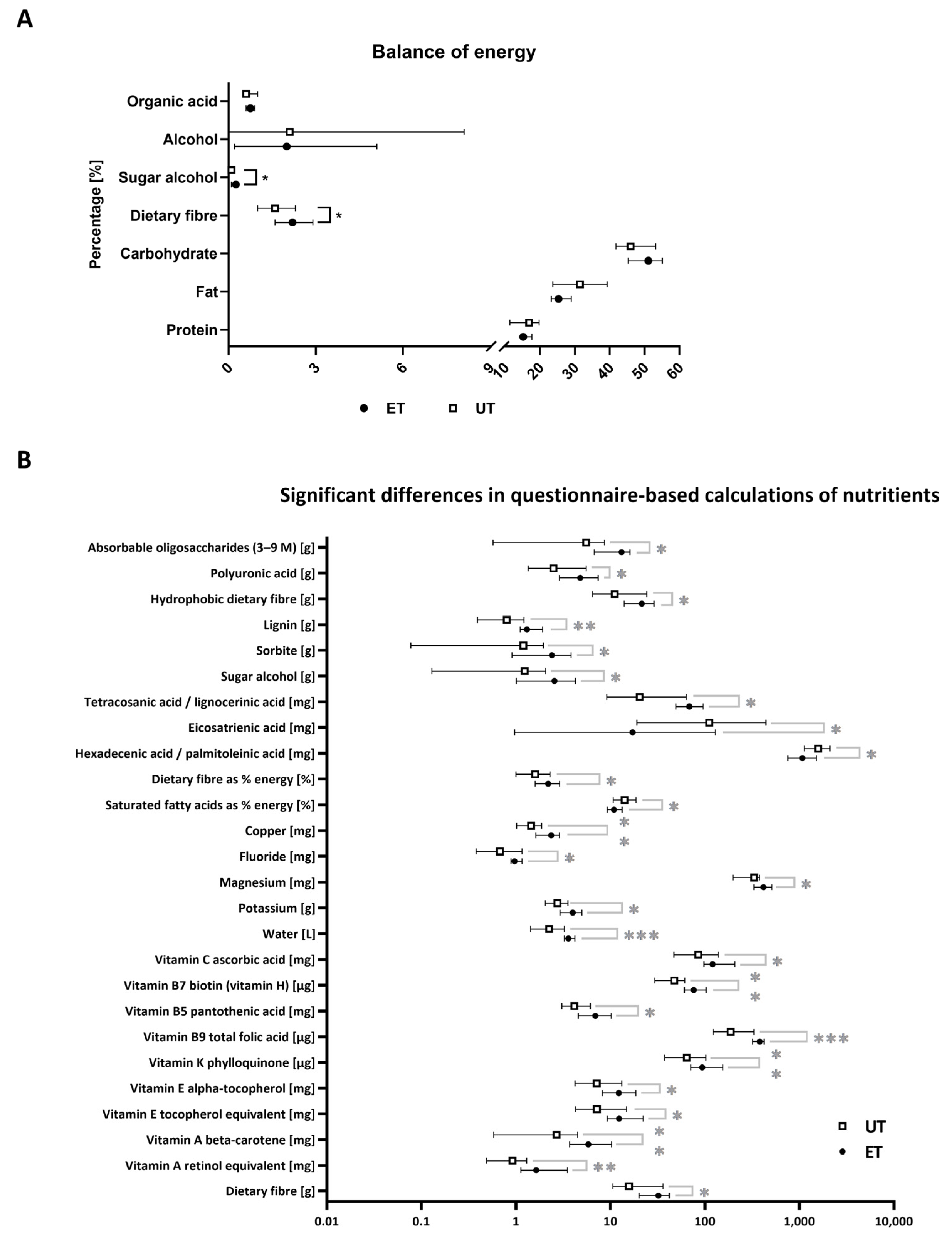
2.3. Nutritional Status
2.4. Blood Sampling Procedure
2.5. Targeted Metabolomics
2.6. Statistical Analysis
3. Results
3.1. Study Design and Descriptive Parameters
3.2. Nutritional Status
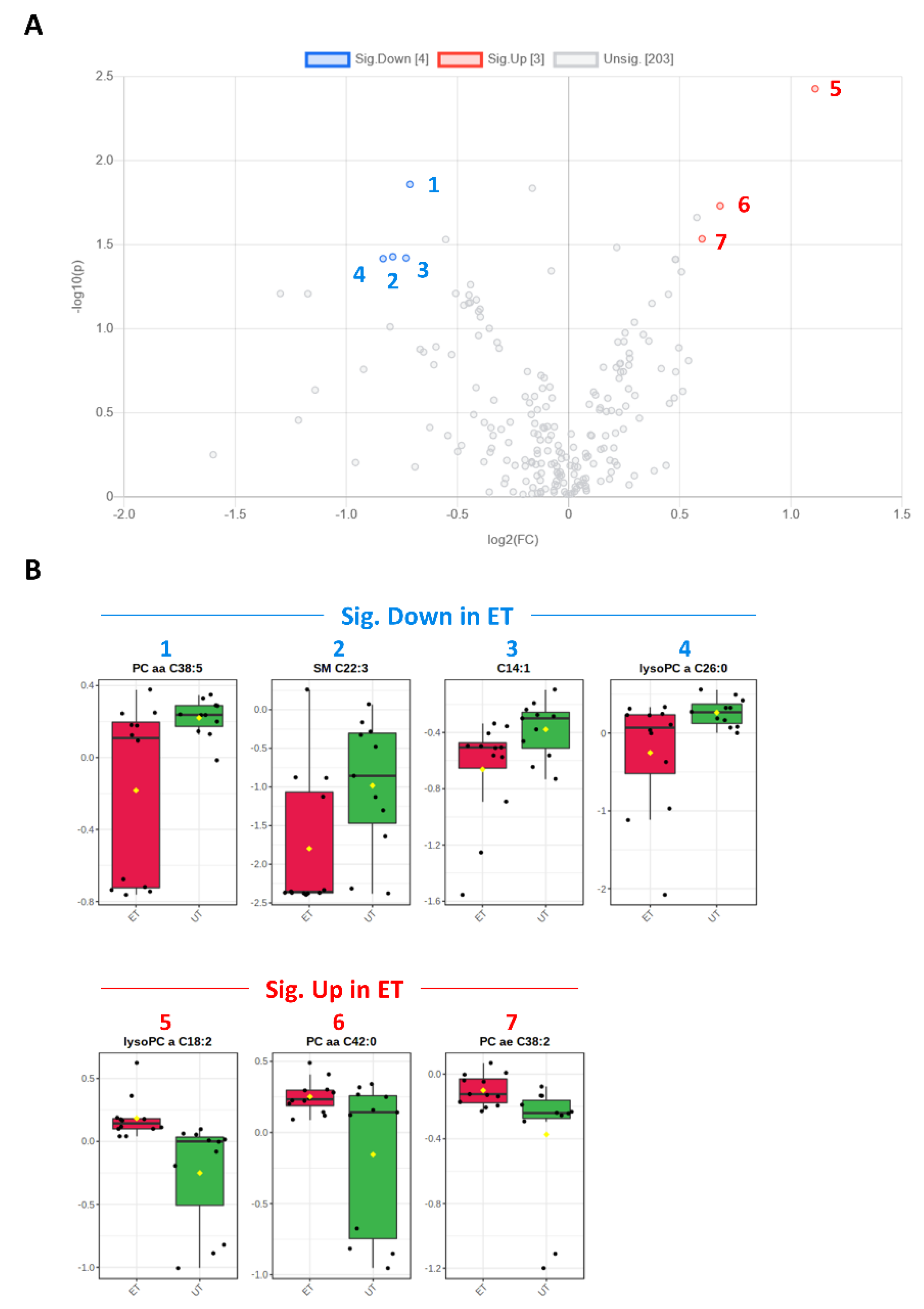
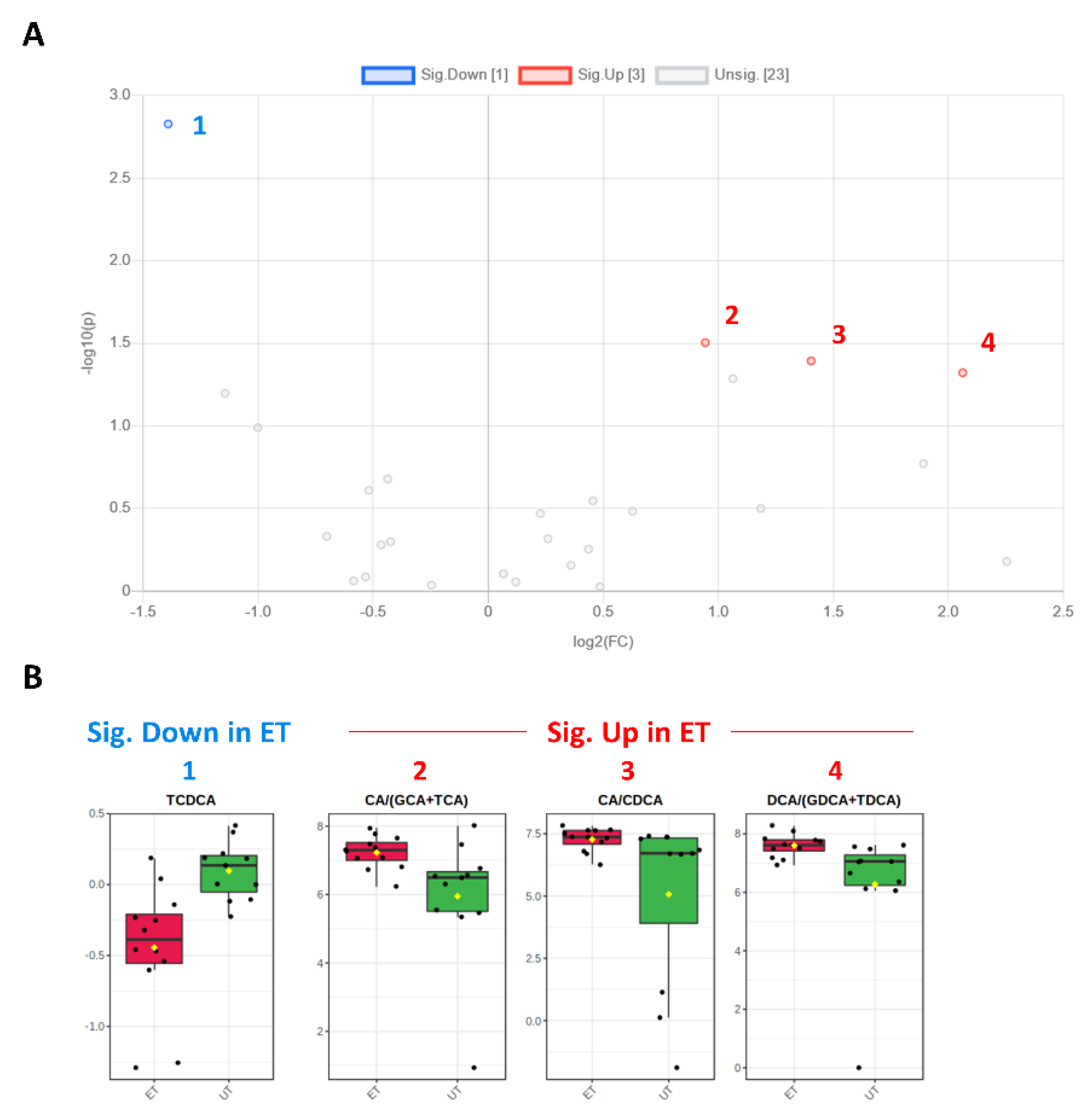
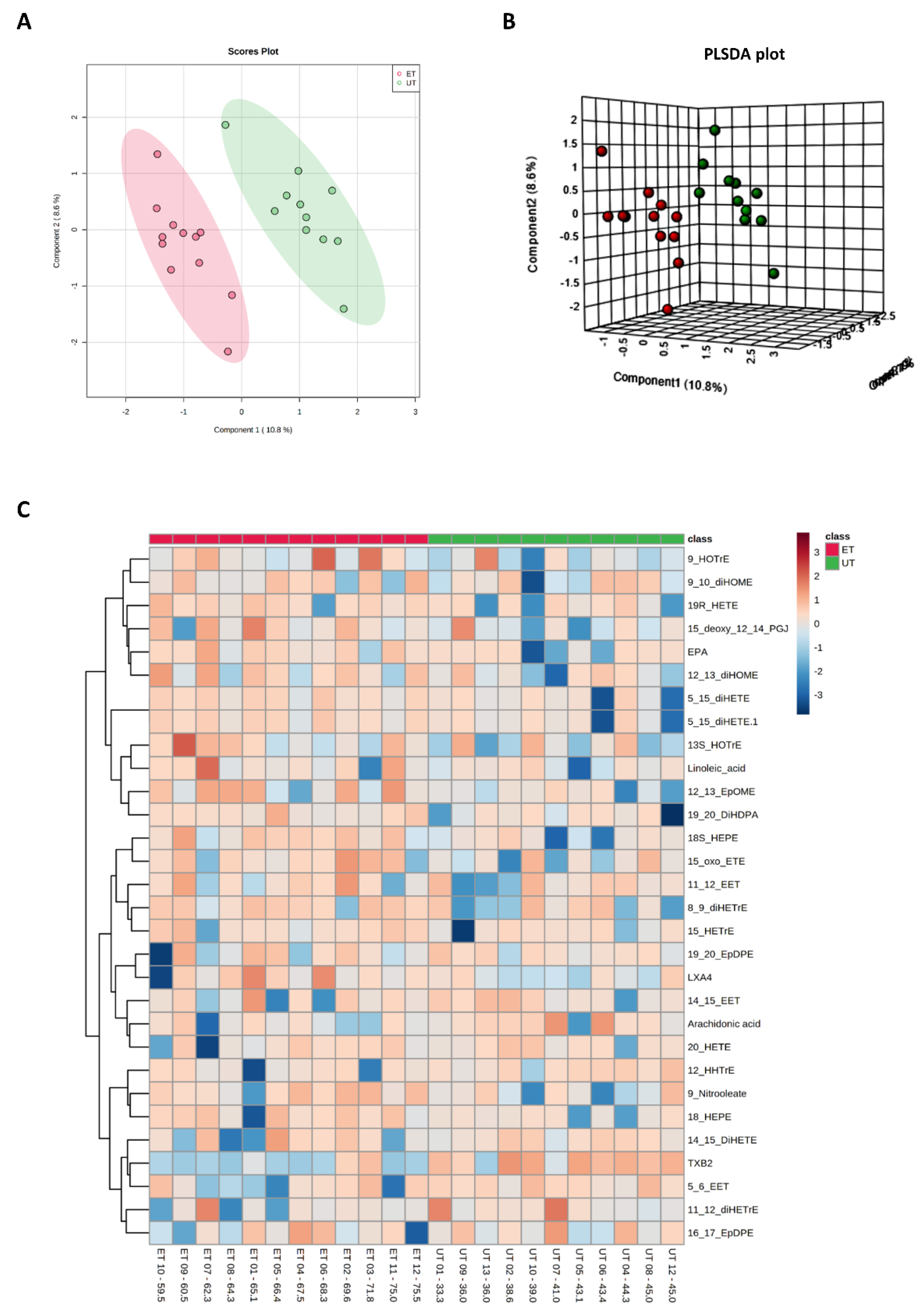
3.3. Targeted Metabolomics Analyses
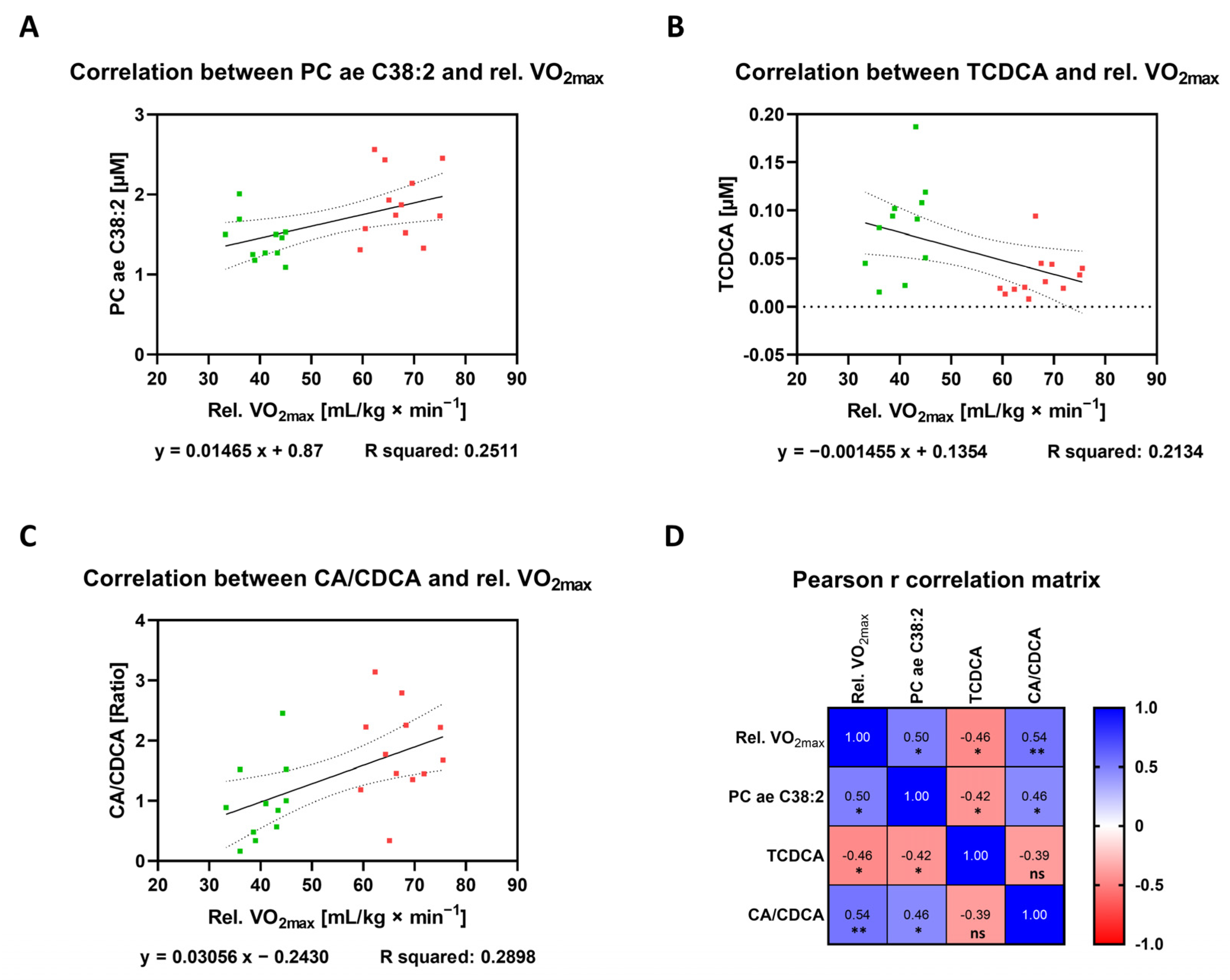
3.4. Correlation between Plasma Metabolites and Endurance Performance
3.5. Identification of Biomarkers for Cardio-Pulmonary Fitness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18-HEPE | hydroxyeicosapentaenoic acid |
| 5,6-EET | 5,6-epoxyeicosatrienoic acid |
| BMI | body mass index |
| CA | cholic acid |
| CDCA | chenodeoxycholic acid |
| DGE | German Nutrition Society (DGE) |
| EDTA | ethylene diamine tetra acetic acid |
| EPA | eicosapentaenoic acid |
| ET | endurance-trained |
| FC | fold change |
| GPCs | glycerophosphocholines |
| LysoPC | lysophosphatidylcholine |
| PCA | principal component analysis |
| PLSDA | partial least squares discriminant analysis |
| PUFAs | polyunsaturated fatty acids |
| SM | sphingomyelin |
| TCA | tricarboxylic cycle |
| TCDCA | taurochenodeoxycholic acid |
| TXB2 | thromboxane B2 |
| UT | untrained |
| VO2max | maximum relative oxygen uptake |
References
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savikj, M.; Zierath, J.R. Train Like an Athlete: Applying Exercise Interventions to Manage Type 2 Diabetes. Diabetologia 2020, 63, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Klapcinska, B.; Waskiewicz, Z.; Chrapusta, S.J.; Sadowska-Krepa, E.; Czuba, M.; Langfort, J. Metabolic Responses to a 48-H Ultra-Marathon Run in Middle-Aged Male Amateur Runners. Eur. J. Appl. Physiol. 2013, 113, 2781–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, Y.; Takeuchi, T.; Hosoi, T.; Yoshizaki, H.; Loeppky, J.A. Effect of a Marathon Run on Serum Lipoproteins, Creatine Kinase, and Lactate Dehydrogenase in Recreational Runners. Res. Q. Exerc. Sport 2005, 76, 450–455. [Google Scholar] [CrossRef]
- Murray, B.; Rosenbloom, C. Fundamentals of Glycogen Metabolism for Coaches and Athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Hodun, K.; Chabowski, A.; Baranowski, M. Sphingosine-1-Phosphate in Acute Exercise and Training. Scand. J. Med. Sci. Sports 2021, 31, 945–955. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Smidt, M.C.F.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise Training Improves Mitochondrial Respiration and Is Associated with an Altered Intramuscular Phospholipid Signature in Women with Obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Danese, E.; Salvagno, G.L.; Tarperi, C.; Negrini, D.; Montagnana, M.; Festa, L.; Sanchis-Gomar, F.; Schena, F.; Lippi, G. Middle-Distance Running Acutely Influences the Concentration and Composition of Serum Bile Acids: Potential Implications for Cancer Risk? Oncotarget 2017, 8, 52775–52782. [Google Scholar] [CrossRef] [Green Version]
- Nix, C.; Hemmati, M.; Cobraiville, G.; Servais, A.C.; Fillet, M. Blood Microsampling to Monitor Metabolic Profiles during Physical Exercise. Front. Mol. Biosci. 2021, 8, 681400. [Google Scholar] [CrossRef]
- Hammond, K.A.; Diamond, J. Maximal Sustained Energy Budgets in Humans and Animals. Nature 1997, 386, 457–462. [Google Scholar] [CrossRef]
- Alack, K.; Kruger, K.; Weiss, A.; Schermuly, R.; Frech, T.; Eggert, M.; Mooren, F.C. Aerobic Endurance Training Status Affects Lymphocyte Apoptosis Sensitivity by Induction of Molecular Genetic Adaptations. Brain Behav. Immun. 2019, 75, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Alack, K.; Weiss, A.; Kruger, K.; Horet, M.; Schermuly, R.; Frech, T.; Eggert, M.; Mooren, F.C. Profiling of Human Lymphocytes Reveals a Specific Network of Protein Kinases Modulated by Endurance Training Status. Sci. Rep. 2020, 10, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zukunft, S.; Prehn, C.; Rohring, C.; Moller, G.; de Angelis, M.H.; Adamski, J.; Tokarz, J. High-Throughput Extraction and Quantification Method for Targeted Metabolomics in Murine Tissues. Metabolomics 2018, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamazhakypov, A.; Weiss, A.; Zukunft, S.; Sydykov, A.; Kojonazarov, B.; Wilhelm, J.; Vroom, C.; Petrovic, A.; Kosanovic, D.; Weissmann, N.; et al. Effects of Macitentan and Tadalafil Monotherapy or Their Combination on the Right Ventricle and Plasma Metabolites in Pulmonary Hypertensive Rats. Pulm. Circ. 2020, 10, 2045894020947283. [Google Scholar] [CrossRef] [PubMed]
- Parry-Williams, G.; Sharma, S. The Effects of Endurance Exercise on the Heart: Panacea or Poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered Levels of Serum Sphingomyelin and Ceramide Containing Distinct Acyl Chains in Young Obese Adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Herrmann, N.; Dinoff, A.; Marzolini, S.; Mielke, M.M.; Andreazza, A.; Oh, P.I.; Venkata, S.L.V.; Haughey, N.J.; Lanctot, K.L. Association between Sphingolipids and Cardiopulmonary Fitness in Coronary Artery Disease Patients Undertaking Cardiac Rehabilitation. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 671–679. [Google Scholar] [CrossRef] [Green Version]
- Sigruener, A.; Kleber, M.E.; Heimerl, S.; Liebisch, G.; Schmitz, G.; Maerz, W. Glycerophospholipid and Sphingolipid Species and Mortality: The Ludwigshafen Risk and Cardiovascular Health (Luric) Study. PLoS ONE 2014, 9, e85724. [Google Scholar] [CrossRef] [Green Version]
- Merz, B.; Nothlings, U.; Wahl, S.; Haftenberger, M.; Schienkiewitz, A.; Adamski, J.; Suhre, K.; Wang-Sattler, R.; Grallert, H.; Thorand, B.; et al. Specific Metabolic Markers Are Associated with Future Waist-Gaining Phenotype in Women. PLoS ONE 2016, 11, e0157733. [Google Scholar] [CrossRef]
- Schranner, D.; Schonfelder, M.; Romisch-Margl, W.; Scherr, J.; Schlegel, J.; Zelger, O.; Riermeier, A.; Kaps, S.; Prehn, C.; Adamski, J.; et al. Physiological Extremes of the Human Blood Metabolome: A Metabolomics Analysis of Highly Glycolytic, Oxidative, and Anabolic Athletes. Physiol. Rep. 2021, 9, e14885. [Google Scholar] [CrossRef]
- Schader, J.F.; Haid, M.; Cecil, A.; Schoenfeld, J.; Halle, M.; Pfeufer, A.; Prehn, C.; Adamski, J.; Nieman, D.C.; Scherr, J. Metabolite Shifts Induced by Marathon Race Competition Differ between Athletes Based on Level of Fitness and Performance: A Substudy of the Enzy-MagIC Study. Metabolites 2020, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcinkiewicz-Siemion, M.; Ciborowski, M.; Ptaszynska-Kopczynska, K.; Szpakowicz, A.; Lisowska, A.; Jasiewicz, M.; Waszkiewicz, E.; Kretowski, A.; Musial, W.J.; Kaminski, K.A. Lc-Ms-Based Serum Fingerprinting Reveals Significant Dysregulation of Phospholipids in Chronic Heart Failure. J. Pharm. Biomed. Anal. 2018, 154, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Kistner, S.; Doring, M.; Kruger, R.; Rist, M.J.; Weinert, C.H.; Bunzel, D.; Merz, B.; Radloff, K.; Neumann, R.; Hartel, S.; et al. Sex-Specific Relationship between the Cardiorespiratory Fitness and Plasma Metabolite Patterns in Healthy Humans-Results of the Karmen Study. Metabolites 2021, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [PubMed] [Green Version]
- Morville, T.; Sahl, R.E.; Trammell, S.A.; Svenningsen, J.S.; Gillum, M.P.; Helge, J.W.; Clemmensen, C. Divergent Effects of Resistance and Endurance Exercise on Plasma Bile Acids, Fgf19, and Fgf21 in Humans. JCI Insight 2018, 3, e122737. [Google Scholar] [CrossRef]
- Bao, L.; Hao, D.; Wang, X.; He, X.; Mao, W.; Li, P. Transcriptome Investigation of Anti-Inflammation and Immuno-Regulation Mechanism of Taurochenodeoxycholic Acid. BMC Pharmacol. Toxicol. 2021, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.C.; Duan, G.Z.; Mao, W.; Liu, Q.; Zhang, Y.L.; Li, P.F. Taurochenodeoxycholic Acid Mediates Camp-Pka-Creb Signaling Pathway. Chin. J. Nat. Med. 2020, 18, 898–906. [Google Scholar] [CrossRef]
- Anwer, M.S. Intracellular Signaling by Bile Acids. J. Biosci. 2012, 20, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Mohr, A.E.; Jager, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The Athletic Gut Microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Todd, M.K.; Goldfarb, A.H.; Boyer, B.T. Effect of Exercise Intensity on 6-Keto-Pgf1 Alpha, Txb2, and 6-Keto-Pgf1 Alpha/Txb2 Ratios. Thromb. Res. 1992, 65, 487–493. [Google Scholar] [CrossRef]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of Platelet Aggregation and Eicosanoid Production by Phenolic Components of Olive Oil. Thromb Res. 1995, 78, 151–160. [Google Scholar] [CrossRef]
- Patelis, N.; Karaolanis, G.; Kouvelos, G.N.; Hart, C.; Metheiken, S. The Effect of Exercise on Coagulation and Fibrinolysis Factors in Patients with Peripheral Arterial Disease. Exp. Biol. Med. 2016, 241, 1699–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welty, F.K.; Schulte, F.; Alfaddagh, A.; Elajami, T.K.; Bistrian, B.R.; Hardt, M. Regression of Human Coronary Artery Plaque Is Associated with a High Ratio of (18-Hydroxy-Eicosapentaenoic Acid + Resolvin E1) to Leukotriene B4. FASEB J. 2021, 35, e21448. [Google Scholar] [CrossRef] [PubMed]
- Alshahawy, R.; Habachi, N.E.; Allam, E.; Jerneren, F.; Refsum, H.; Elshorbagy, A. Changes in Plasma Fatty Acids and Related Biomarkers During Transition to an Exclusively Plant- and Fish-Based Diet in Healthy Adults. Nutrition 2021, 90, 111306. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acids as Metabolic Regulators and Nutrient Sensors. Annu. Rev. Nutr. 2019, 39, 175–200. [Google Scholar] [CrossRef] [PubMed]


| Metabolite | HMDB ID | Category | FC | Log2 (FC) | p-Value | −Log10 (p-Value) | ET vs. UT |
|---|---|---|---|---|---|---|---|
| SM C22:3 | 0013468 | SM | 0.578 | −0.791 | 0.037 | 1.428 | Down (2) |
| C14:1 | 0002014 | AC | 0.602 | −0.732 | 0.038 | 1.421 | Down (3) |
| lysoPC a C18:2 | 0010386 | GPL | 2.157 | 1.109 | 0.004 | 2.428 | Up (5) |
| PC aa C42:0 | 0008537 | GPL | 1.604 | 0.682 | 0.019 | 1.731 | Up (6) |
| PC ae C38:2 | 0013436 0013431 | GPL | 1.516 | 0.600 | 0.029 | 1.535 | Up (7) |
| PC aa C38:5 | 0008114 | GPL | 0.610 | −0.714 | 0.014 | 1.859 | Down (1) |
| lysoPC a C26:0 | 0029205 | GPL | 0.560 | −0.835 | 0.038 | 1.417 | Down (4) |
| Metabolite (or Ratio) | HMDB ID | Category | FC | Log2 (FC) | p-Value | −Log10 (p-Value) | ET vs. UT |
|---|---|---|---|---|---|---|---|
| TCDCA | 0000951 | Bile acid | 0.381 | −1.392 | 0.002 | 2.828 | Down (1) |
| CA/CDCA | 0000619/ 0000518 | Bile acid | 2.645 | 1.403 | 0.041 | 1.393 | Up (3) |
| CA/(GCA+TCA) | 0000619/ (0000138 + 0000036) | Bile acid | 1.923 | 0.943 | 0.031 | 1.504 | Up (2) |
| DCA/(GDCA+TDCA) | 0000626/ (0252868 + 0000896) | Bile acid | 4.175 | 2.062 | 0.048 | 1.321 | Up (4) |
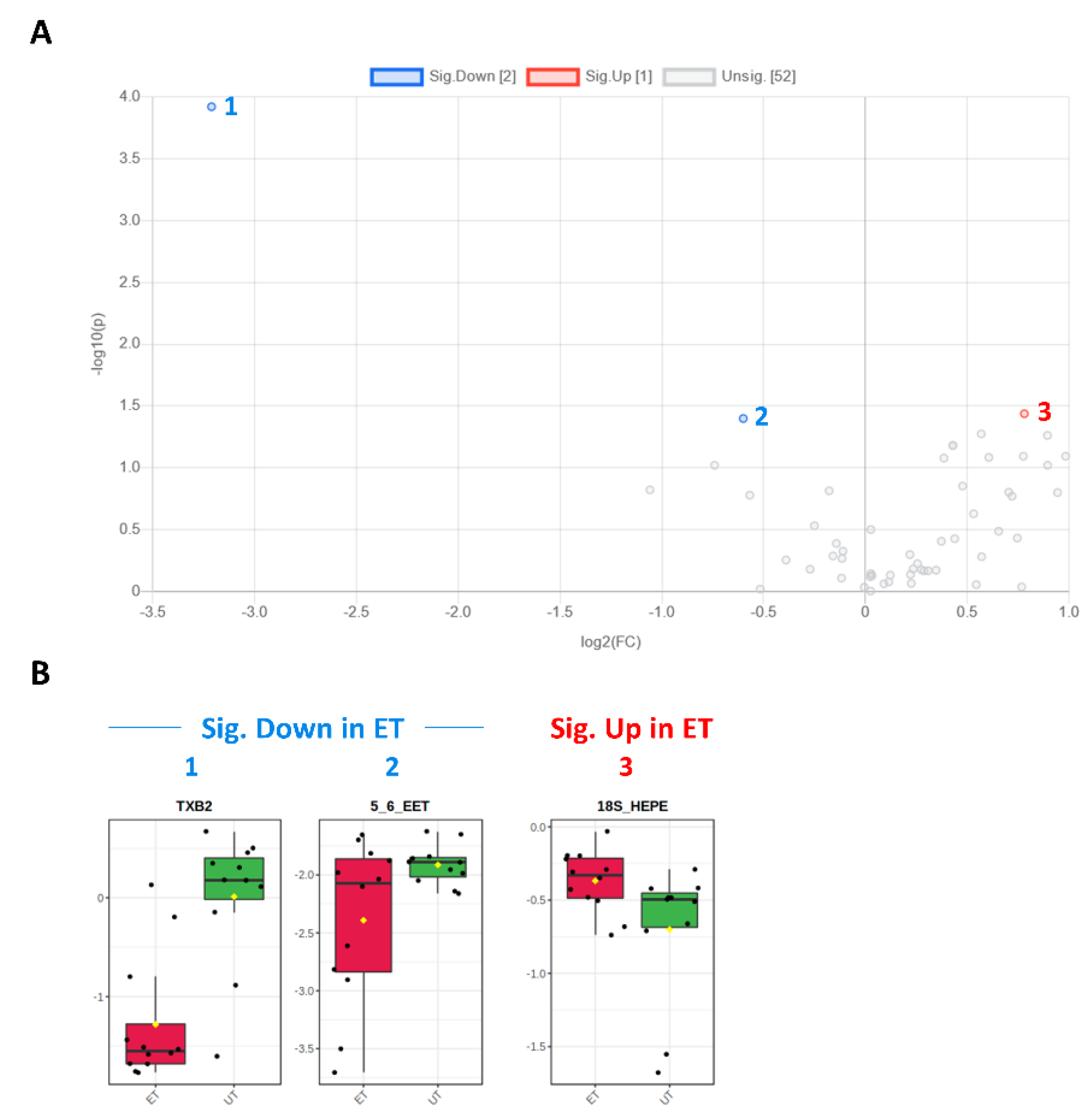
| Metabolite | HMDB ID | Category | FC | Log2 (FC) | p-Value | −Log10 (p-Value) | ET vs. UT |
|---|---|---|---|---|---|---|---|
| TXB2 | 0003252 | Eicosanoid | 0.107 | −3.212 | 0.001 | 3.922 | Down (1) |
| 5,6-EET | 0246887 | Eicosanoid | 0.660 | −0.600 | 0.040 | 1.398 | Down (2) |
| 18S-HEPE | 0257623 (S-Enantiomer) | PUFA | 1.718 | 0.781 | 0.037 | 1.437 | Up (3) |
| Metabolite | HMDB ID | Category | Pearson R | 95% CI | R Squared | p Value | Sig. Level |
|---|---|---|---|---|---|---|---|
| Asp | 0000191 | AA | −0.5046 | −0.7589 to −0.1167 | 0.2546 | 0.0141 | * |
| Glu | 0000148 | AA | −0.5268 | −0.7715 to −0.1464 | 0.2776 | 0.0098 | ** |
| Cit/Orn | Cit: 0000904 Orn: 0000214 | AA | 0.5518 | 0.1808 to 0.7854 | 0.3045 | 0.0063 | ** |
| α-AAA | 0000510 | BA | −0.4722 | −0.7403 to −0.07449 | 0.223 | 0.0229 | * |
| Taurine | 0000251 | BA | −0.4512 | −0.7280 to −0.04785 | 0.2035 | 0.0307 | * |
| SM C18:0 | 0001348 | SM | −0.421 | −0.7100 to −0.01066 | 0.1773 | 0.0454 | * |
| SM C18:1 | 0012101 | SM | −0.5023 | −0.7576 to −0.1136 | 0.2523 | 0.0146 | * |
| C16 | 0000222 | AC | −0.4414 | −0.7222 to −0.03565 | 0.1948 | 0.035 | * |
| PC aa C38:4 | 0008048 | GPL | −0.4283 | −0.7144 to −0.01958 | 0.1835 | 0.0414 | * |
| PC ae C38:2 | 0013431 0013436 | GPL | 0.5011 | 0.1121 to 0.7570 | 0.2511 | 0.0149 | * |
| PC ae C42:2 | 0013438 | GPL | 0.4517 | 0.0485 to 0.7283 | 0.204 | 0.0305 | * |
| lysoPC a C18:1 | 0010385 0010408 0002815 | GPL | 0.4914 | 0.0993 to 0.7514 | 0.2415 | 0.0173 | * |
| lysoPCtotal/PCtotal | GPL | 0.5409 | 0.1657 to 0.7794 | 0.2926 | 0.0077 | ** | |
| GCDCA | 0000637 | Bile acid | −0.4482 | −0.7262 to −0.04413 | 0.2009 | 0.032 | * |
| TCDCA | 0000951 | Bile acid | −0.462 | −0.7343 to −0.06148 | 0.2134 | 0.0265 | * |
| CA/CDCA | CA: 0000619 CDCA: 000518 | Bile acid | 0.5383 | 0.1620 to 0.7779 | 0.2898 | 0.0081 | ** |
| LXA4 | 0004385 | PUFA | 0.4313 | 0.0232 to 0.7161 | 0.186 | 0.0399 | * |
| 18-HEPE | 0257623 | PUFA | 0.466 | 0.0552 to 0.7419 | 0.2171 | 0.0288 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, A.; Alack, K.; Klatt, S.; Zukunft, S.; Schermuly, R.; Frech, T.; Mooren, F.-C.; Krüger, K. Sustained Endurance Training Leads to Metabolomic Adaptation. Metabolites 2022, 12, 658. https://doi.org/10.3390/metabo12070658
Weiss A, Alack K, Klatt S, Zukunft S, Schermuly R, Frech T, Mooren F-C, Krüger K. Sustained Endurance Training Leads to Metabolomic Adaptation. Metabolites. 2022; 12(7):658. https://doi.org/10.3390/metabo12070658
Chicago/Turabian StyleWeiss, Astrid, Katharina Alack, Stephan Klatt, Sven Zukunft, Ralph Schermuly, Torsten Frech, Frank-Christoph Mooren, and Karsten Krüger. 2022. "Sustained Endurance Training Leads to Metabolomic Adaptation" Metabolites 12, no. 7: 658. https://doi.org/10.3390/metabo12070658
APA StyleWeiss, A., Alack, K., Klatt, S., Zukunft, S., Schermuly, R., Frech, T., Mooren, F.-C., & Krüger, K. (2022). Sustained Endurance Training Leads to Metabolomic Adaptation. Metabolites, 12(7), 658. https://doi.org/10.3390/metabo12070658






